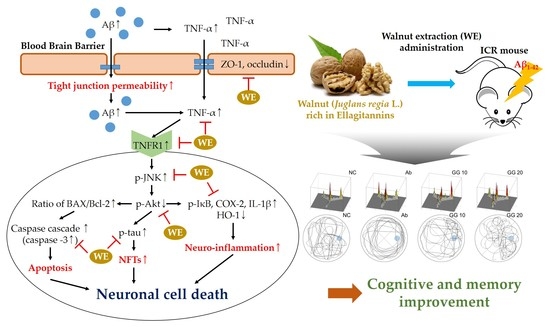
Anti-Amnesic Effect of Walnut via the Regulation of BBB Function and Neuro-Inflammation in Aβ1-42-Induced Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. UPLC Q-TOF/MS2
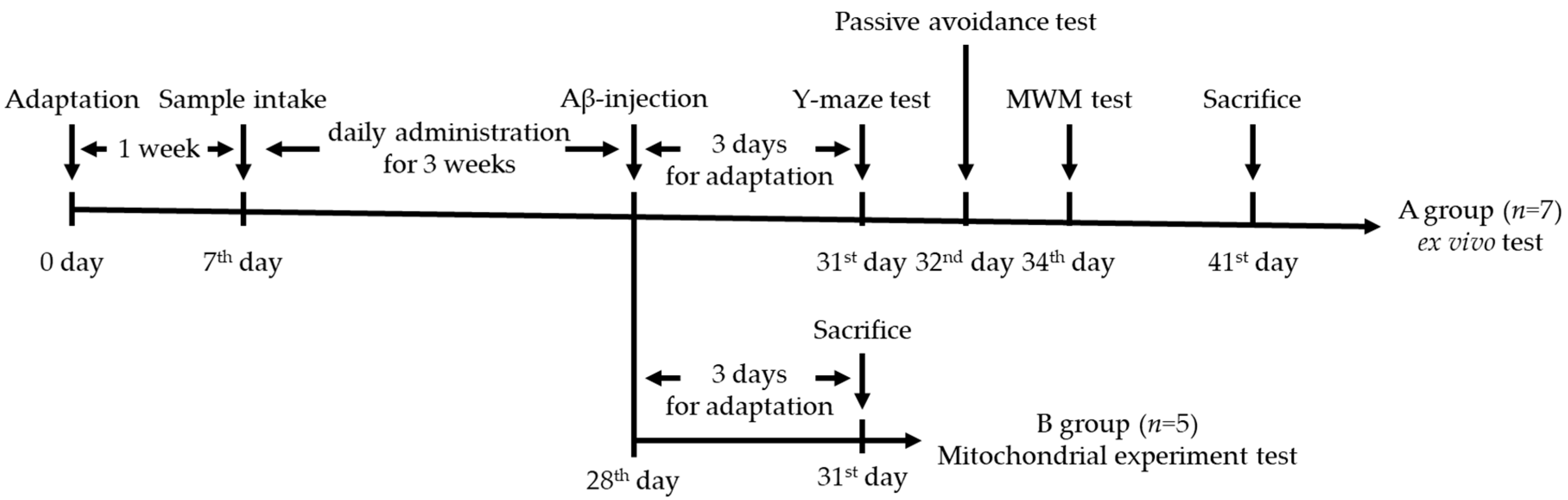
2.4. Animal Design
2.5. Behavioral Tests
2.5.1. Y-maze Test
2.5.2. Passive Avoidance Test
2.5.3. Morris Water Maze Test
2.6. Preparation of Tissue
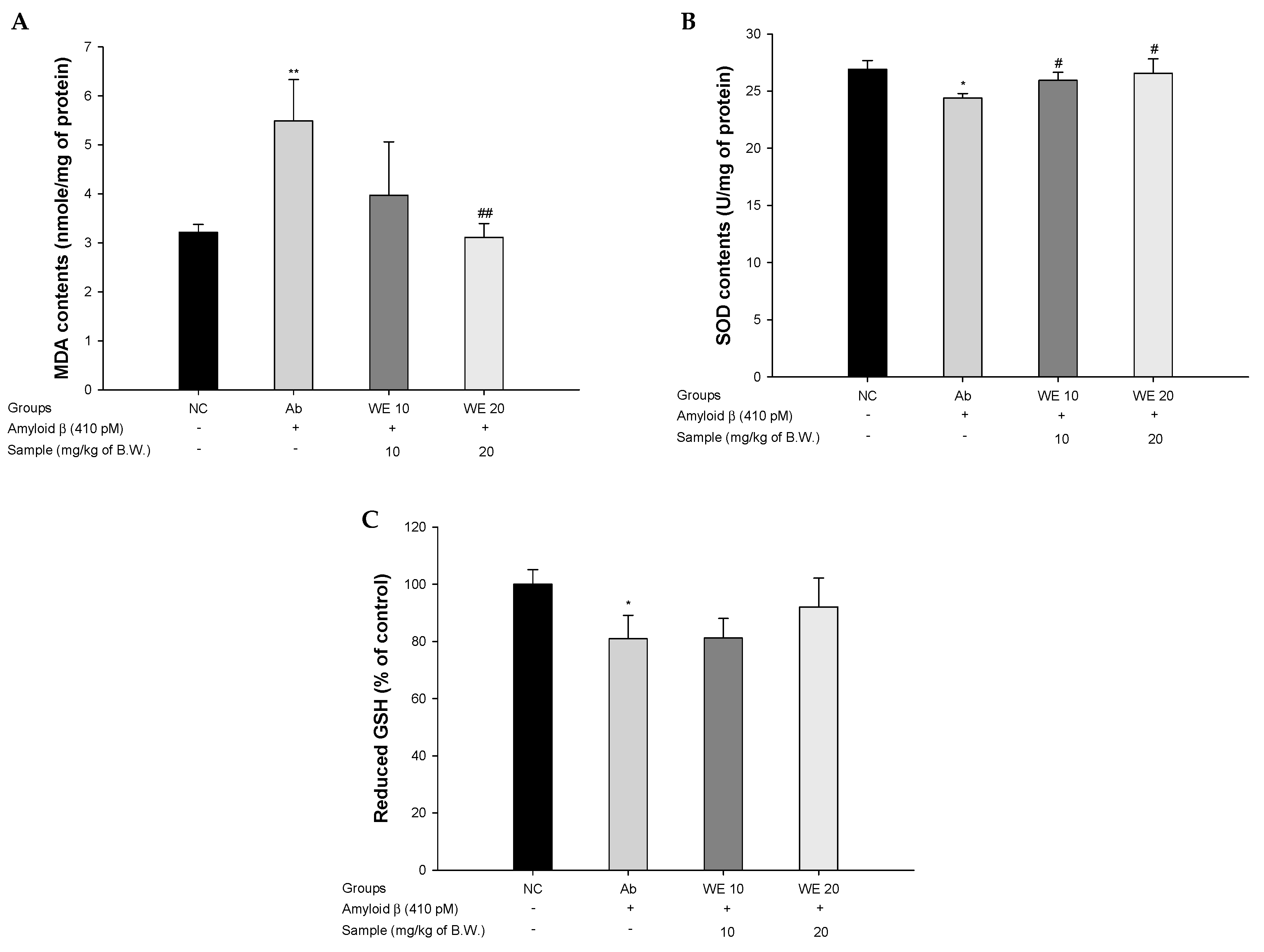
2.7. Antioxidant System
2.7.1. SOD Levels
2.7.2. Reduced GSH Levels
2.7.3. MDA Levels
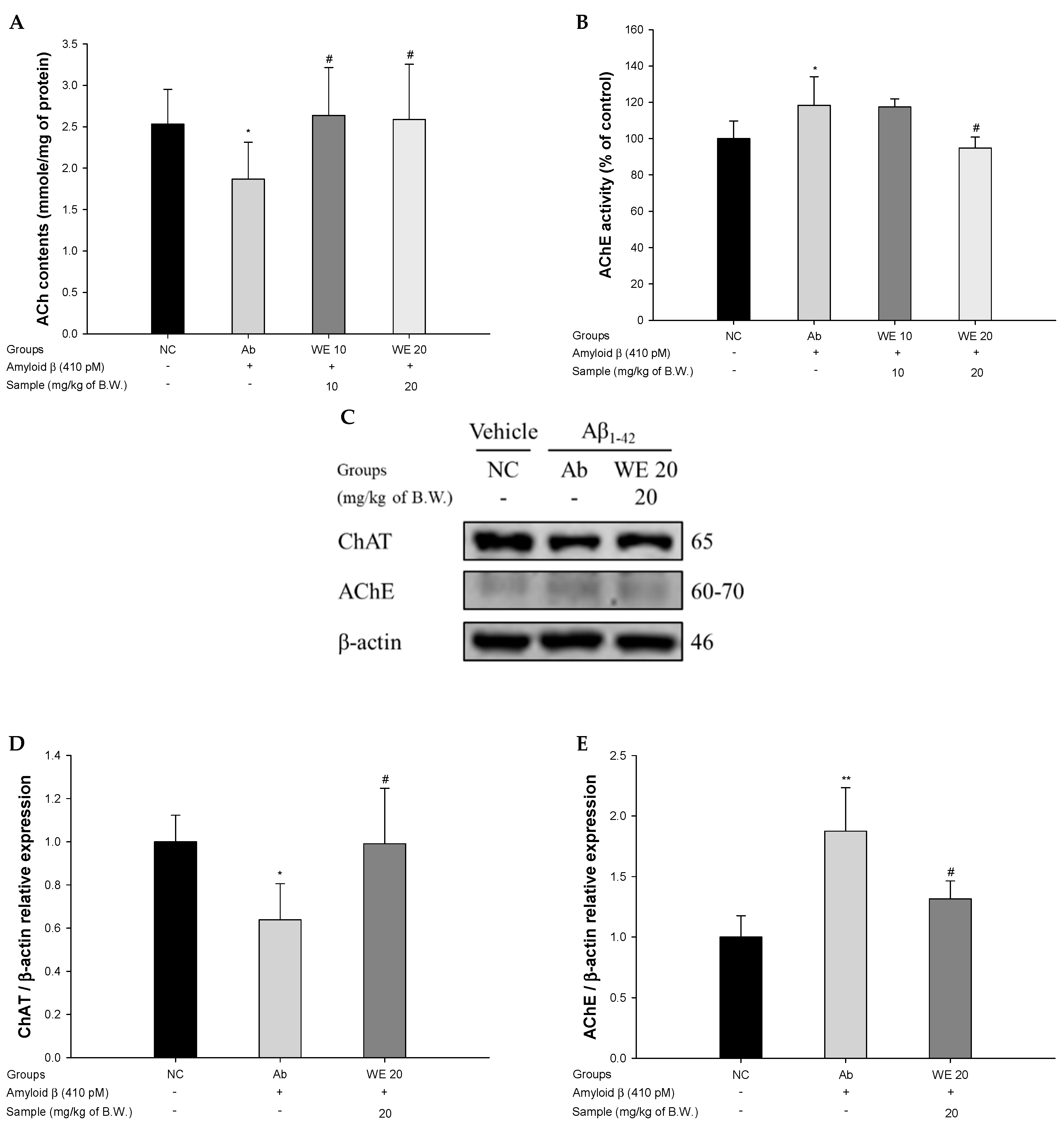
2.8. Cholinergic System
2.8.1. ACh Levels
2.8.2. AChE Activities
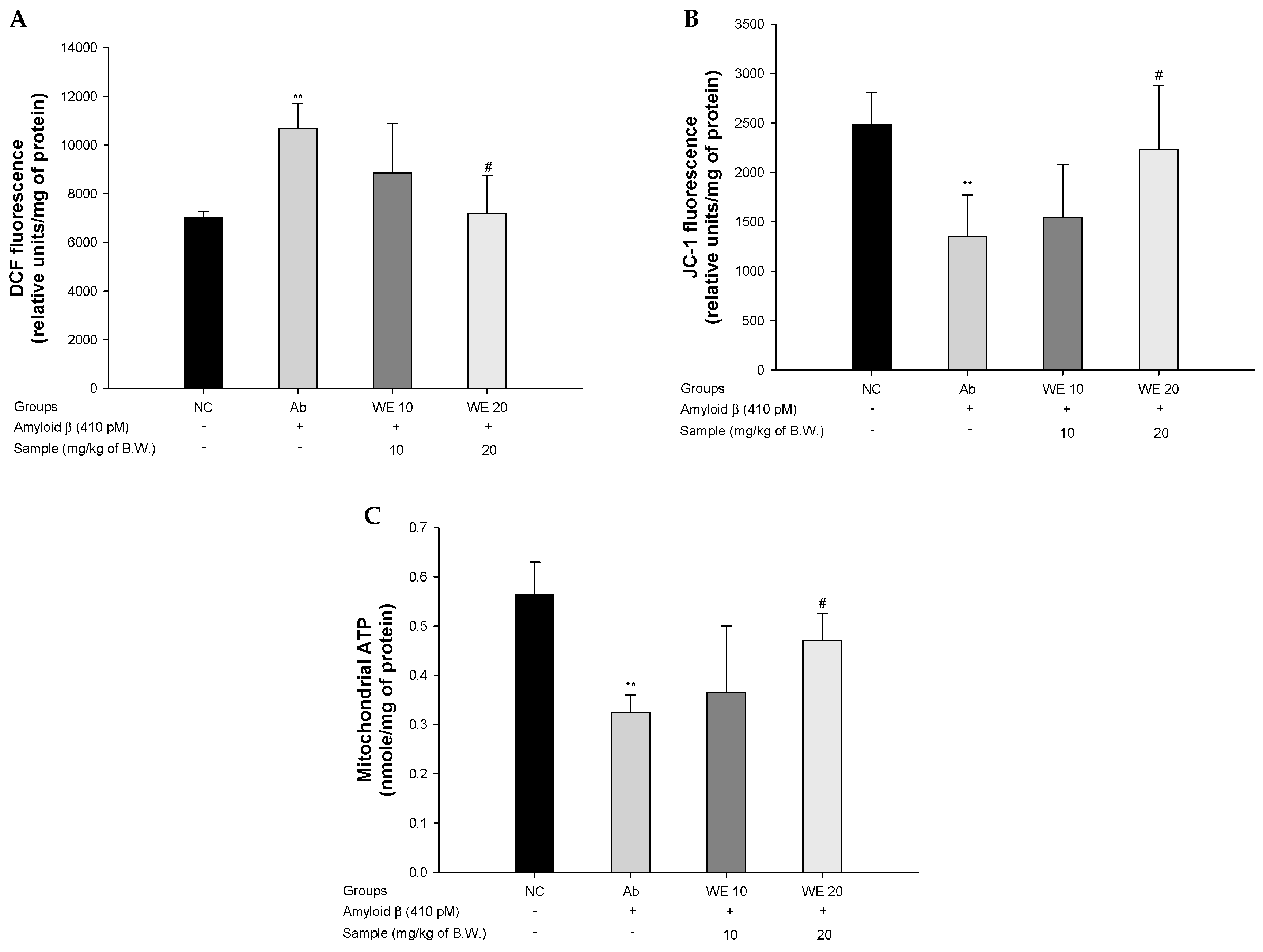
2.9. Mitochondrial Function
2.9.1. Extration of Mitochondria
2.9.2. Mitochondrial ROS Contents
2.9.3. Mitochondrial Membrane Potential
2.9.4. Mitochondrial ATP Contents
2.10. Western Blot
2.11. Statistical Analysis
3. Results
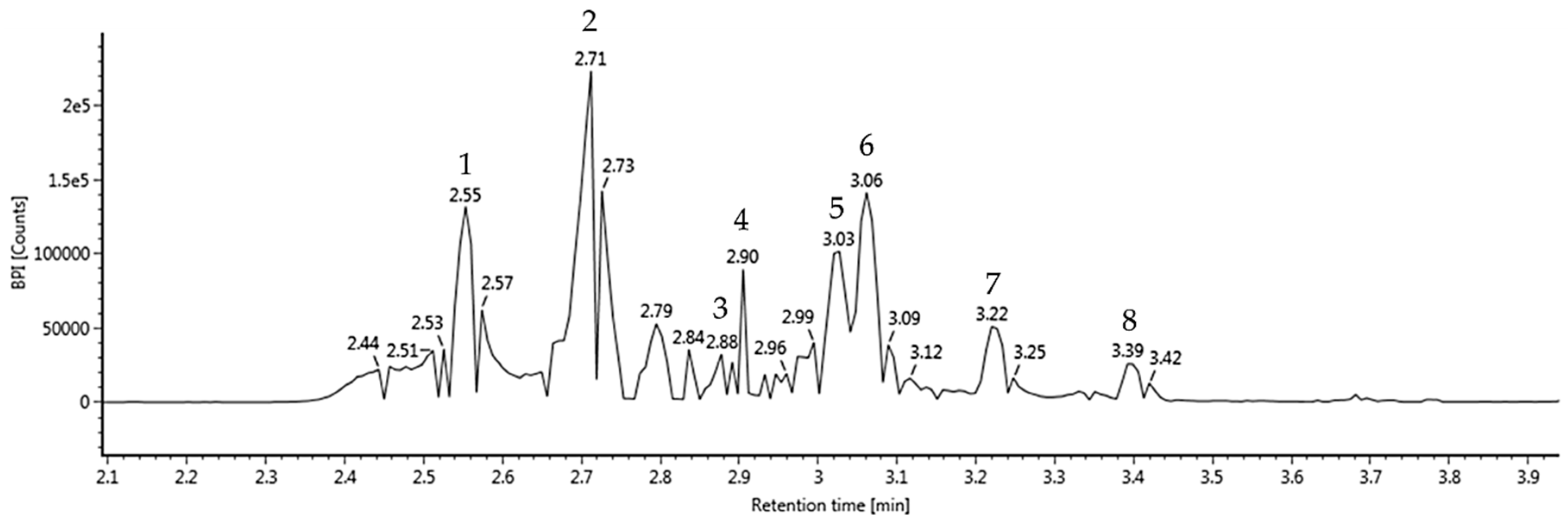
3.1. UPLC Q-TOF/MS2
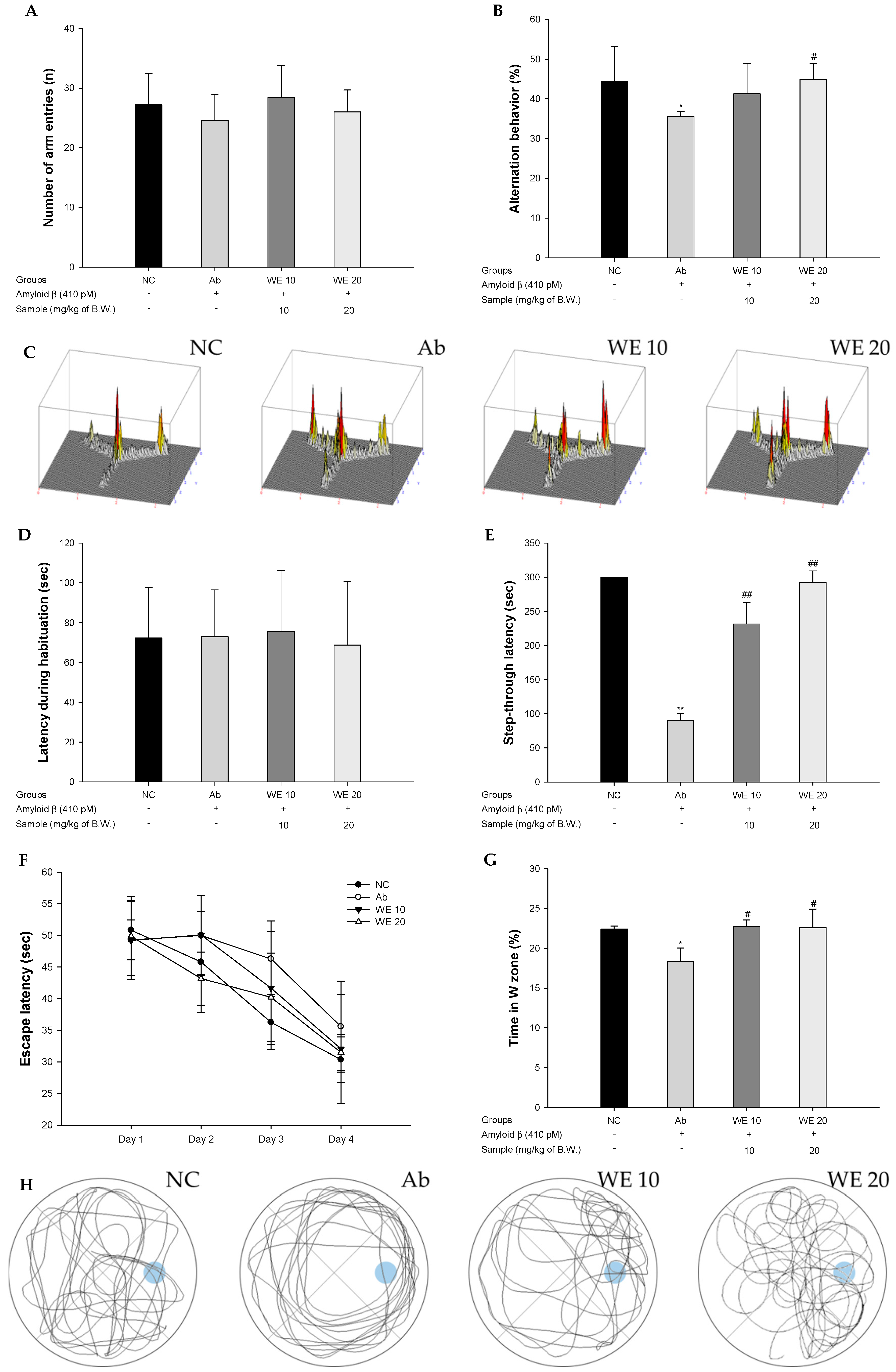
3.2. Behavioral Tests
3.3. Antioxidant System
3.4. Cholinergic System
3.5. Mitochondrial Function
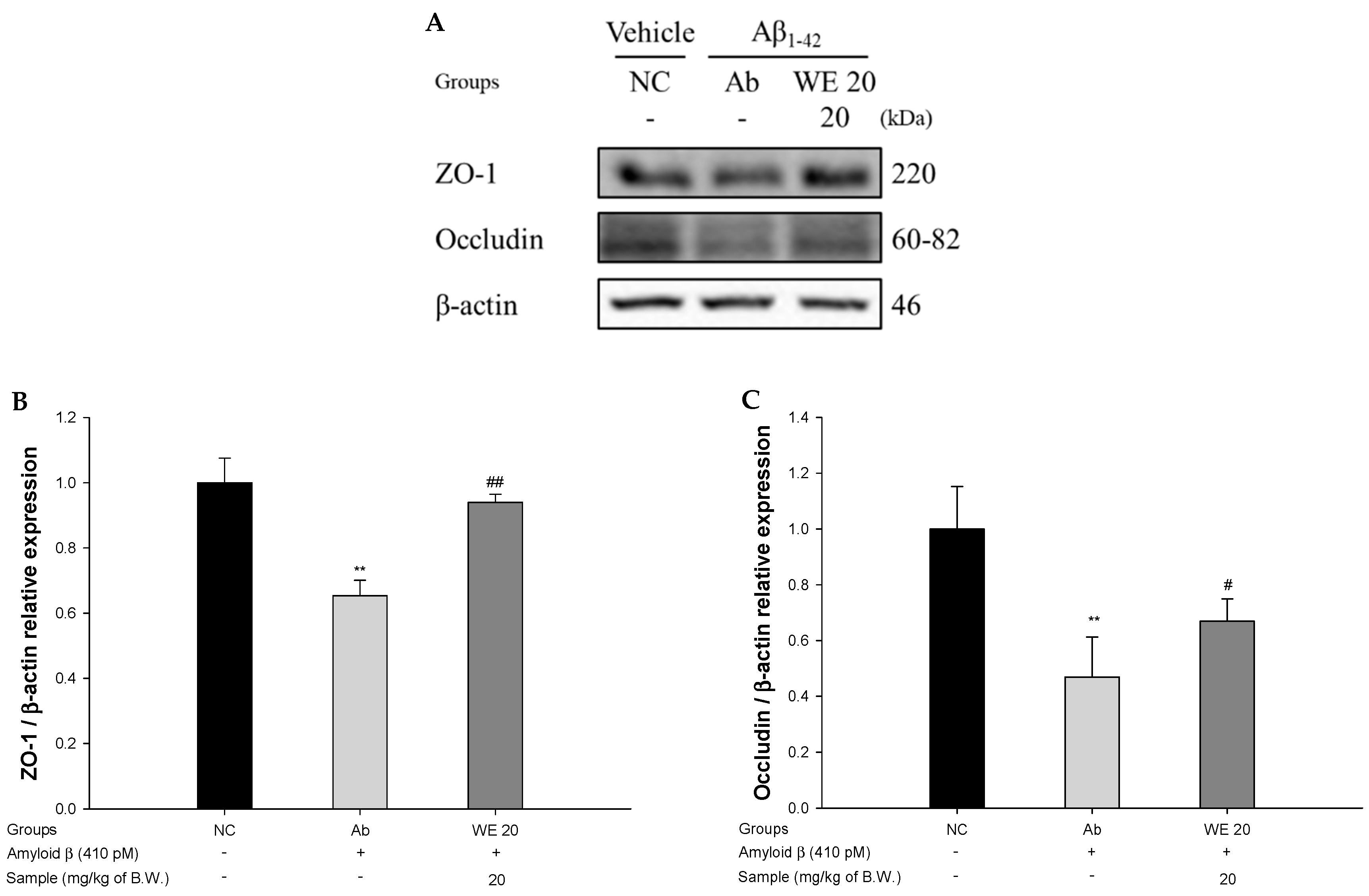
3.6. Blood–Brain Barrier (BBB) Function
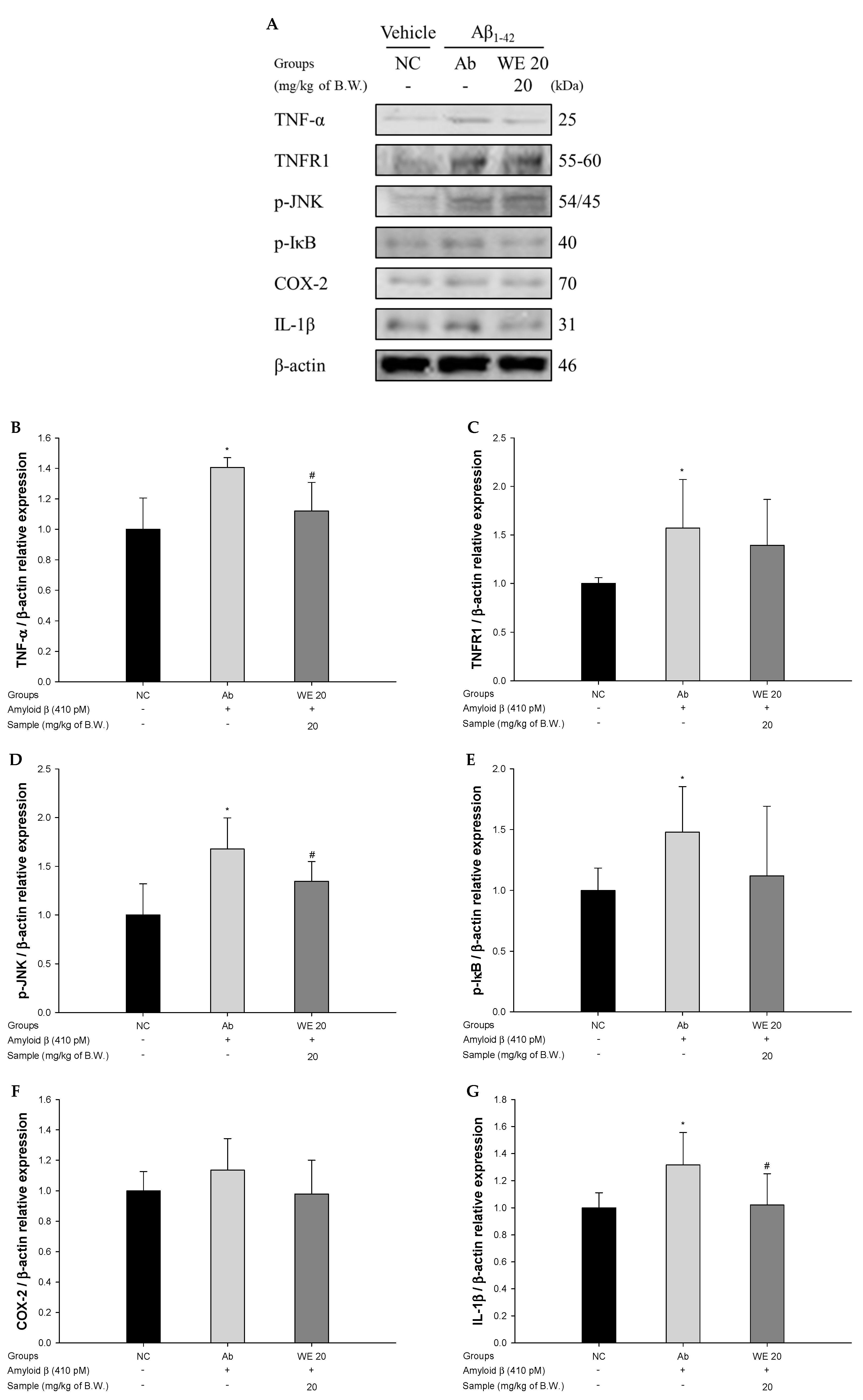
3.7. Neuroinflammation Pathway
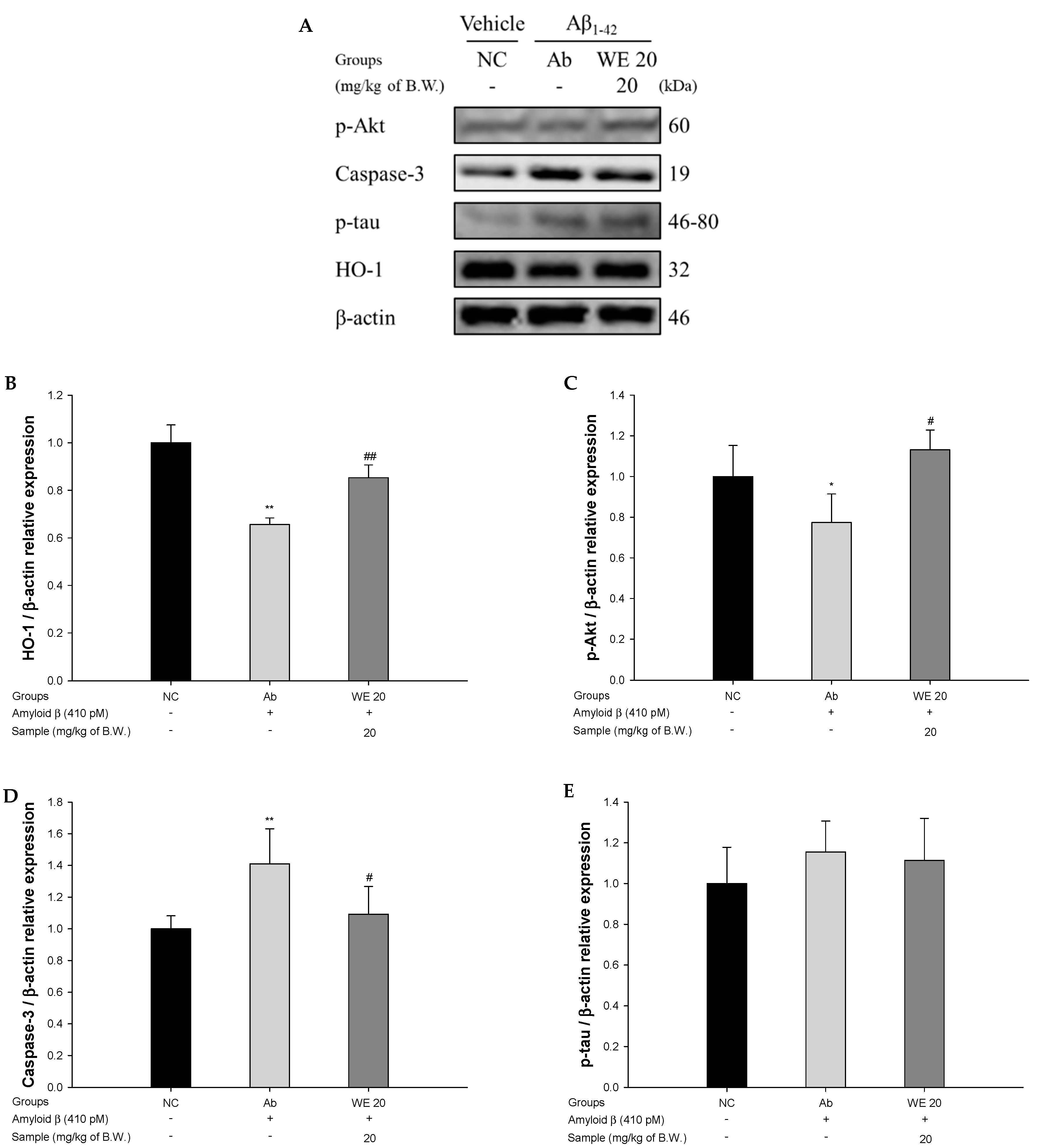
3.8. Aβ-Related Akt Pathway
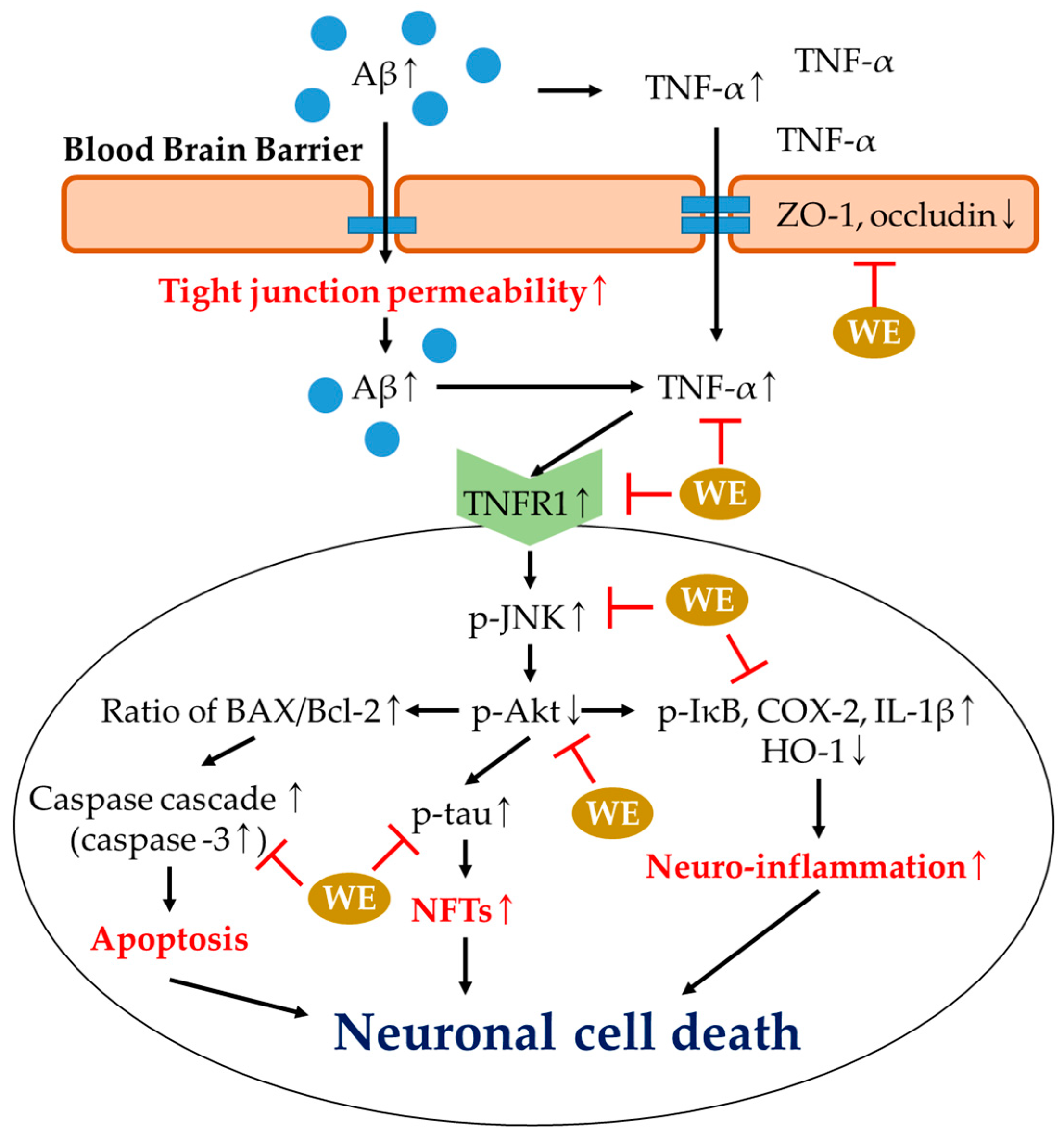
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Su, B.; Wang, X.; Smith, M.A.; Perry, G. Causes of oxidative stress in Alzheimer disease. Cell. Mol. Life Sci. 2007, 64, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Hyman, B.T. The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer’s Disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Tsai, M.Y.; Wolynes, P.G. Comparing the aggregation free energy landscapes of amyloid beta (1–42) and amyloid beta (1–40). J. Am. Chem. Soc. 2017, 139, 16666–16676. [Google Scholar] [CrossRef] [PubMed]
- Malito, E.; Hulse, R.E.; Tang, W.-J. Amyloid β-degrading cryptidases: Insulin degrading enzyme, presequence peptidase, and neprilysin. Cell. Mol. Life Sci. 2008, 65, 2574–2585. [Google Scholar] [CrossRef]
- Yang, R.; Liu, S.; Zhou, J.; Bu, S.; Zhang, J. Andrographolide attenuates microglia-mediated Aβ neurotoxicity partially through inhibiting NF-κB and JNK MAPK signaling pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 276–284. [Google Scholar] [CrossRef]
