Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression of Genes Related to VITD Metabolism
2.2. Cell Culture
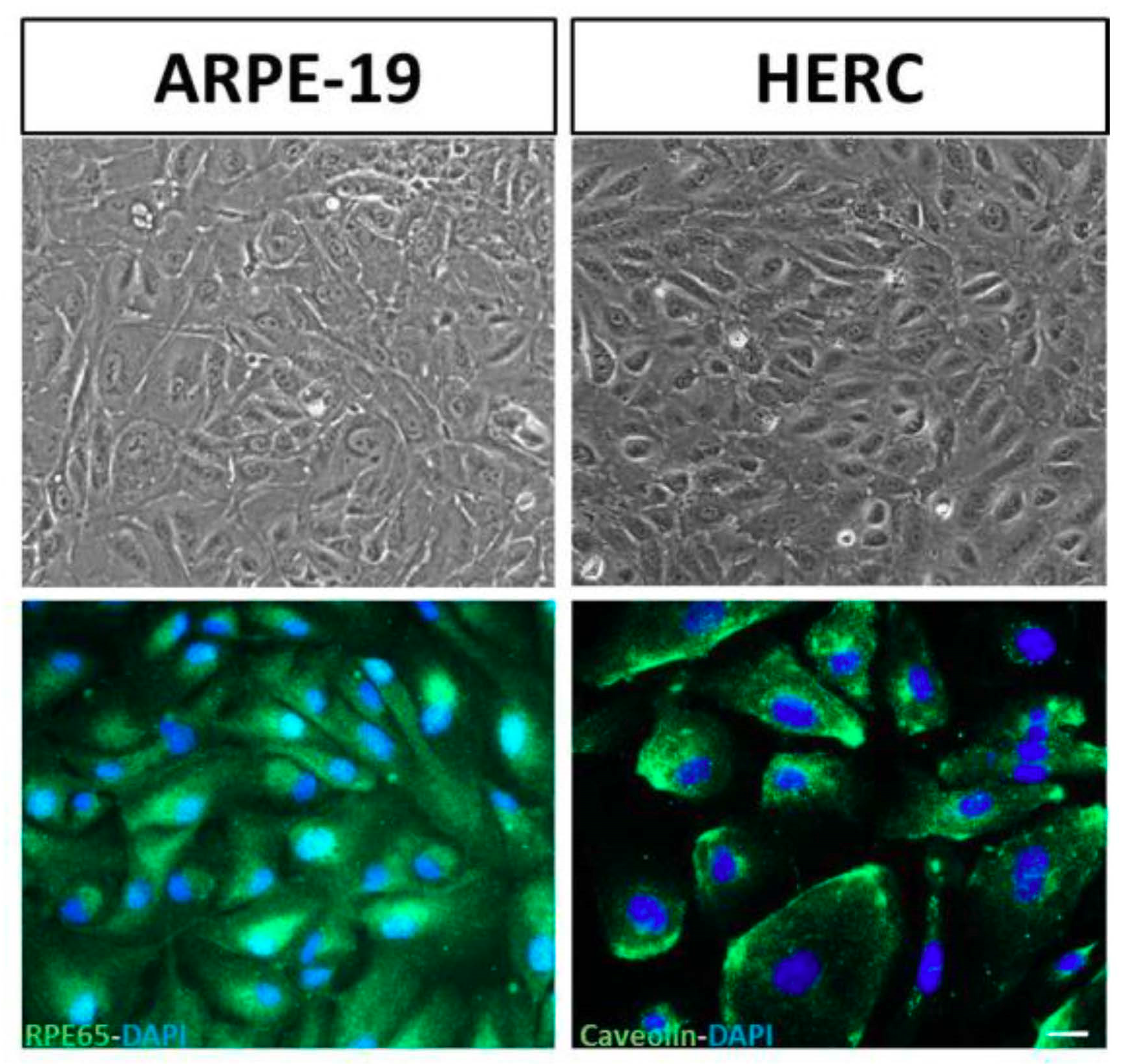
2.3. Validation of the Cell Lines: Stable Phenotypic Characterization
2.4. Treatments and Experimental Design: Oxidative Stress and Inflammation-Like Conditions
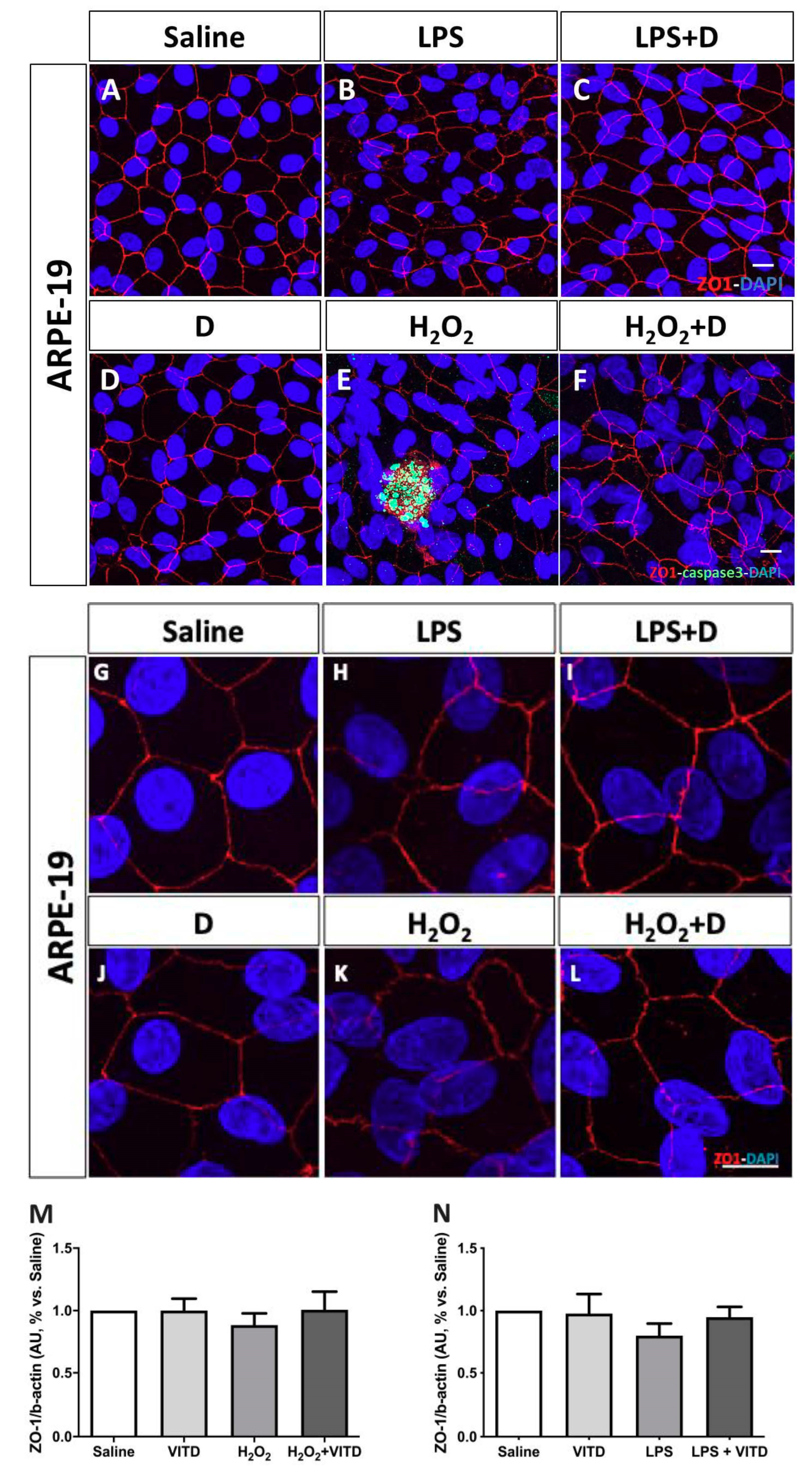
2.5. Cell Structure and Integrity: Zonula Occludens (ZO)-1 Immunofluorescence and Western Blot
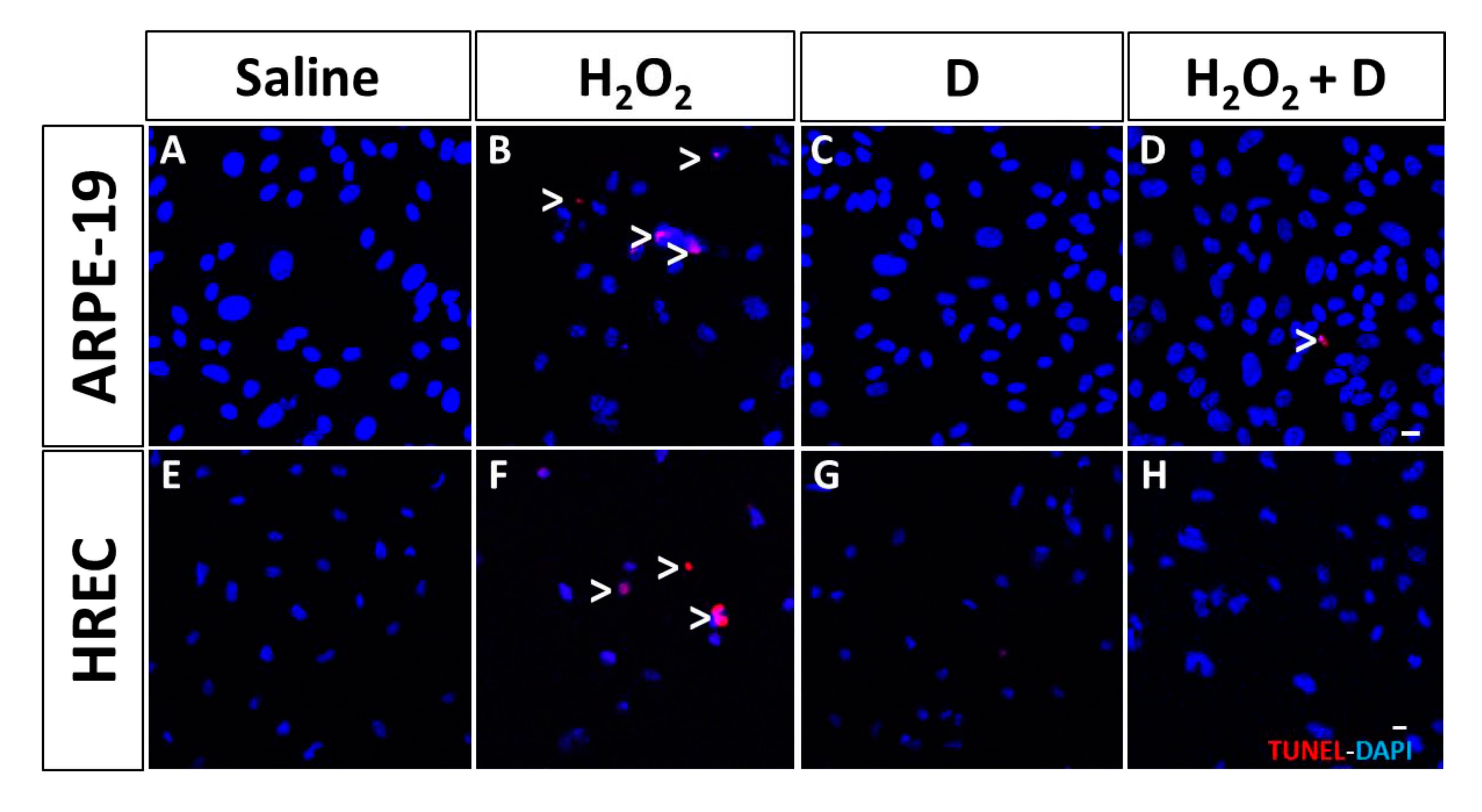
2.6. Assay to Detect Cell Apoptosis
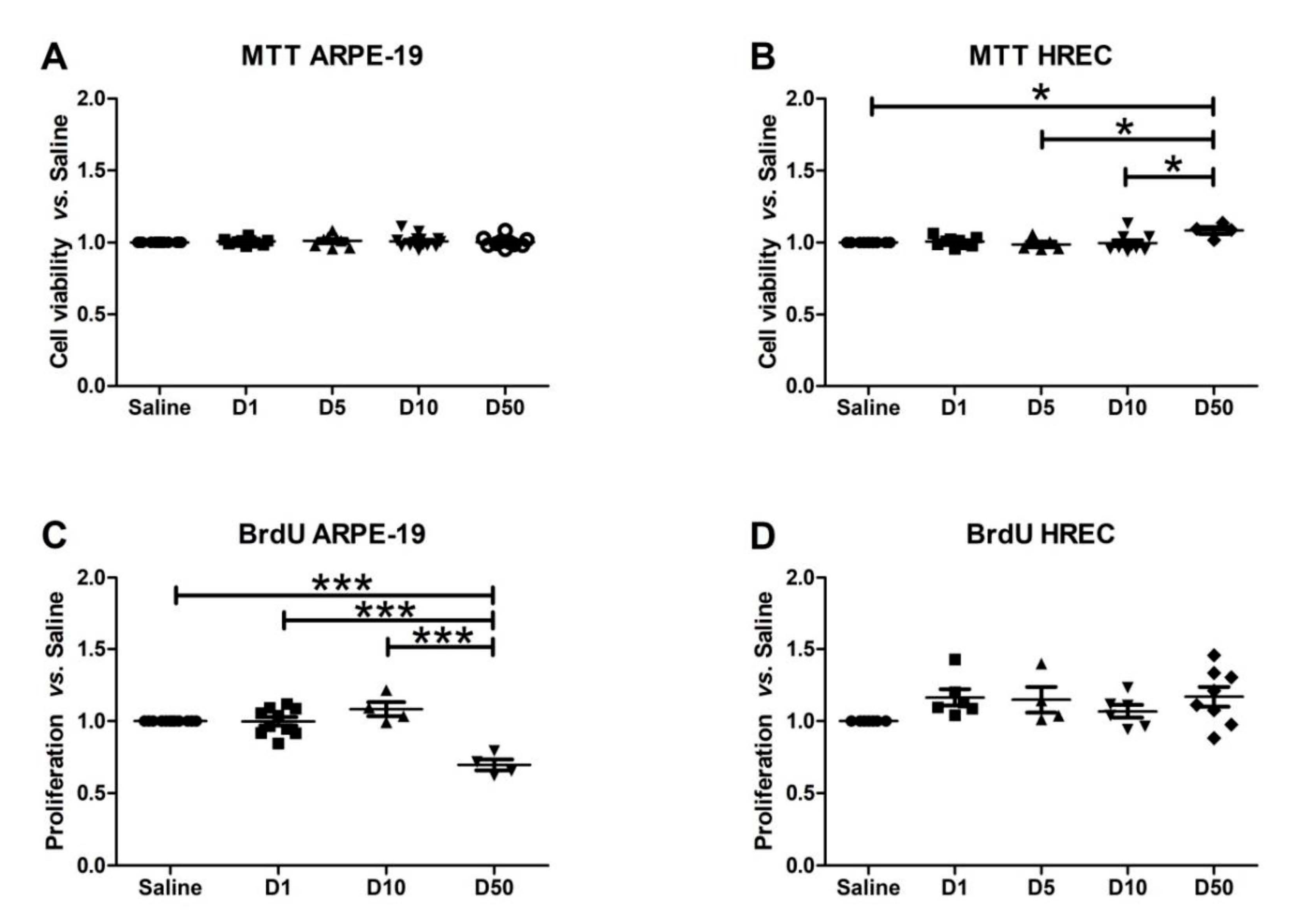
2.7. Viability/Toxicity Assay (MTT)
2.8. Proliferation Assay (Bromodeoxyuridine, BrdU)
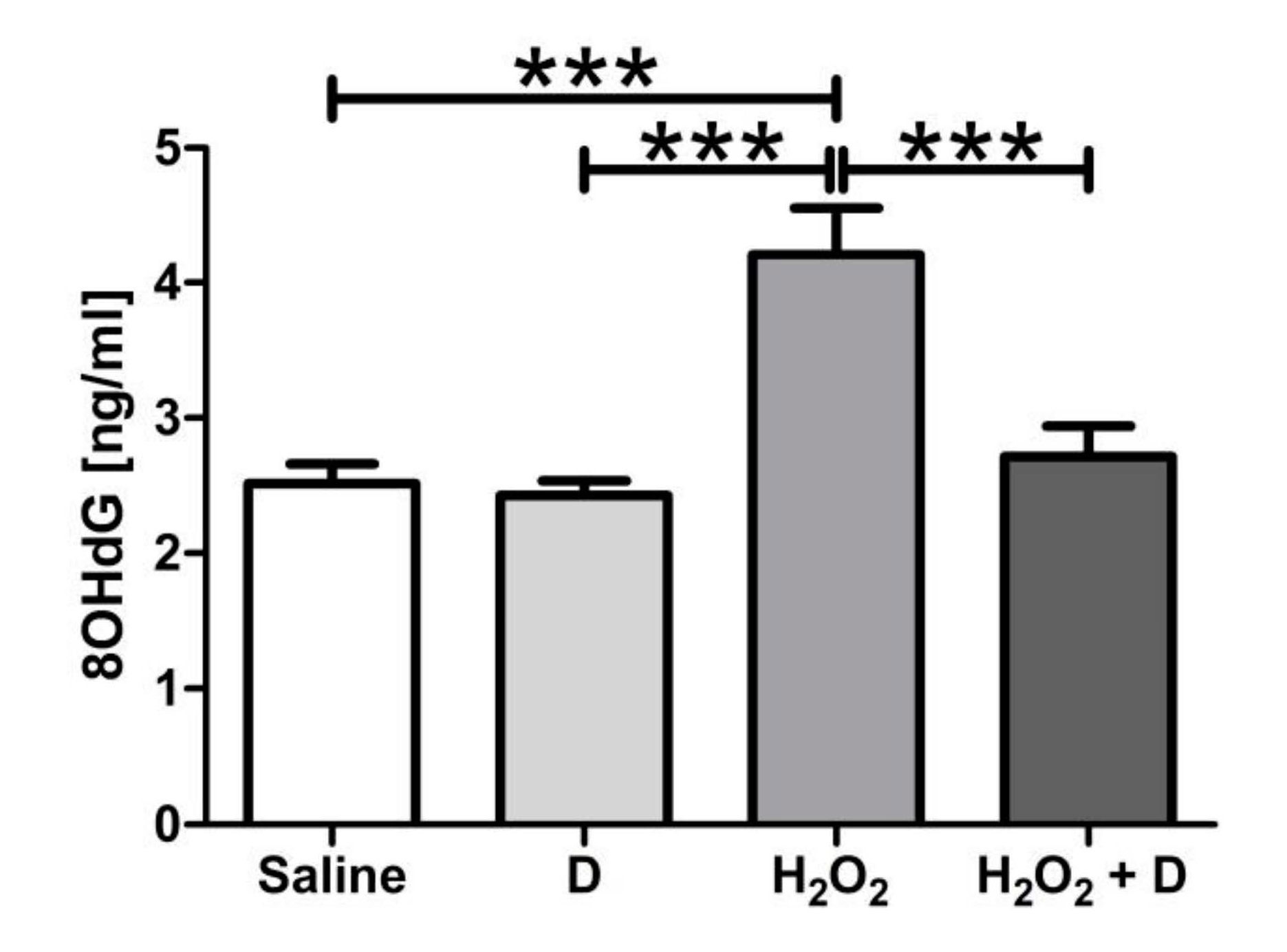
2.9. Measurement of 8-Hydroxidioxiguanosine (8-OHdG) under Oxidative Stress Conditions
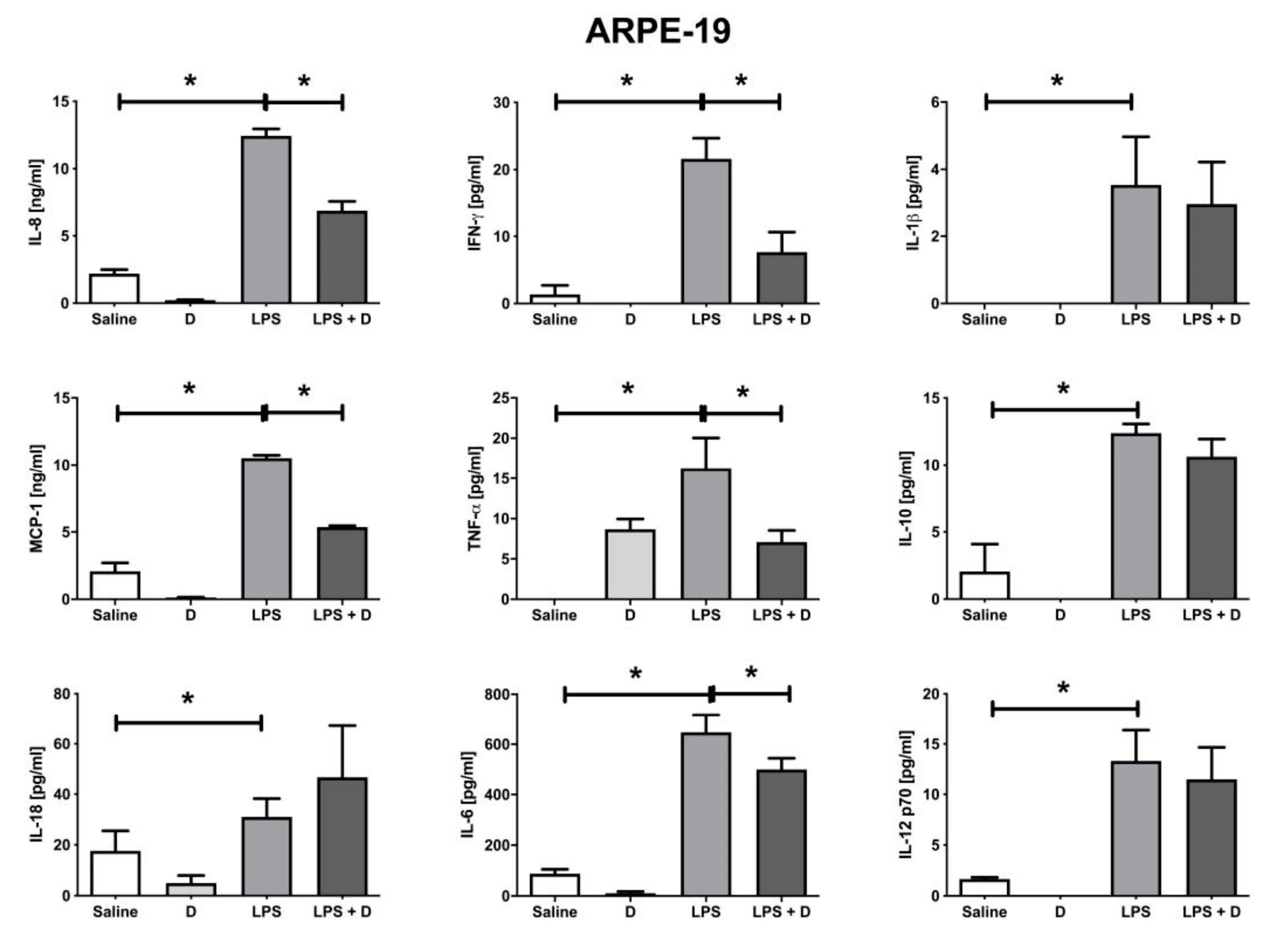
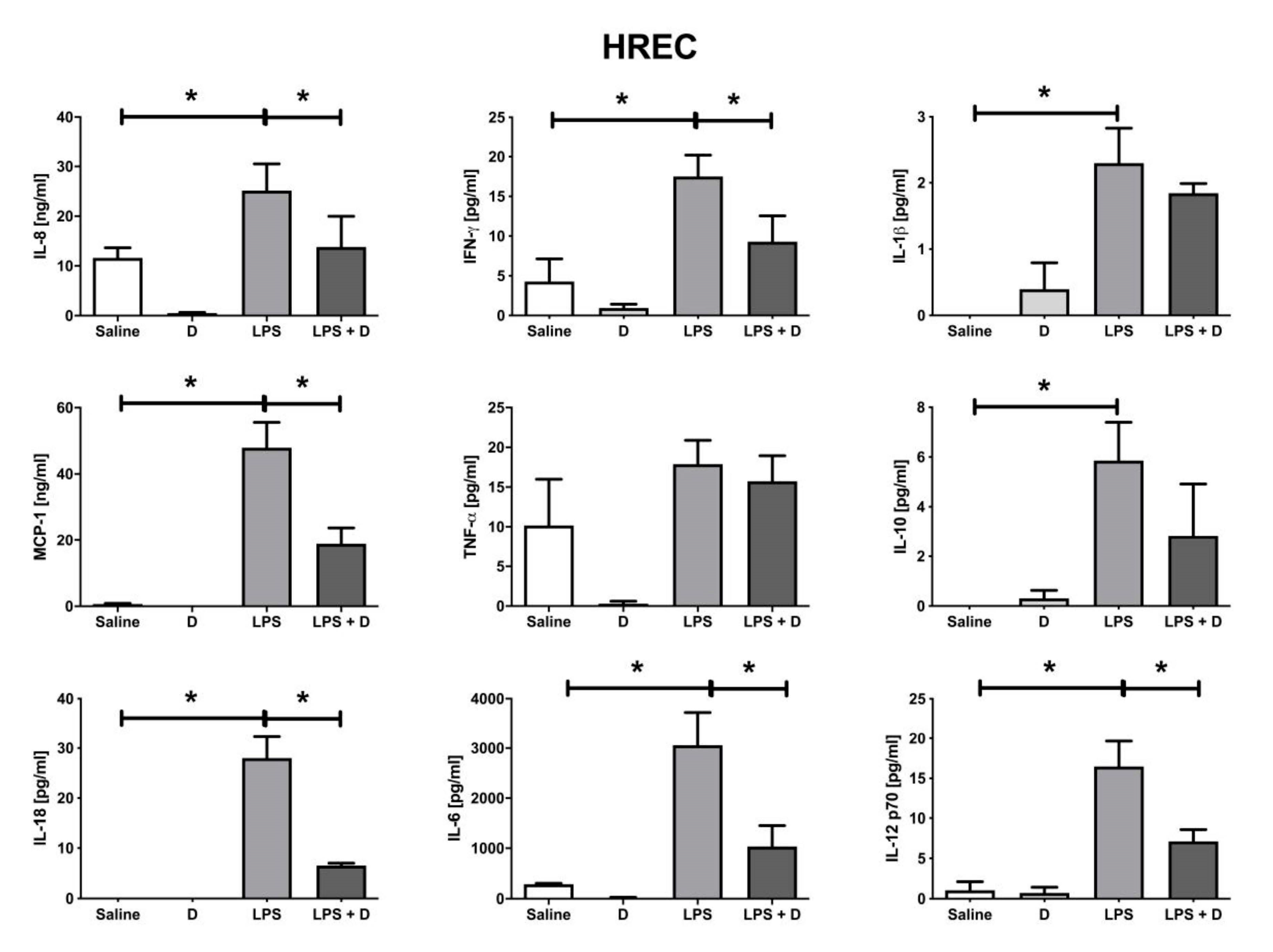
2.10. Multiplex Cytokine Analysis under Inflammatory and Basal Conditions: Interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-12p70, and IL-18; Interferon (IFN)-γ; Monocyte Chemoattractant Protein (MCP)-1; and Tumor Necrosis Factor (TNF)-α
2.11. Statistical Analysis
3. Results
3.1. Confirmation of VITD Receptor Expression in ARPE-19 and HREC Cell Lines
3.2. Validation of the Cell Lines: Stable Phenotypic Characterization
3.3. Effect of VITD Addition on Cytoxicity and Proliferation
3.4. Effect of VITD Addition on Integrity and Apoptosis of Cells
3.5. Antioxidative and Anti-Inflammatory Properties of VITD Addition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratton, I.M. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The ACCORD Study Group and ACCORD Eye Study Group Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes. N. Engl. J. Med. 2010, 363, 233–244. [CrossRef] [PubMed] [Green Version]
- The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [CrossRef] [PubMed]
- Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: Attractive, elusive, and resilient. Exp. Diabetes Res. 2007, 2007, 61038. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp. Mol. Pathol. 2003, 75, 95–108. [Google Scholar] [CrossRef]
