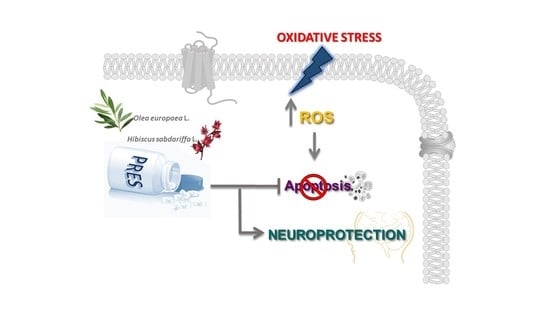
Olive Leaves and Hibiscus Flowers Extracts-Based Preparation Protect Brain from Oxidative Stress-Induced Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. PRES
2.2. Experiments on Human Neuroblastoma SH-SY5Y Cells
2.2.1. Cell Cultures
2.2.2. H2O2-Induced Injury
2.2.3. PRES Treatments
2.2.4. Cell Viability Assays
2.2.5. Apoptosis Assays
2.2.6. Intracellular ROS Content
2.2.7. Mitochondrial Electrochemical Potential Gradient (Δψ)
2.2.8. Caspase-3, Caspase-8 and Caspase-9
2.3. Experiments on Brain Slices
2.3.1. Preparation of Cortical Slices
2.3.2. PRES Treatment
2.3.3. Brain Slices Viability Assays
2.4. In Silico Prediction of Main PRES Component Pharmacokinetic Properties
2.5. Statistical Analysis
3. Results
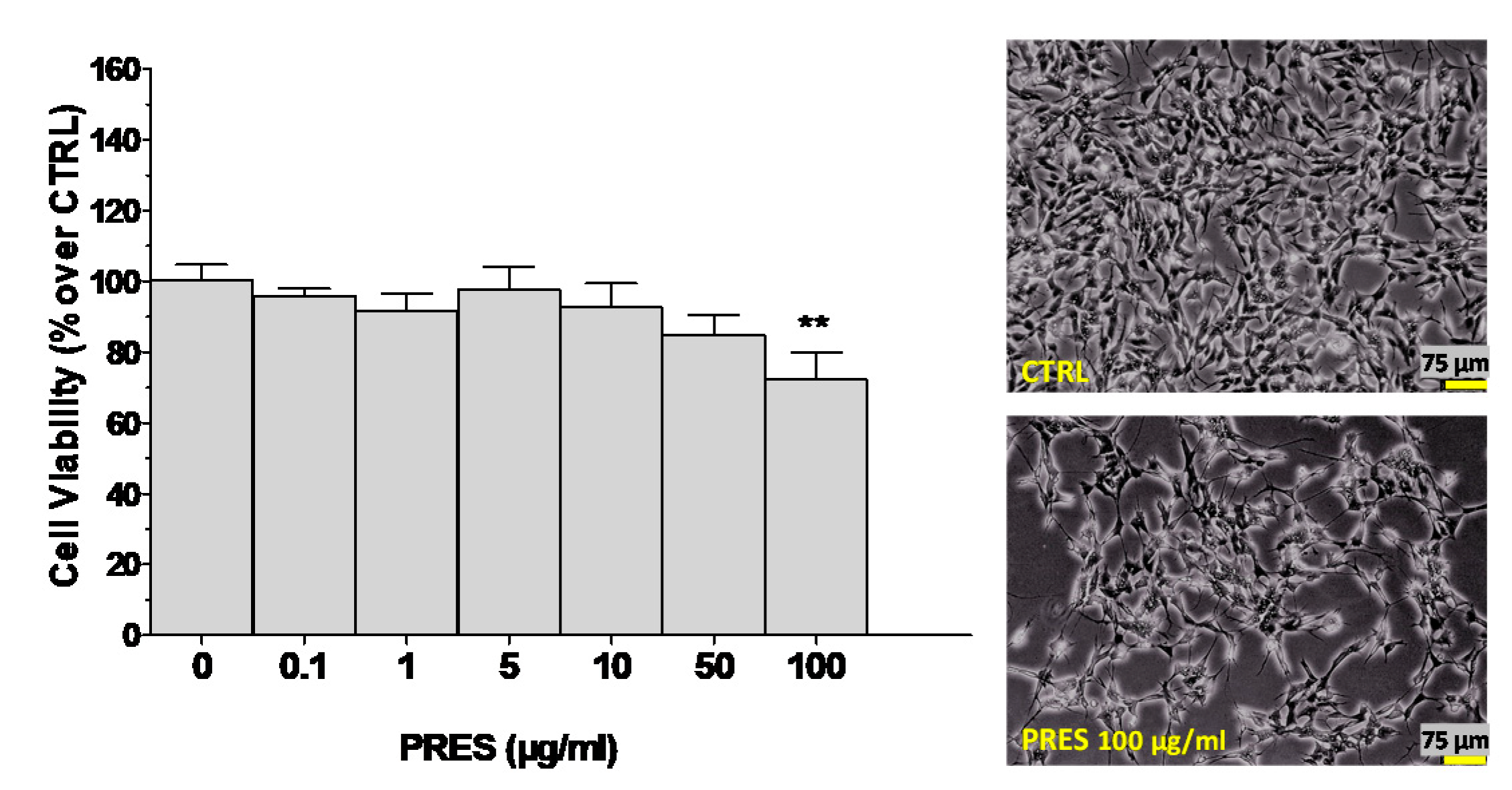
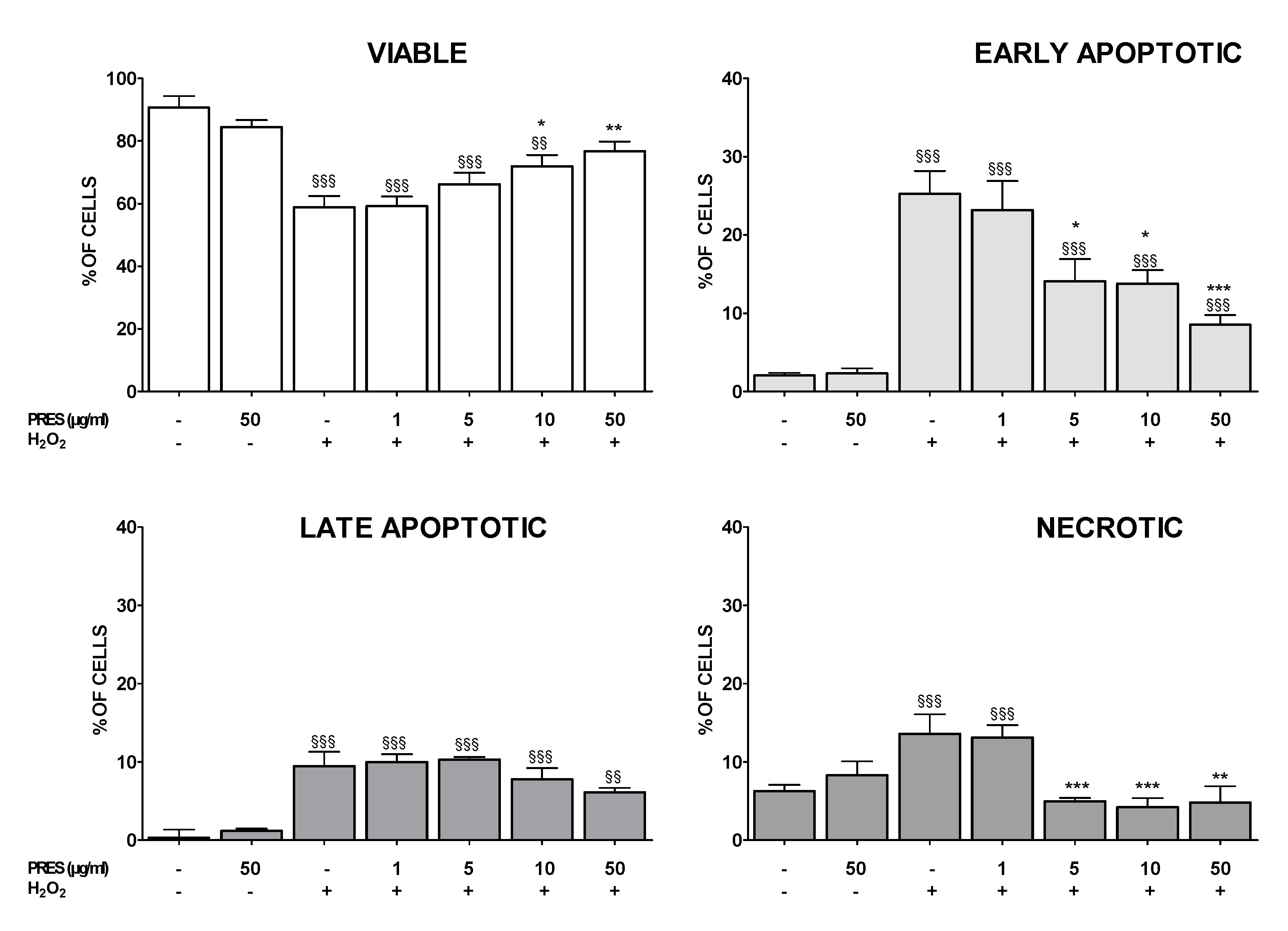
3.1. Effects of PRES on SH-SY5Y Cell Viability
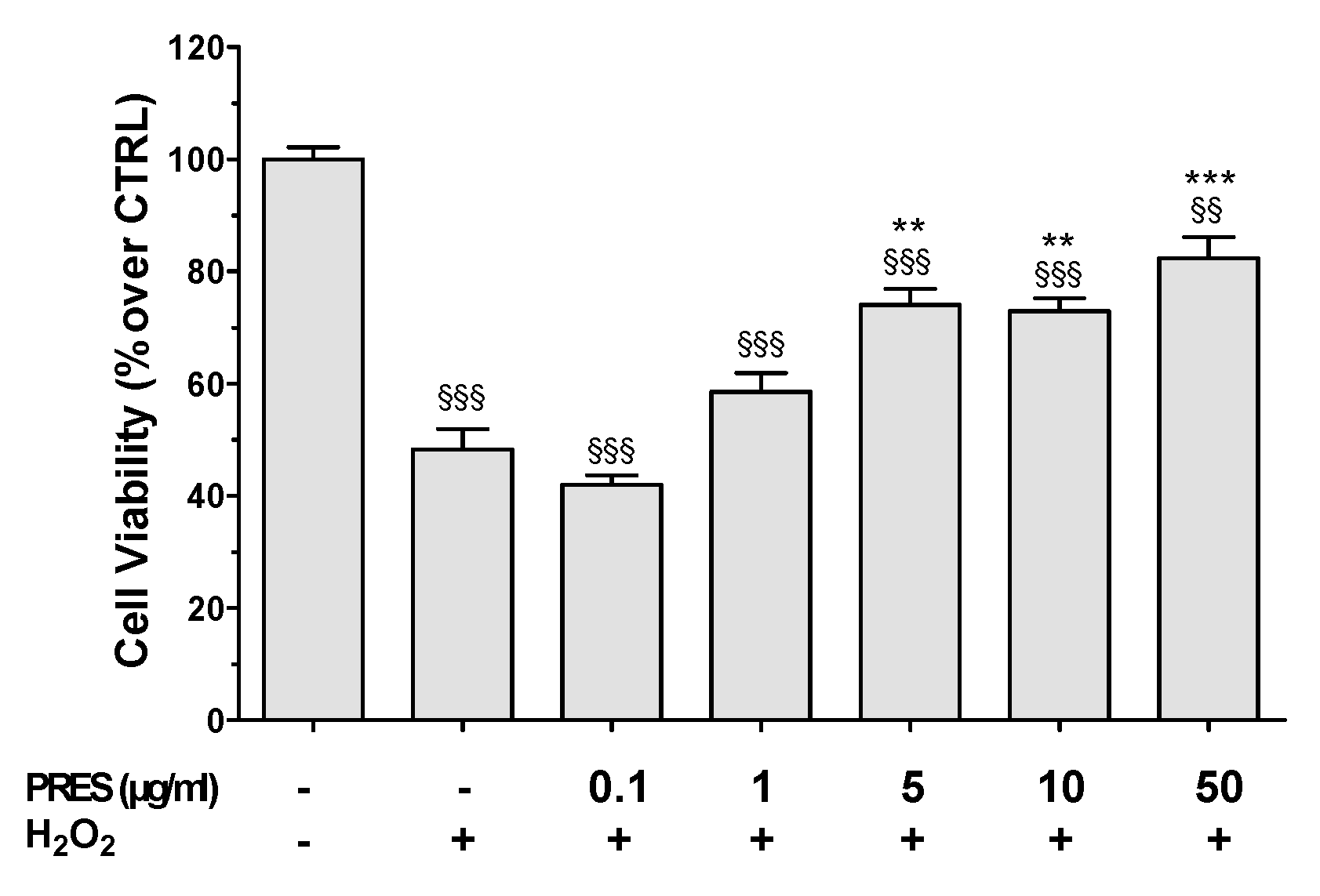
3.2. PRES Reverted OS-Induced Decrease in SH-SY5Y Cells Viability
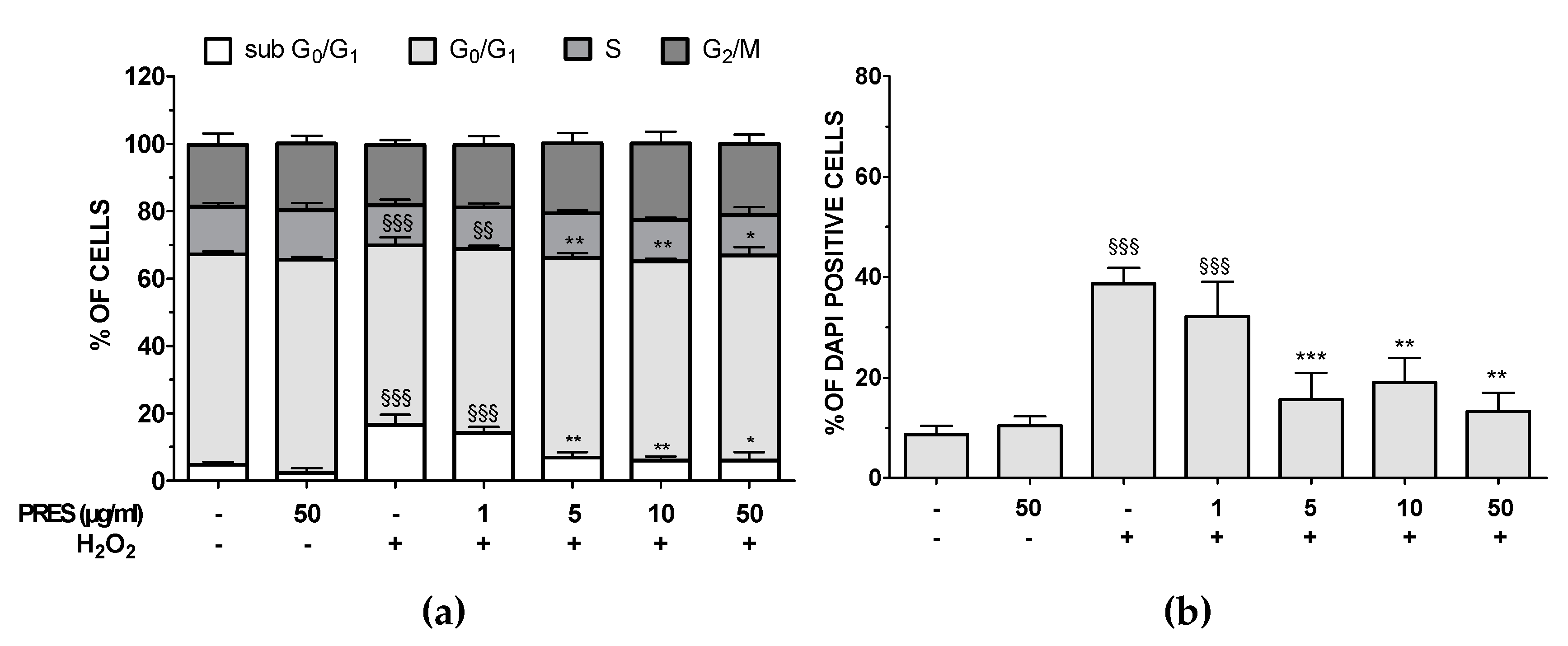
3.3. PRES Reduced Apoptotic-Mediated SH-SY5Y Cell Death Caused by OS
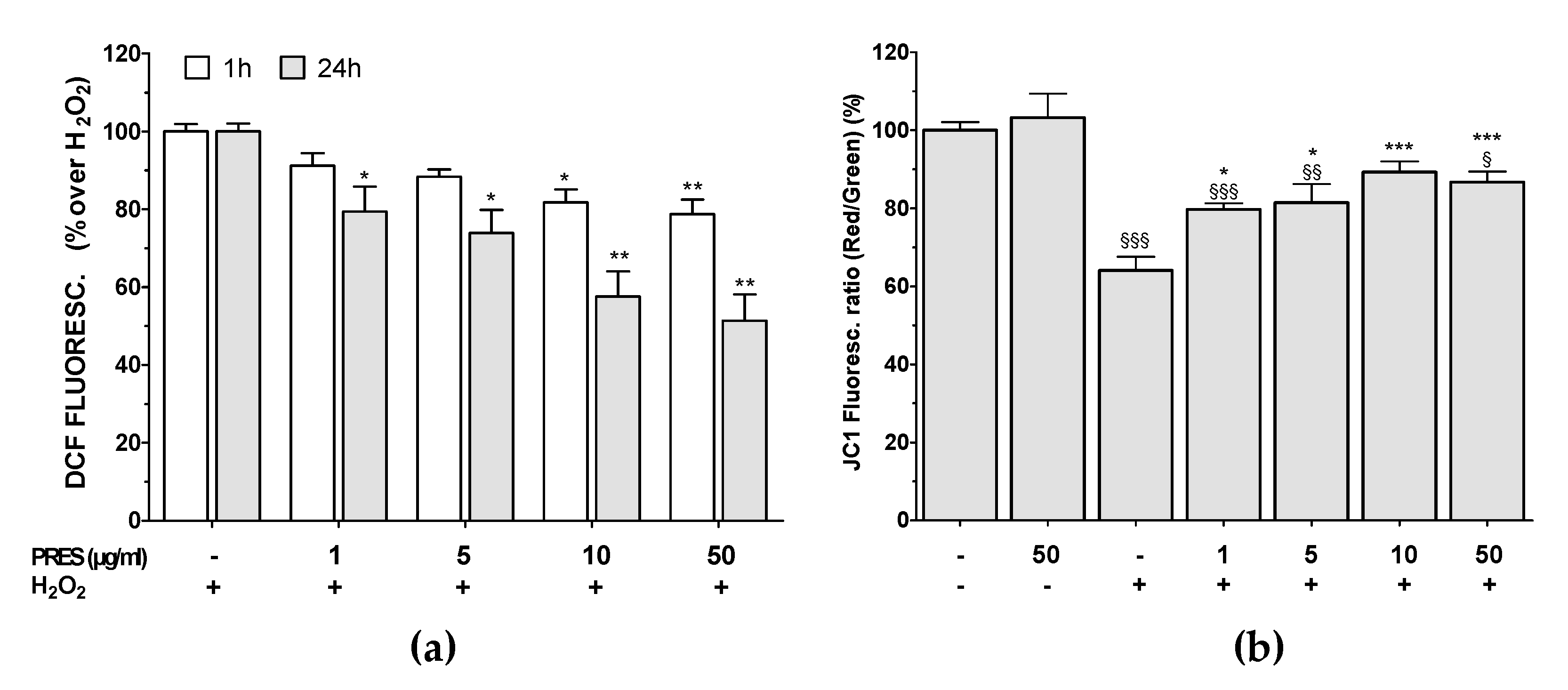
3.4. PRES Hampered the Formation of ROS Along with Loss in Mitochondria Membrane Potential Caused by OS
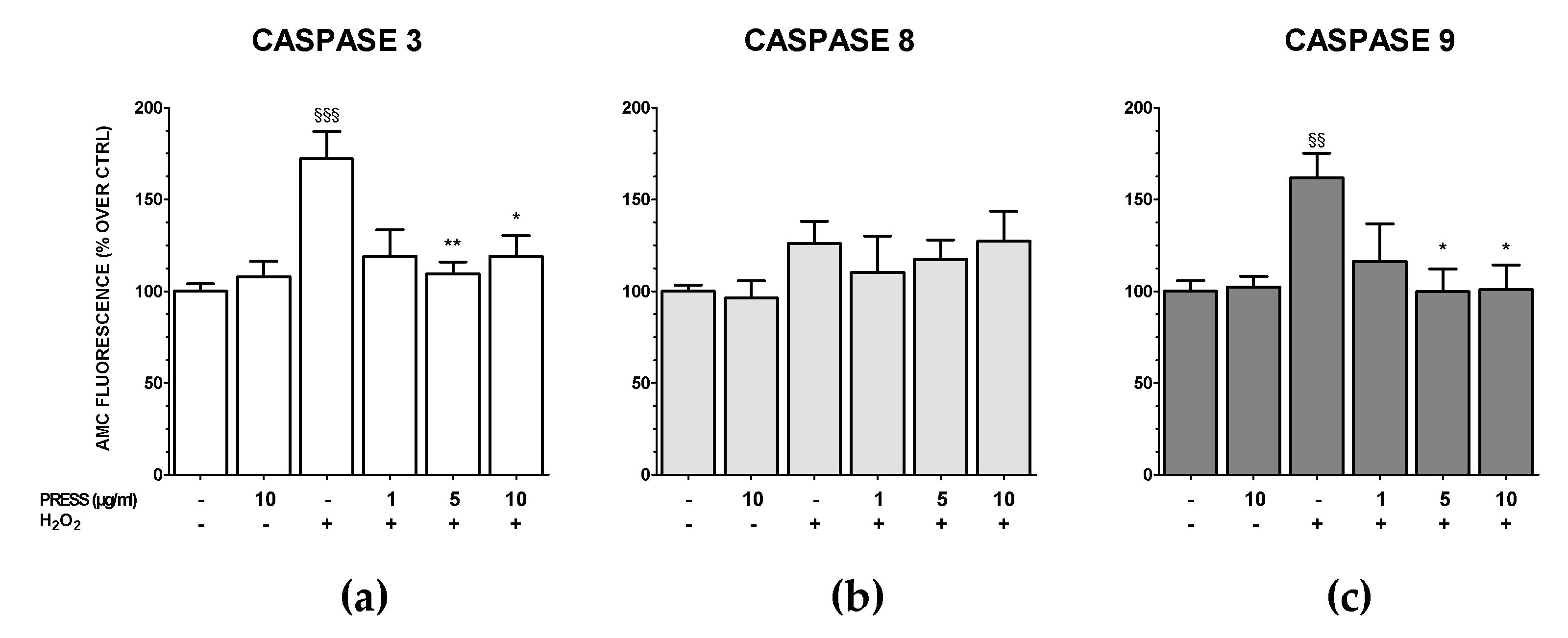
3.5. The Increase in Caspase Activity Caused by OS Was Reduced by PRES
3.6. PRES Neuroprotection Occurred Also in Rat Brain Slices Subjected to OS
3.7. In Silico Prediction of PRES Component’s Drug-Like Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef]