- Ali, T.; Yoon, G.H.; Shah, S.A.; Lee, H.Y.; Kim, M.O. Osmotin attenuates amyloid beta-induced memory impairment, tau phosphorylation and neurodegeneration in the mouse hippocampus. Sci. Rep. 2015, 5, 11708. [Google Scholar] [CrossRef]
- Wong-Riley, M.T. Cytochrome oxidase: An endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989, 12, 94–101. [Google Scholar] [CrossRef]
- Meda, L.; Cassatella, M.A.; Szendrei, G.I.; Otvos, L.; Baron, P.; Villalba, M.; Ferrari, D.; Rossi, F. Activation of microglial cells by β-amyloid protein and interferon-γ. Nat. Cell Biol. 1995, 374, 647–650. [Google Scholar] [CrossRef]
- Du Yan, S.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef]
- Kook, S.Y.; Hong, H.S.; Moon, M.; Ha, C.M.; Chang, S.; Mook-Jung, I. Aβ1–42-RAGE interaction disrupts tight junctions of the blood–brain barrier via Ca2+-calcineurin signaling. J. Neurosci. 2012, 32, 8845–8854. [Google Scholar] [CrossRef] [PubMed]
- Du Yan, S.; Roher, A.; Chaney, M.; Zlokovic, B.; Schmidt, A.M.; Stern, D. Cellular cofactors potentiating induction of stress and cytotoxicity by amyloid β-peptide. Biochim. Biophys. Acta—Mol. Basis Dis. 2000, 1502, 145–157. [Google Scholar] [CrossRef]
- Bednarczyk, J.; Lukasiuk, K. Tight junctions in neurological diseases. Acta Neurobiol. Exp. 2011, 71, 4. [Google Scholar]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2011, 49, 173–183. [Google Scholar] [CrossRef]
- Payghamzadeh, K.; Kazemitabar, S.K. In vitro propagation of walnut-A review. Afr. J. Biotechnol. 2011, 10, 290–311. [Google Scholar]
- Sala-Vila, A.; Cofán, M.; Núñez, I.; Gilabert, R.; Junyent, M.; Ros, E. Carotid and femoral plaque burden is inversely associated with the α-linolenic acid proportion of serum phospholipids in Spanish subjects with primary dyslipidemia. Atherosclerosis 2011, 214, 209–214. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011, 131, 49–57. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Ciudad, C.J.; Noé, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.-J. Preventive effects of dietary walnuts on high-fat-induced hepatic fat accumulation, oxidative stress and apoptosis in mice. J. Nutr. Biochem. 2016, 38, 70–80. [Google Scholar] [CrossRef]
- Van Der Borght, K.; Havekes, R.; Boß, T.; Eggen, B.J.L.; Van Der Zee, E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behav. Neurosci. 2007, 121, 324–334. [Google Scholar] [CrossRef]
- Newman, J.P.; Kosson, D.S. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J. Abnorm. Psychol. 1986, 95, 252. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, F.; Ng, T.B. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem. Cell Biol. 2000, 78, 447–453. [Google Scholar] [CrossRef]