- Donnelly, R.; Idris, I.; Forrester, J.V. Protein kinase C inhibition and diabetic retinopathy: A shot in the dark at translational research. Br. J. Ophthalmol. 2004, 88, 145–151. [Google Scholar] [CrossRef]
- Madsen-Bouterse, S.A.; Kowluru, R.A. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev. Endocr. Metab. Disord. 2008, 9, 315–327. [Google Scholar] [CrossRef]
- Calderon, G.D.; Juarez, O.H.; Hernandez, G.E.; Punzo, S.M.; De la Cruz, Z.D. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2017, 31, 1122–1130. [Google Scholar] [CrossRef]
- Cecilia, O.-M.; José Alberto, C.-G.; José, N.-P.; Ernesto Germán, C.-M.; Ana Karen, L.-C.; Luis Miguel, R.-P.; Ricardo Raúl, R.-R.; Adolfo Daniel, R.-C. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J. Diabetes Res. 2019, 2019, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Miao, X.; Li, F.; Wang, S.; Liu, Q.; Wang, Y.; Sun, J. Oxidative Stress-Related Mechanisms and Antioxidant Therapy in Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquida, M.; Drawnel, F.; Fauser, S. The role of inflammation in diabetic eye disease. Semin. Immunopathol. 2019, 41, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr. J. Ophthalmol. 2012, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Hamden, K.; Carreau, S.; Jamoussi, K.; Miladi, S.; Lajmi, S.; Aloulou, D.; Ayadi, F.; Elfeki, A. 1α,25 Dihydroxyvitamin D3: Therapeutic and Preventive Effects against Oxidative Stress, Hepatic, Pancreatic and Renal Injury in Alloxan-Induced Diabetes in Rats. J. Nutr. Sci. Vitaminol. 2009, 55, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Tohari, A.M.; Almarhoun, M.; Alhasani, R.H.; Biswas, L.; Zhou, X.; Reilly, J.; Zeng, Z.; Shu, X. Protection by vitamin D against high-glucose-induced damage in retinal pigment epithelial cells. Exp. Cell Res. 2020, 392, 112023. [Google Scholar] [CrossRef]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D 3 Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef] [Green Version]