- Vassalle, C.; Maltinti, M.; Sabatino, L. Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here. Molecules 2020, 25, 2653. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species--the good, the bad and the ugly. Acta Physiol. Oxf. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Di Paolo, M.; Papi, L.; Gori, F.; Turillazzi, E. Natural Products in Neurodegenerative Diseases: A Great Promise but an Ethical Challenge. Int. J. Mol. Sci. 2019, 20, 5170. [Google Scholar] [CrossRef]
- Pohl, F.; Kong Thoo Lin, P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef]
- Micucci, M.; Malaguti, M.; Toschi, T.G.; Di Lecce, G.; Aldini, R.; Angeletti, A.; Chiarini, A.; Budriesi, R.; Hrelia, S. Cardiac and Vascular Synergic Protective Effect of Olea europea L. Leaves and Hibiscus sabdariffa L. Flower Extracts. Oxid. Med. Cell Longev. 2015, 2015, 318125. [Google Scholar] [CrossRef] [PubMed]
- Micucci, M.; Angeletti, A.; Cont, M.; Corazza, I.; Aldini, R.; Donadio, E.; Chiarini, A.; Budriesi, R. Hibiscus Sabdariffa, L. Flowers and Olea Europea L. Leaves Extract-Based Formulation for Hypertension Care: In Vitro Efficacy and Toxicological Profile. J. Med. Food 2016, 19, 504–512. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharmacother. 2020, 128, 110303. [Google Scholar] [CrossRef]
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Jakobusic Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, Z.; Solati, K.; Amini-Khoei, H. Phytotherapy in treatment of Parkinson’s disease: A review. Pharm. Biol. 2019, 57, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Santulli, C.; Brizi, C.; Micucci, M.; Del Genio, A.; De Cristofaro, A.; Bracco, F.; Pepe, G.L.; di Perna, I.; Budriesi, R.; Chiarini, A.; et al. Castanea sativa Mill. Bark Extract Protects U-373 MG Cells and Rat Brain Slices Against Ischemia and Reperfusion Injury. J. Cell Biochem. 