- Heo, H.J.; Cho, H.Y.; Hong, B.; Kim, H.K.; Kim, E.K.; Kim, B.G.; Shin, D.H. Protective effect of 4′, 5-dihydroxy-3′,6,7-trimethoxyflavone from Artemisia asiatica against Aβ-induced oxidative stress in PC12 cells. Amyloid J. Protein Fold. Disord. 2001, 8, 194–201. [Google Scholar] [CrossRef]
- Vincent, D.; Segonzac, G.; Vincent, M.C. Colorimetric determination of acetylcholine by the Hestrin hydroxylamine reaction and its application in pharmacy. Ann. Pharm. Françaises 1958, 16, 179–185. [Google Scholar]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Brown, M.R.; Geddes, J.W.; Sullivan, P.G. Brain Region-Specific, Age-Related, Alterations in Mitochondrial Responses to Elevated Calcium. J. Bioenerg. Biomembr. 2004, 36, 401–406. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Rogers, J.; Strohmeyer, R.; Kovelowski, C.; Li, R. Microglia and inflammatory mechanisms in the clearance of amyloid? peptide. Glia 2002, 40, 260–269. [Google Scholar] [CrossRef]
- Dasuri, K.; Zhang, L.; Keller, J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free. Radic. Biol. Med. 2013, 62, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; MacGarvey, U.; Beal, M.F. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann. Neurol. 1994, 36, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Melchor, J.P.; Pawlak, R.; Strickland, S. The Tissue Plasminogen Activator-Plasminogen Proteolytic Cascade Accelerates Amyloid-β (Aβ) Degradation and Inhibits Aβ-Induced Neurodegeneration. J. Neurosci. 2003, 23, 8867–8871. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G. What the locus of brain lesion tells us about the nature of the cognitive defect underlying category-specific disorders: A review. Cortex 2000, 36, 539–559. [Google Scholar] [CrossRef]
- Shekarian, M.; Komaki, A.; Shahidi, S.; Sarihi, A.; Salehi, I.; Raoufi, S. The protective and therapeutic effects of vinpocetine, a PDE1 inhibitor, on oxidative stress and learning and memory impairment induced by an intracerebroventricular (ICV) injection of amyloid beta (aβ) peptide. Behav. Brain Res. 2020, 383, 112512. [Google Scholar] [CrossRef] [PubMed]