- Alatawi, F.S.; Faridi, U.A.; Alatawi, M.S. Effect of treatment with vitamin D plus calcium on oxidative stress in streptozotocin-induced diabetic rats. Saudi Pharm. J. 2018, 26, 1208–1213. [Google Scholar] [CrossRef]
- Cyganek, K.; Mirkiewicz-Sieradzka, B.; Malecki, M.T.; Wolkow, P.; Skupien, J.; Bobrek, J.; Czogala, M.; Klupa, T.; Sieradzki, J. Clinical risk factors and the role of VDR gene polymorphisms in diabetic retinopathy in Polish type 2 diabetes patients. Acta Diabetol. 2006, 43, 114–119. [Google Scholar] [CrossRef]
- Zhong, X.; Du, Y.; Lei, Y.; Liu, N.; Guo, Y.; Pan, T. Effects of vitamin D receptor gene polymorphism and clinical characteristics on risk of diabetic retinopathy in Han Chinese type 2 diabetes patients. Gene 2015, 566, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Taverna, M.J.; Sola, A.; Guyot-Argenton, C.; Pacher, N.; Bruzzo, F.; Slama, G.; Reach, G.; Selam, J.-L. Taq I polymorphism of the vitamin D receptor and risk of severe diabetic retinopathy. Diabetologia 2002, 45, 436–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taverna, M.J.; Selam, J.-L.; Slama, G. Association between a Protein Polymorphism in the Start Codon of the Vitamin D Receptor Gene and Severe Diabetic Retinopathy in C-Peptide-Negative Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2005, 90, 4803–4808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afarid, M.; Ghattavi, N.; Karim Johari, M. Serum Levels of Vitamin D in Diabetic Patients with and without Retinopathy. J. Ophthalmic Vis. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ashinne, B.; Rajalakshmi, R.; Anjana, R.M.; Narayan, K.M.V.; Jayashri, R.; Mohan, V.; Hendrick, A.M. Association of serum vitamin D levels and diabetic retinopathy in Asian Indians with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 139, 308–313. [Google Scholar] [CrossRef]
- Wu, W.; Weng, Y.; Guo, X.; Feng, L.; Xia, H.; Jiang, Z.; Lou, J. The Association between Serum Vitamin D Levels and Age-Related Macular Degeneration: A Systematic Meta-Analytic Review. Investig. Opthalmol. Vis. Sci. 2016, 57, 2168. [Google Scholar] [CrossRef] [Green Version]