2017, 118, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Brizi, C.; Santulli, C.; Micucci, M.; Budriesi, R.; Chiarini, A.; Aldinucci, C.; Frosini, M. Neuroprotective Effects of Castanea sativa Mill. Bark Extract in Human Neuroblastoma Cells Subjected to Oxidative Stress. J. Cell Biochem. 2016, 117, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Santulli, C.; Brizi, C.; Durante, M.; Micucci, M.; Budriesi, R.; Chiarini, A.; Frosini, M. Apoptotic-induced effects of castanea sativa bark extract in human SH-SY5Y neuroblastoma cells. Nat. Prod. Commun. 2018, 13, 887–890. [Google Scholar] [CrossRef]
- Chiaino, E.; Micucci, M.; Durante, M.; Budriesi, R.; Gotti, R.; Marzetti, C.; Chiarini, A.; Frosini, M. Apoptotic-Induced Effects of Acacia Catechu Willd. Extract in Human Colon Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2102. [Google Scholar] [CrossRef]
- Durante, M.; Frosini, M.; Fusi, F.; Gamberucci, A.; Chegaev, K.; Rolando, B.; Riganti, C.; Fruttero, R.; Saponara, S. In vitro vascular toxicity assessment of NitDOX, a novel NO-releasing doxorubicin. Eur. J. Pharmacol. 2020, 880, 173164. [Google Scholar] [CrossRef] [PubMed]
- Meini, A.; Garcia, J.B.; Pessina, G.P.; Aldinucci, C.; Frosini, M.; Palmi, M. Role of intracellular Ca2+ and calmodulin/MAP kinase kinase/extracellular signal-regulated protein kinase signalling pathway in the mitogenic and antimitogenic effect of nitric oxide in glia- and neurone-derived cell lines. Eur. J. Neurosci. 2006, 23, 1690–1700. [Google Scholar] [CrossRef]
- Contartese, A.; Valoti, M.; Corelli, F.; Pasquini, S.; Mugnaini, C.; Pessina, F.; Aldinucci, C.; Sgaragli, G.; Frosini, M. A novel CB2 agonist, COR167, potently protects rat brain cortical slices against OGD and reperfusion injury. Pharmacol. Res. 2012, 66, 555–563. [Google Scholar] [CrossRef]