- Asadbegi, M.; Komaki, A.; Salehi, I.; Yaghmaei, P.; Ebrahim-Habibi, A.; Shahidi, S.; Sarihi, A.; Asl, S.S.; Golipoor, Z. Effects of thymol on amyloid-β-induced impairments in hippocampal synaptic plasticity in rats fed a high-fat diet. Brain Res. Bull. 2018, 137, 338–350. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Cho, K.H.; Kim, J.C.; Heo, H.J. Green Tea Seed Oil Suppressed Aβ1–42-Induced Behavioral and Cognitive Deficit via the Aβ-Related Akt Pathway. Int. J. Mol. Sci. 2019, 20, 1865. [Google Scholar] [CrossRef]
- He, M.T.; Kim, J.-H.; Park, C.H.; Cho, E.J. Combination of Carthamus tinctorius L. seed and Taraxacum coreanum exerts synergistic effects on learning and memory function by regulating metabolism of amyloid beta in mice. J. Funct. Foods 2020, 72, 104048. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Y.; Sun, B.; Su, G.; Zhao, M. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, F.; Liu, C.; Wang, J.; Guo, Y.; Liu, J.; Min, W. Antioxidant hydrolyzed peptides from Manchurian walnut (Juglans mandshurica Maxim.) attenuate scopolamine-induced memory impairment in mice. J. Sci. Food Agric. 2018, 98, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Setzer, W.N.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Braidy, N.; Gortzi, O. Neuroprotective Effects of Ellagitannins: A Brief Review. Curr. Drug Targets 2017, 18, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Kiasalari, Z.; Heydarifard, R.; Khalili, M.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Zahedi, E.; Sanaierad, A.; Roghani, M. Ellagic acid ameliorates learning and memory deficits in a rat model of Alzheimer’s disease: An exploration of underlying mechanisms. Psychopharmacology 2017, 234, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Augusto, E.; Oliveira, C.; Agostinho, P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: Involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience 2008, 156, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Amyloid precursor protein-mediated free radicals and oxidative damage: Implications for the development and progression of Alzheimer’s disease. J. Neurochem. 2006, 96, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Diplock, A.T.; Charuleux, J.-L.; Crozier-Willi, G.; Kok, F.J.; Rice-Evans, C.; Roberfroid, M.; Stahl, W.; Viña-Ribes, J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998, 80, S77–S112. [Google Scholar] [CrossRef]