- Tohari, A.M.; Alhasani, R.H.; Biswas, L.; Patnaik, S.R.; Reilly, J.; Zeng, Z.; Shu, X. Vitamin D Attenuates Oxidative Damage and Inflammation in Retinal Pigment Epithelial Cells. Antioxidants 2019, 8, 341. [Google Scholar] [CrossRef] [Green Version]
- Layana, A.; Minnella, A.; Garhöfer, G.; Aslam, T.; Holz, F.; Leys, A.; Silva, R.; Delcourt, C.; Souied, E.; Seddon, J. Vitamin D and Age-Related Macular Degeneration. Nutrients 2017, 9, 1120. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.K.; Keenan, H.A.; Cavallerano, J.D.; Asztalos, B.F.; Schaefer, E.J.; Sell, D.R.; Strauch, C.M.; Monnier, V.M.; Doria, A.; Aiello, L.P.; et al. Protection From Retinopathy and Other Complications in Patients With Type 1 Diabetes of Extreme Duration: The Joslin 50-Year Medalist Study. Diabetes Care 2011, 34, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Keenan, H.A.; Costacou, T.; Sun, J.K.; Doria, A.; Cavellerano, J.; Coney, J.; Orchard, T.J.; Aiello, L.P.; King, G.L. Clinical Factors Associated With Resistance to Microvascular Complications in Diabetic Patients of Extreme Disease Duration: The 50-year Medalist Study. Diabetes Care 2007, 30, 1995–1997. [Google Scholar] [CrossRef] [Green Version]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voronova, V.; Zhudenkov, K.; Helmlinger, G.; Peskov, K. Interpretation of metabolic memory phenomenon using a physiological systems model: What drives oxidative stress following glucose normalization? PLoS ONE 2017, 12, e0171781. [Google Scholar] [CrossRef] [PubMed]
- Jha, J.C.; Ho, F.; Dan, C.; Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. 2018, 132, 1811–1836. [Google Scholar] [CrossRef]
- Aghadavod, E.; Khodadadi, S.; Baradaran, A.; Nasri, P.; Bahmani, M.; Rafieian-Kopaei, M. Role of Oxidative Stress and Inflammatory Factors in Diabetic Kidney Disease. Iran. J. Kidney Dis. 2016, 10, 337–343. [Google Scholar] [PubMed]