- Pessina, F.; Frosini, M.; Marcolongo, P.; Fusi, F.; Saponara, S.; Gamberucci, A.; Valoti, M.; Giustarini, D.; Fiorenzani, P.; Gorelli, B.; et al. Antihypertensive, cardio- and neuro-protective effects of Tenebrio molitor (Coleoptera: Tenebrionidae) defatted larvae in spontaneously hypertensive rats. PLoS ONE 2020, 15, e0233788. [Google Scholar] [CrossRef]
- Dhuriya, Y.K.; Sharma, D.; Naik, A.A. Cellular demolition: Proteins as molecular players of programmed cell death. Int. J. Biol. Macromol. 2019, 138, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Yang, H.; Zhang, H.; Zhu, Y. 1291 cases of cholelithiasis treated with electric shock on otoacupoints. J. Tradit. Chin. Med. 1991, 11, 101–109. [Google Scholar]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural. Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Nunez-Sanchez, M.A.; Tomas-Barberan, F.A.; Espin, J.C. Neuroprotective Effects of Bioavailable Polyphenol-Derived Metabolites against Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Lee, J.Y.; Choi, Y.K.; Kim, G.S.; Han, B.H. Neuroprotective effects of flavones on hydrogen peroxide-induced apoptosis in SH-SY5Y neuroblostoma cells. Bioorg. Med. Chem. Lett. 2004, 14, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Garcimartin, A.; Merino, J.J.; Gonzalez, M.P.; Sanchez-Reus, M.I.; Sanchez-Muniz, F.J.; Bastida, S.; Benedi, J. Organic silicon protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide effects. BMC Complement. Altern. Med. 2014, 14, 384. [Google Scholar] [CrossRef]