- Haider, S.; Batool, Z.; Ahmad, S.; Siddiqui, R.A.; Haleem, D.J. Walnut supplementation reverses the scopolamine-induced memory impairment by restoration of cholinergic function via mitigating oxidative stress in rats: A potential therapeutic intervention for age related neurodegenerative disorders. Metab. Brain Dis. 2017, 33, 39–51. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Xu, C.; Zhao, Q.; Ma, Y.; Zhao, S.; Chen, C. Glycolipid metabolism and liver transcriptomic analysis of the therapeutic effects of pressed degreased walnut meal extracts on type 2 diabetes mellitus rats. Food Funct. 2020, 11, 5538–5552. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, W.; Chen, D.; Lin, Y.; Ma, Y.; Chen, C.; Zhao, S. Optimization of simultaneous microwave/ultrasonic-assisted extraction of phenolic compounds from walnut flour using response surface methodology. Pharm. Biol. 2017, 55, 1999–2004. [Google Scholar] [CrossRef]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef]
- Geerts, H. Indicators of neuroprotection with galantamine. Brain Res. Bull. 2005, 64, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; LaFerla, F.M. The role of nicotinic acetylcholine receptors in Alzheimer’s disease. J. Physiol. 2006, 99, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Kawai, H.; Berg, D.K. β-Amyloid peptide blocks the response of α7-containing nicotinic receptors on hippocampal neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.N.; Song, J.H.; Lee, J.S. Screening of a potent antidementia acetylcholinesterase inhibitor-containing fruits and optimal extraction conditions. Korean J. Food Nutr. 2010, 23, 318–323. [Google Scholar]
- Zeisel, S.H. Choline: Critical Role During Fetal Development and Dietary Requirements in Adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Maguire, L.S.; O’Sullivan, S.M.; Galvin, K.; O’Connor, T.P.; Maguire, L.S. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Bogie, J.F.J.; Mailleux, J.; Vanmol, J.; Lütjohann, D.; Mulder, M.; Hendriks, J.J.A. Plant sterols: Friend or foe in CNS disorders? Prog. Lipid Res. 2015, 58, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Xin, G.; Li, B.; Li, D.; Sun, X.; Yan, T.; Li, L.; Shi, L.; Cao, S.; Meng, X. Anthocyanins Extracted from Aronia melanocarpa Protect SH-SY5Y Cells against Amyloid-beta (1–42)-Induced Apoptosis by Regulating Ca2+ Homeostasis and Inhibiting Mitochondrial Dysfunction. J. Agric. Food Chem. 2018, 66, 12967–12977. [Google Scholar] [CrossRef]