- Jha, J.C.; Banal, C.; Chow, B.S.M.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J. Diabetes Res. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Liyanagamage, D.S.N.K.; Martinus, R.D. Role of Mitochondrial Stress Protein HSP60 in Diabetes-Induced Neuroinflammation. Mediators Inflamm. 2020, 2020, 8073516. [Google Scholar] [CrossRef]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Robay, A.; Chidiac, O.M.; Jayyousi, A.; Al Suwaidi, J.; Crystal, R.G.; Atkin, S.L.; et al. Association of vitamin D2 and D3 with type 2 diabetes complications. BMC Endocr. Disord. 2020, 20, 65. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. N. Am. 2010, 39, 243–253. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Labudzynskyi, D.O.; Zaitseva, O.V.; Latyshko, N.V.; Gudkova, O.O.; Veliky, M.M. Vitamin D3 contribution to the regulation of oxidative metabolism in the liver of diabetic mice. Ukr. Biochem. J. 2015, 87, 75–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, K.; Fujii, H.; Nakai, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Shinohara, M.; Hirata, M.; Fukagawa, M.; Nishi, S. Anti-oxidative effect of vitamin D analog on incipient vascular lesion in non-obese type 2 diabetic rats. Am. J. Nephrol. 2013, 37, 167–174. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Nameni, G.; Hajiluian, G.; Mesgari-Abbasi, M. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat-diet induced obese rats. BMC Cardiovasc. Disord. 2017, 17, 161. [Google Scholar] [CrossRef] [Green Version]
- Hajiluian, G.; Abbasalizad Farhangi, M.; Nameni, G.; Shahabi, P.; Megari-Abbasi, M. Oxidative stress-induced cognitive impairment in obesity can be reversed by vitamin D administration in rats. Nutr. Neurosci. 2018, 21, 744–752. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Hajiluian, G.; Nameni, G.; Shahabi, P. Adipose Tissue Inflammation and Oxidative Stress: The Ameliorative Effects of Vitamin D. Inflammation 2017, 40, 1688–1697. [Google Scholar] [CrossRef]
- Lin, Y.; Ubels, J.L.; Schotanus, M.P.; Yin, Z.; Pintea, V.; Hammock, B.D.; Watsky, M.A. Enhancement of Vitamin D Metabolites in the Eye Following Vitamin D3 Supplementation and UV-B Irradiation. Curr. Eye Res. 2012, 37, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Toda, R.; Kawazu, K.; Oyabu, M.; Miyazaki, T.; Kiuchi, Y. Comparison of drug permeabilities across the blood-retinal barrier, blood-aqueous humor barrier, and blood-brain barrier. J. Pharm. Sci. 2011, 100, 3904–3911. [Google Scholar] [CrossRef]