- Law, B.N.; Ling, A.P.; Koh, R.Y.; Chye, S.M.; Wong, Y.P. Neuroprotective effects of orientin on hydrogen peroxideinduced apoptosis in SHSY5Y cells. Mol. Med. Rep. 2014, 9, 947–954. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Omar, S.H. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi. Pharm. J. 2010, 18, 111–121. [Google Scholar] [CrossRef]
- Yuan, J.J.; Wang, C.Z.; Ye, J.Z.; Tao, R.; Zhang, Y.S. Enzymatic hydrolysis of oleuropein from Olea europea (olive) leaf extract and antioxidant activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.H.; Ho, C.T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [PubMed]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice. Int. J. Mol. Sci. 2018, 20, 125. [Google Scholar] [CrossRef]
- Seung, T.W.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Park, S.H.; Kwon, B.S.; Lee, C.J.; Kang, J.E.; Kim, D.O.; Lee, U.; et al. Ethyl acetate fraction from Hibiscus sabdariffa L. attenuates diabetes-associated cognitive impairment in mice. Food Res. Int. 2018, 105, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Goudarzvand, M.; Afraei, S.; Yaslianifard, S.; Ghiasy, S.; Sadri, G.; Kalvandi, M.; Alinia, T.; Mohebbi, A.; Yazdani, R.; Azarian, S.K.; et al. Hydroxycitric acid ameliorates inflammation and oxidative stress in mouse models of multiple sclerosis. Neural. Regen. Res. 2016, 11, 1610–1616. [Google Scholar] [CrossRef]
- Cho, S.; Wood, A.; Bowlby, M.R. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr. Neuropharmacol. 2007, 5, 19–33. [Google Scholar] [CrossRef]
- Carito, V.; Venditti, A.; Bianco, A.; Ceccanti, M.; Serrilli, A.M.; Chaldakov, G.; Tarani, L.; De Nicolo, S.; Fiore, M. Effects of olive leaf polyphenols on male mouse brain NGF, BDNF and their receptors TrkA, TrkB and p75. Nat. Prod. Res. 2014, 28, 1970–1984. [Google Scholar] [CrossRef]