- Salimi, A.; Ayatollahi, A.; Seydi, E.; Khomeisi, N.; Pourahmad, J.; Seydi, E.; Khomaisi, N.; Ayatollahi, S.A. Direct toxicity of amyloid beta peptide on rat brain mitochondria: Preventive role ofMangifera indicaandJuglans regia. Toxicol. Environ. Chem. 2015, 97, 1–14. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Lu, H.; Fang, L.; Qin, H.; Liu, C.; Min, W. Neuroprotection by Walnut-Derived Peptides through Autophagy Promotion via Akt/mTOR Signaling Pathway against Oxidative Stress in PC12 Cells. J. Agric. Food Chem. 2020, 68, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M. Cerebral amyloid angiopathy. Prog. Mol. Biol. Transl. 2004, 107, 41–78. [Google Scholar]
- Re, F.; Cambianica, I.; Sesana, S.; Salvati, E.; Cagnotto, A.; Salmona, M.; Couraud, P.-O.; Moghimi, S.M.; Masserini, M.; Sancini, G. Functionalization with ApoE-derived peptides enhances the interaction with brain capillary endothelial cells of nanoliposomes binding amyloid-beta peptide. J. Biotechnol. 2011, 156, 341–346. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, L.; Quiros, M.; Díaz-Coránguez, M. ZO Proteins and Redox-Dependent Processes. Antioxidants Redox Signal. 2011, 15, 1235–1253. [Google Scholar] [CrossRef] [PubMed]
- Farbood, Y.; Sarkaki, A.; Dianat, M.; Khodadadi, A.; Haddad, M.K.; Mashhadizadeh, S. Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci. 2015, 124, 120–127. [Google Scholar] [CrossRef]
- Jahangiri, H.M.; Sarkaki, A.; Farbood, Y.; Dianat, M.; Goudarzi, G. Gallic acid affects blood-brain barrier permeability, behaviors, hippocampus local EEG, and brain oxidative stress in ischemic rats exposed to dusty particulate matter. Environ. Sci. Pollut. Res. 2019, 27, 5281–5292. [Google Scholar] [CrossRef]
- De Felice, F.G. Alzheimer’s disease and insulin resistance: Translating basic science into clinical applications. J. Clin. Investig. 2013, 123, 531–539. [Google Scholar] [CrossRef]
- Meraz Rios, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernández, J.; Campos-Peña, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef]
- Poulose, S.M.; Bielinski, N.F.; Shukitt-Hale, B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J. Nutr. Biochem. 2013, 24, 912–919. [Google Scholar] [CrossRef]
- Carey, A.N.; Fisher, D.R.; Joseph, J.A.; Shukitt-Hale, B. The ability of walnut extract and fatty acids to protect against the deleterious effects of oxidative stress and inflammation in hippocampal cells. Nutr. Neurosci. 2013, 16, 13–20. [Google Scholar] [CrossRef]