- Packer, L.; Cadenas, E. Oxidants and antioxidants revisited. New concepts of oxidative stress. Free Radic. Res. 2007, 41, 951–952. [Google Scholar] [CrossRef] [PubMed]
- Polidoro, L.; Properzi, G.; Marampon, F.; Gravina, G.L.; Festuccia, C.; Di Cesare, E.; Scarsella, L.; Ciccarelli, C.; Zani, B.M.; Ferri, C. Vitamin D Protects Human Endothelial Cells from H2O2 Oxidant Injury Through the Mek/Erk-Sirt1 Axis Activation. J Cardiovasc. Transl. Res. 2013, 6, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.-Y.; Ting, H.-J.; Hsu, J.-W.; Lee, Y.-F. Protective role of 1α, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int. J. Cancer 2008, 122, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Vaishnav, A.; Murillo, G.; Alimirah, F.; Torres, K.E.O.; Mehta, R.G. Protection against cellular stress by 25-hydroxyvitamin D3 in breast epithelial cells. J. Cell. Biochem. 2010, 110, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Namkoong, K.; Kim, D.-H.; Kim, K.-J.; Cheong, Y.-H.; Kim, S.-S.; Lee, W.-B.; Kim, K.-Y. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc. Res. 2004, 68, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.; Weger, M.; Haller-Schober, E.-M.; El-Shabrawi, Y.; Wedrich, A.; Theisl, A.; Aigner, R.; Barth, A.; Haas, A. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol. Vis. 2008, 14, 637–643. [Google Scholar]
- Koleva-Georgieva, D.N.; Sivkova, N.P.; Terzieva, D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med. 2011, 53, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, T.; Sonoda, K.; Sugahara, M.; Mochizuki, Y.; Enaida, H.; Oshima, Y.; Ueno, A.; Hata, Y.; Yoshida, H.; Ishibashi, T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE 2009, 4, e8158. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.G.; Das, A. Diabetic retinopathy and inflammation: Novel therapeutic targets. Middle East Afr. J. Ophthalmol. 