- Mezni, A.; Mhadhbi, L.; Khazri, A.; Sellami, B.; Dellali, M.; Mahmoudi, E.; Beyrem, H. The protective effect of Hibiscus sabdariffa calyxes extract against cypermethrin induced oxidative stress in mice. Pestic. Biochem. Physiol. 2020, 165, 104463. [Google Scholar] [CrossRef] [PubMed]
- Pantano, D.; Luccarini, I.; Nardiello, P.; Servili, M.; Stefani, M.; Casamenti, F. Oleuropein aglycone and polyphenols from olive mill waste water ameliorate cognitive deficits and neuropathology. Br. J. Clin. Pharmacol. 2017, 83, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Ventrice, D.; Giannetto, F.; Cirinna, S.; Santagati, N.A.; Procopio, A.; Mollace, V.; Muscoli, C. Antioxidant activity of oleuropein and semisynthetic acetyl-derivatives determined by measuring malondialdehyde in rat brain. J. Pharm. Pharmacol. 2017, 69, 1502–1512. [Google Scholar] [CrossRef]
- Palagati, S.; Satyanarayana, S.V.; Kesavan, B.R. Application of computational tools for the designing of Oleuropein loaded nanostructured lipid carrier for brain targeting through nasal route. DARU 2019, 27, 695–708. [Google Scholar] [CrossRef]
- Wu, Y.T.; Lin, L.C.; Tsai, T.H. Measurement of free hydroxytyrosol in microdialysates from blood and brain of anesthetized rats by liquid chromatography with fluorescence detection. J. Chromatogr. A 2009, 1216, 3501–3507. [Google Scholar] [CrossRef]
- Perneczky, R.; Kempermann, G.; Korczyn, A.D.; Matthews, F.E.; Ikram, M.A.; Scarmeas, N.; Chetelat, G.; Stern, Y.; Ewers, M. Translational research on reserve against neurodegenerative disease: Consensus report of the International Conference on Cognitive Reserve in the Dementias and the Alzheimer’s Association Reserve, Resilience and Protective Factors Professional Interest Area working groups. BMC Med. 2019, 17, 47. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid. Based Complement Alternat. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef]