- Haramiishi, R.; Okuyama, S.; Yoshimura, M.; Nakajima, M.; Furukawa, Y.; Ito, H.; Amakura, Y. Identification of the characteristic components in walnut and anti-inflammatory effect of glansreginin A as an indicator for quality evaluation. Biosci. Biotechnol. Biochem. 2019, 84, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Schabbauer, G.; Tencati, M.; Pedersen, B.; Pawlinski, R.; Mackman, N. PI3K-Akt Pathway Suppresses Coagulation and Inflammation in Endotoxemic Mice. Arter. Thromb. Vasc. Biol. 2004, 24, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Roy, A.M.; Baliga, M.S. Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol. Cancer Ther. 2005, 4, 207–216. [Google Scholar] [PubMed]
- Crespo-Biel, N.; Canudas, A.; Camins, A.; Pallàs, M. Kainate induces AKT, ERK and cdk5/GSK3β pathway deregulation, phosphorylates tau protein in mouse hippocampus. Neurochem. Int. 2007, 50, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xie, Y.; Meng, Y.; Ma, W.; Tong, Z.; Yang, X.; Lai, S.; Zhou, Y.; He, M.; Liao, Z. Resveratrol protects cardiomyocytes against anoxia/reoxygenation via dephosphorylation of VDAC1 by Akt-GSK3 β pathway. Eur. J. Pharmacol. 2019, 843, 80–87. [Google Scholar] [CrossRef]
- Hannan, A.; Dash, R.; Sohag, A.A.M.; Haque, N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Min, W.; Guo, Y.; Zhao, F.; Qin, H.; Lu, H.; Fang, L.; Wang, J.; Min, W. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 2019, 10, 3491–3501. [Google Scholar] [CrossRef]
- Xu, M.; Du, Q.; Hao, Y.; Fan, R.; Li, Y. Long-term walnut oligopeptides prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and down-regulating the PI3K/Akt signaling pathway in hippocampus. Curr. Dev. Nutr. 2020, 4, 92. [Google Scholar] [CrossRef]
- Park, G.; Jang, D.-S.; Oh, M.S. Juglans mandshurica leaf extract protects skin fibroblasts from damage by regulating the oxidative defense system. Biochem. Biophys. Res. Commun. 2012, 421, 343–348. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, B.; Zhou, K.; Chen, M.; Wang, M.; Jia, Y.; Song, Y.; Li, Y.; Wen, A. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: Role of Nrf2 activation. Int. J. Cardiol. 2014, 175, 508–514. [Google Scholar] [CrossRef]
| Antibody | Catalog | Conc. | Manufacturer |
|---|---|---|---|
| β-actin | sc-69879 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| AChE | sc-373901 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| p-JNK | sc-6254 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| p-Akt | sc-514032 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| p-tau | sc-12952 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| ZO-1 | sc-33725 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| Occludin | sc-133256 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| HO-1 | sc-136960 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| COX-2 | sc-376861 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| p-IκB | sc-8404 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| IL-1β | sc-4592 | 1:1000 | Santa Cruz Biotech (Dallas, TX, United States) |