2012, 19, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.-Z. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, T.; Felinski, E.A.; Antonetti, D.A. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem. 2009, 284, 21036–21046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.-R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Shab-Bidar, S.; Neyestani, T.R.; Djazayery, A.; Eshraghian, M.-R.; Houshiarrad, A.; Kalayi, A.; Shariatzadeh, N.; Khalaji, N.; Gharavi, A. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes: Vitamin D and Systemic Inflammation. Diabetes Metab. Res. Rev. 2012, 28, 424–430. [Google Scholar] [CrossRef]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.-C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Zhang, H.; Yu, J.; Chen, X.; Yang, L. Methylene Blue Attenuates Diabetic Retinopathy by Inhibiting NLRP3 Inflammasome Activation in STZ-Induced Diabetic Rats. Ocul. Immunol. Inflamm. 2019, 27, 836–843. [Google Scholar] [CrossRef]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, S.; Xia, X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr. Eye Res. 2012, 37, 416–420. [Google Scholar] [CrossRef]
- Loukovaara, S.; Piippo, N.; Kinnunen, K.; Hytti, M.; Kaarniranta, K.; Kauppinen, A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017, 95, 803–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, E.; Gordish-Dressman, H.; Hathout, Y. Effect of TNF-alpha on human ARPE-19-secreted proteins. Mol. Vis. 2008, 14, 2292–2303. [Google Scholar] [PubMed]
- Kutty, R.K.; Samuel, W.; Boyce, K.; Cherukuri, A.; Duncan, T.; Jaworski, C.; Nagineni, C.N.; Redmond, T.M. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol. Vis. 2016, 22, 1156–1168. [Google Scholar] [PubMed]