- Angeloni, C.; Vauzour, D. Natural Products and Neuroprotection. Int. J. Mol. Sci. 2019, 20, 5570. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Hrelia, S. Combined Treatment with Three Natural Antioxidants Enhances Neuroprotection in a SH-SY5Y 3D Culture Model. Antioxid. Basel 2019, 8, 420. [Google Scholar] [CrossRef]
- Cooper, E.L.; Ma, M.J. Alzheimer Disease: Clues from traditional and complementary medicine. J. Tradit. Complement Med. 2017, 7, 380–385. [Google Scholar] [CrossRef]

| Calculated Parameters | Compounds | Range of Recommended Values a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rutin | Elenolic Acid Glucoside | Hydroxyoleuropein | Hibiscus Acid | Verbascoside | Ligstroside | Oleuropein | Oleurpoein Aglycone | Luteolin-7-O-Rutinoside | Luteolin-7-O-Glucoside | ||
| #rotor b | 15 | 10 | 17 | 3 | 20 | 14 | 15 | 9 | 16 | 10 | 0–15 |
| Lipinski Rule of 5 violations c | 3 | 1 | 3 | 0 | 3 | 2 | 3 | 0 | 3 | 2 | N.A. |
| mol_MW d | 610.5 | 404.4 | 556.5 | 190.1 | 624.6 | 524.5 | 540.5 | 378.4 | 610.5 | 448.4 | 130.0–725.0 |
| dipole e | 10.3 | 5.0 | 7.9 | 4.1 | 4.7 | 5.5 | 9.6 | 5.2 | 10.8 | 6.7 | 1.0–12.5 |
| SASA f | 895.4 | 622.9 | 824.8 | 333.6 | 924.4 | 801.8 | 808.6 | 612.8 | 820.5 | 702.0 | 300.0–1000.0 |
| donorHB g | 9.0 | 5.0 | 7.0 | 2.0 | 9.0 | 5.0 | 6.0 | 3.0 | 9.0 | 6.0 | 0.0–6.0 |
| accptHB h | 20.6 | 15.9 | 19.1 | 6.8 | 20.3 | 16.7 | 17.4 | 8.9 | 21.5 | 13.0 | 2.0–20.0 |
| QPlogPo/w i | −2.4 | −1.3 | −1.5 | −0.8 | −1.5 | 0.0 | −0.5 | 1.0 | −2.7 | −1.0 | −2.0–6.5 |