| ChAT | 20747-1AP | 1:1000 | Bioneer (Daejeon, Korea) |
| TNF-α | 5178SC | 1:1000 | Cell Signaling Tech (Danvers, MA, United States) |
| Caspase-3 | CSB-PA05689A0Rb | 1:1000 | Cusabio (Hubei, China) |
| TNFR1 | CSB-PA621879EA01HU | 1:1000 | Cusabio (Hubei, China) |
| No. | RT a | Parent Ion b | MS2 Ions c | Compound |
|---|---|---|---|---|
| (min) | (m/z) | (m/z) | ||
| 1 | 2.55 | 783 | 481, 301, 275 | Pedunculagin/casuariin isomer (bis-HHDP–glucose) |
| 2 | 2.71 | 783 | 481, 301, 275 | Pedunculagin/casuariin isomer (bis-HHDP–glucose) |
| 3 | 2.88 | 951 | 907, 783, 301, 275 | Praecoxin A/platycariin isomer (trigalloyl-HHDP–glucose) |
| 4 | 2.90 | 785 | 633, 483, 301, 275 | Tellimagrandin I isomer (digalloyl-HHDP–glucose) |
| 5 | 3.03 | 935 | 785, 633, 481, 301, 275 | Casuarinin/casuarictin isomer |
| 6 | 3.06 | 433 | 301 | Ellagic acid pentoside |
| 7 | 3.22 | 1085 | 633, 451, 301 | Eucalbanin A/cornusiin B isomer |
| 8 | 3.39 | 592 | 567, 403, 343, 283, 241, 197 | Glansreginin A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Lee, U.; Kang, J.Y.; Park, S.K.; Shin, E.J.; Kim, H.-J.; Kim, C.-W.; Kim, M.-J.; Heo, H.J. Anti-Amnesic Effect of Walnut via the Regulation of BBB Function and Neuro-Inflammation in Aβ1-42-Induced Mice. Antioxidants 2020, 9, 976. https://doi.org/10.3390/antiox9100976
Kim JM, Lee U, Kang JY, Park SK, Shin EJ, Kim H-J, Kim C-W, Kim M-J, Heo HJ. Anti-Amnesic Effect of Walnut via the Regulation of BBB Function and Neuro-Inflammation in Aβ1-42-Induced Mice. Antioxidants. 2020; 9(10):976. https://doi.org/10.3390/antiox9100976
Chicago/Turabian StyleKim, Jong Min, Uk Lee, Jin Yong Kang, Seon Kyeong Park, Eun Jin Shin, Hyun-Jin Kim, Chul-Woo Kim, Mahn-Jo Kim, and Ho Jin Heo. 2020. "Anti-Amnesic Effect of Walnut via the Regulation of BBB Function and Neuro-Inflammation in Aβ1-42-Induced Mice" Antioxidants 9, no. 10: 976. https://doi.org/10.3390/antiox9100976
APA StyleKim, J. M., Lee, U., Kang, J. Y., Park, S. K., Shin, E. J., Kim, H.-J., Kim, C.-W., Kim, M.-J., & Heo, H. J. (2020). Anti-Amnesic Effect of Walnut via the Regulation of BBB Function and Neuro-Inflammation in Aβ1-42-Induced Mice. Antioxidants, 9(10), 976. https://doi.org/10.3390/antiox9100976