- Desjardins, D.M.; Yates, P.W.; Dahrouj, M.; Liu, Y.; Crosson, C.E.; Ablonczy, Z. Progressive Early Breakdown of Retinal Pigment Epithelium Function in Hyperglycemic Rats. Investig. Opthalmol. Vis. Sci. 2016, 57, 2706. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Chen, H.; Zheng, S. Alterations of Retinal Pigment Epithelium–Photoreceptor Complex in Patients with Type 2 Diabetes Mellitus without Diabetic Retinopathy: A Cross-Sectional Study. J. Diabetes Res. 2020, 2020, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Mao, C. Roles of elevated intravitreal IL-1β and IL-10 levels in proliferative diabetic retinopathy. Indian J. Ophthalmol. 2014, 62, 699. [Google Scholar] [CrossRef]
- Gee, K.; Guzzo, C.; Che Mat, N.; Ma, W.; Kumar, A. The IL-12 Family of Cytokines in Infection, Inflammation and Autoimmune Disorders. Inflamm. Allergy-Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef]
- Santos, J.M.; Mohammad, G.; Zhong, Q.; Kowluru, R.A. Diabetic Retinopathy, Superoxide Damage and Antioxidants. Curr. Pharm. Biotechnol. 2011, 12, 352–361. [Google Scholar] [CrossRef] [Green Version]
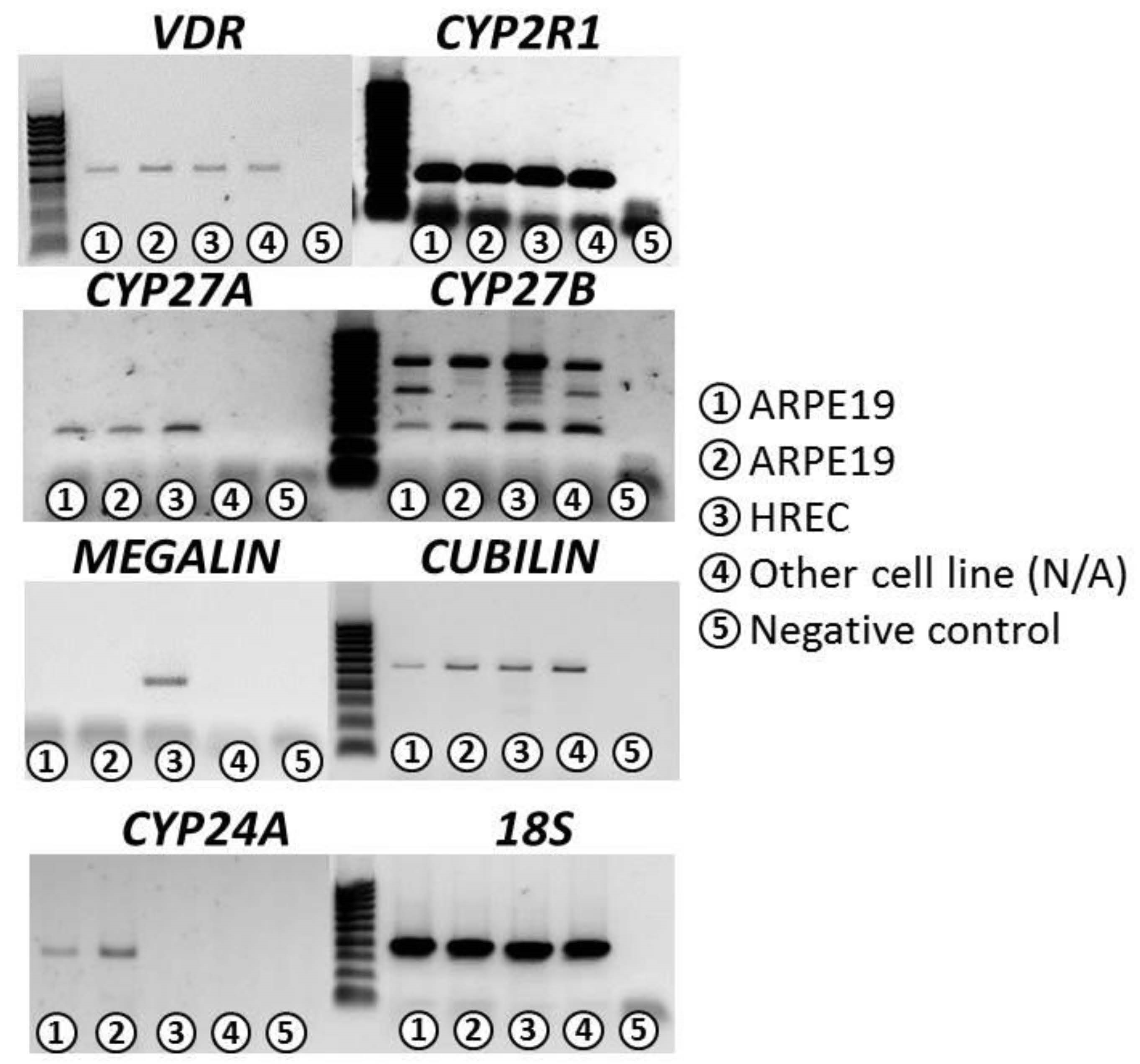
| VDR | CYP2R1 | CYP27A | CYP27B | CYP24A | Cubilin | Megalin | 18S | |
|---|---|---|---|---|---|---|---|---|
| ARPE-19 | ++ | +++ | ++ | +++ | +++ | ++ | − | +++ |
| HREC | ++ | +++ | ++ | +++ | − | ++ | ++ | +++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Robredo, P.; González-Zamora, J.; Recalde, S.; Bilbao-Malavé, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020, 9, 838. https://doi.org/10.3390/antiox9090838
Fernandez-Robredo P, González-Zamora J, Recalde S, Bilbao-Malavé V, Bezunartea J, Hernandez M, Garcia-Layana A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants. 2020; 9(9):838. https://doi.org/10.3390/antiox9090838
Chicago/Turabian StyleFernandez-Robredo, Patricia, Jorge González-Zamora, Sergio Recalde, Valentina Bilbao-Malavé, Jaione Bezunartea, Maria Hernandez, and Alfredo Garcia-Layana. 2020. "Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells" Antioxidants 9, no. 9: 838. https://doi.org/10.3390/antiox9090838
APA StyleFernandez-Robredo, P., González-Zamora, J., Recalde, S., Bilbao-Malavé, V., Bezunartea, J., Hernandez, M., & Garcia-Layana, A. (2020). Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants, 9(9), 838. https://doi.org/10.3390/antiox9090838