| QPlogS j | −3.3 | −1.8 | −2.4 | −0.6 | −2.8 | −3.0 | −2.8 | −3.0 | −2.1 | −3.2 | −6.5–0.5 |
| QPPCaco k | 0.3 | 6.7 | 3.6 | 1.4 | 1.0 | 29.6 | 12.5 | 68.0 | 0.9 | 3.6 | <25 poor, >500 great |
| QPlogBB l | −5.7 | −2.5 | −4.3 | −1.7 | −5.5 | −3.1 | −3.5 | −2.1 | −4.8 | −3.8 | −3.0–1.2 |
| QPPMDCK m | 0.1 | 2.8 | 1.1 | 0.7 | 0.3 | 11.0 | 4.3 | 27.1 | 0.3 | 1.1 | <25 poor, >500 great |
| Jorgensen Rule of 3 violations n | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 0 | 2 | 2 | N.A. |
| #metab o | 10 | 7 | 10 | 3 | 10 | 9 | 10 | 6 | 10 | 7 | 1–8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaino, E.; Micucci, M.; Cosconati, S.; Novellino, E.; Budriesi, R.; Chiarini, A.; Frosini, M. Olive Leaves and Hibiscus Flowers Extracts-Based Preparation Protect Brain from Oxidative Stress-Induced Injury. Antioxidants 2020, 9, 806. https://doi.org/10.3390/antiox9090806
Chiaino E, Micucci M, Cosconati S, Novellino E, Budriesi R, Chiarini A, Frosini M. Olive Leaves and Hibiscus Flowers Extracts-Based Preparation Protect Brain from Oxidative Stress-Induced Injury. Antioxidants. 2020; 9(9):806. https://doi.org/10.3390/antiox9090806
Chicago/Turabian StyleChiaino, Elda, Matteo Micucci, Sandro Cosconati, Ettore Novellino, Roberta Budriesi, Alberto Chiarini, and Maria Frosini. 2020. "Olive Leaves and Hibiscus Flowers Extracts-Based Preparation Protect Brain from Oxidative Stress-Induced Injury" Antioxidants 9, no. 9: 806. https://doi.org/10.3390/antiox9090806
APA StyleChiaino, E., Micucci, M., Cosconati, S., Novellino, E., Budriesi, R., Chiarini, A., & Frosini, M. (2020). Olive Leaves and Hibiscus Flowers Extracts-Based Preparation Protect Brain from Oxidative Stress-Induced Injury. Antioxidants, 9(9), 806. https://doi.org/10.3390/antiox9090806