Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis vinifera var. Merlot, Tannat, and Syrah)
Abstract
:1. Introduction
- −
- The peak current which is proportional to the concentration of antioxidant.
- −
- The peak potential which indicates the type of reductant (the more the oxidation potential is low, the more the reductant is strong and easy to oxidize).
- −
- The charge (area under the curve) is in accordance with the antioxidant capacity of samples [28].
- To determine the polyphenol content of skin and seed extracts (Merlot, Tannat, and Syrah) during ripening.
- To measure the antioxidant capacity (DPPH, ABTS, and FRAP) of these extracts.
- To determine the cyclic voltammetry behavior of these extracts by using disposable single walled carbon nanotubes electrodes for electrochemical tests.
- To determine the correlations of electrochemical parameters with the other antioxidant assays as well as with the phenolic contents.
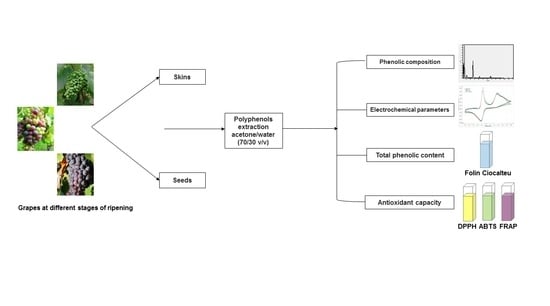
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Samples Preparation
2.4. Determination of Phenolic Composition
2.4.1. Flavanols
2.4.2. Anthocyanins
2.5. Determination of Total Phenolic Content
2.6. Determination of Antioxidant Capacities
2.6.1. Radical Scavenging Activity: DPPH• Assay
2.6.2. Radical Scavenging Activity: ABTS Assay
2.6.3. Ferric-Reducing Antioxidant Power: FRAP Assay
2.6.4. Electrochemical Apparatus and Measurements
2.7. Statistical Analysis
3. Results and Discussion
3.1. Flavanol and Anthocyanin Content of Skin and Seed Grape Extracts during Ripening
3.1.1. Flavanols
3.1.2. Anthocyanins
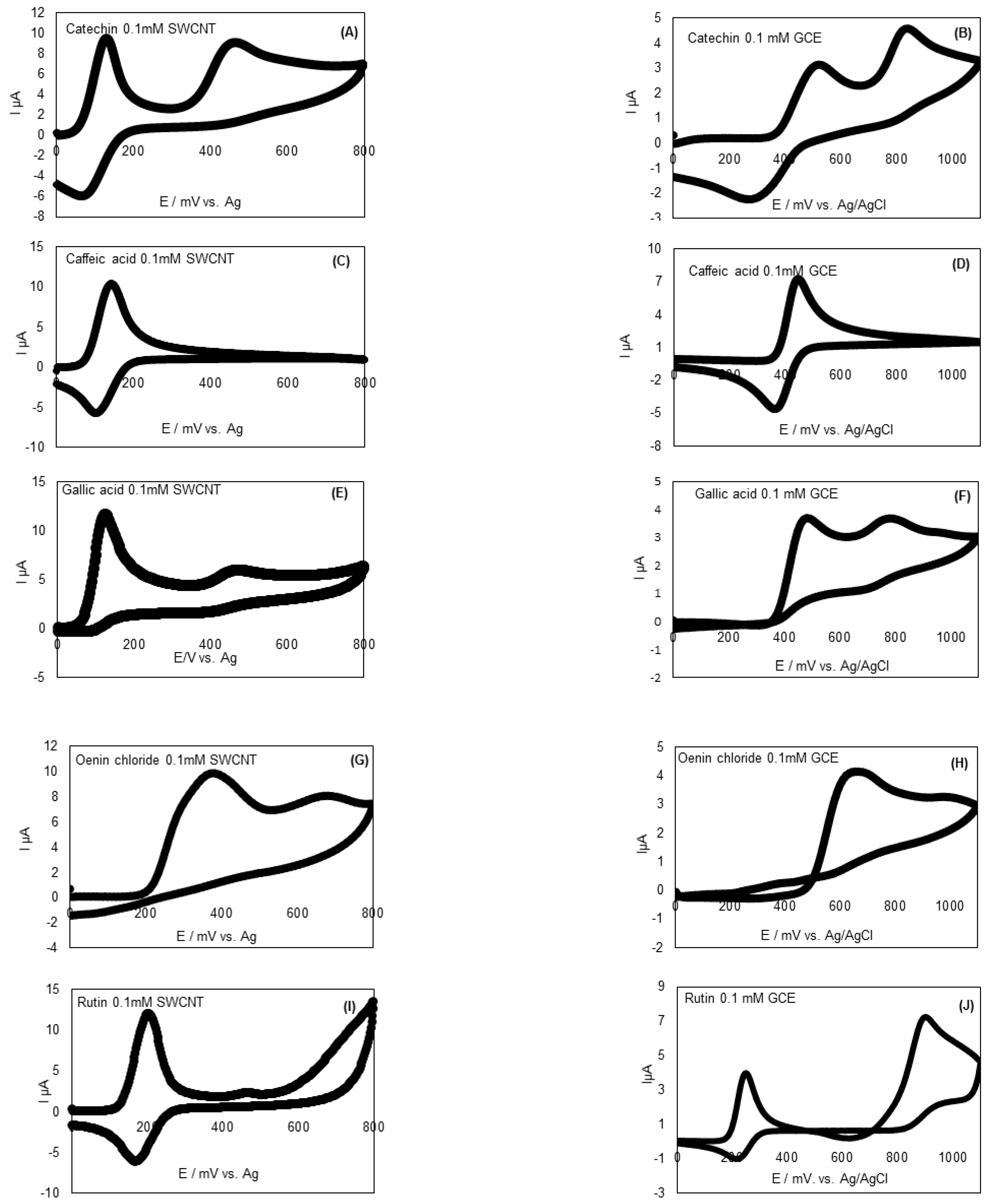
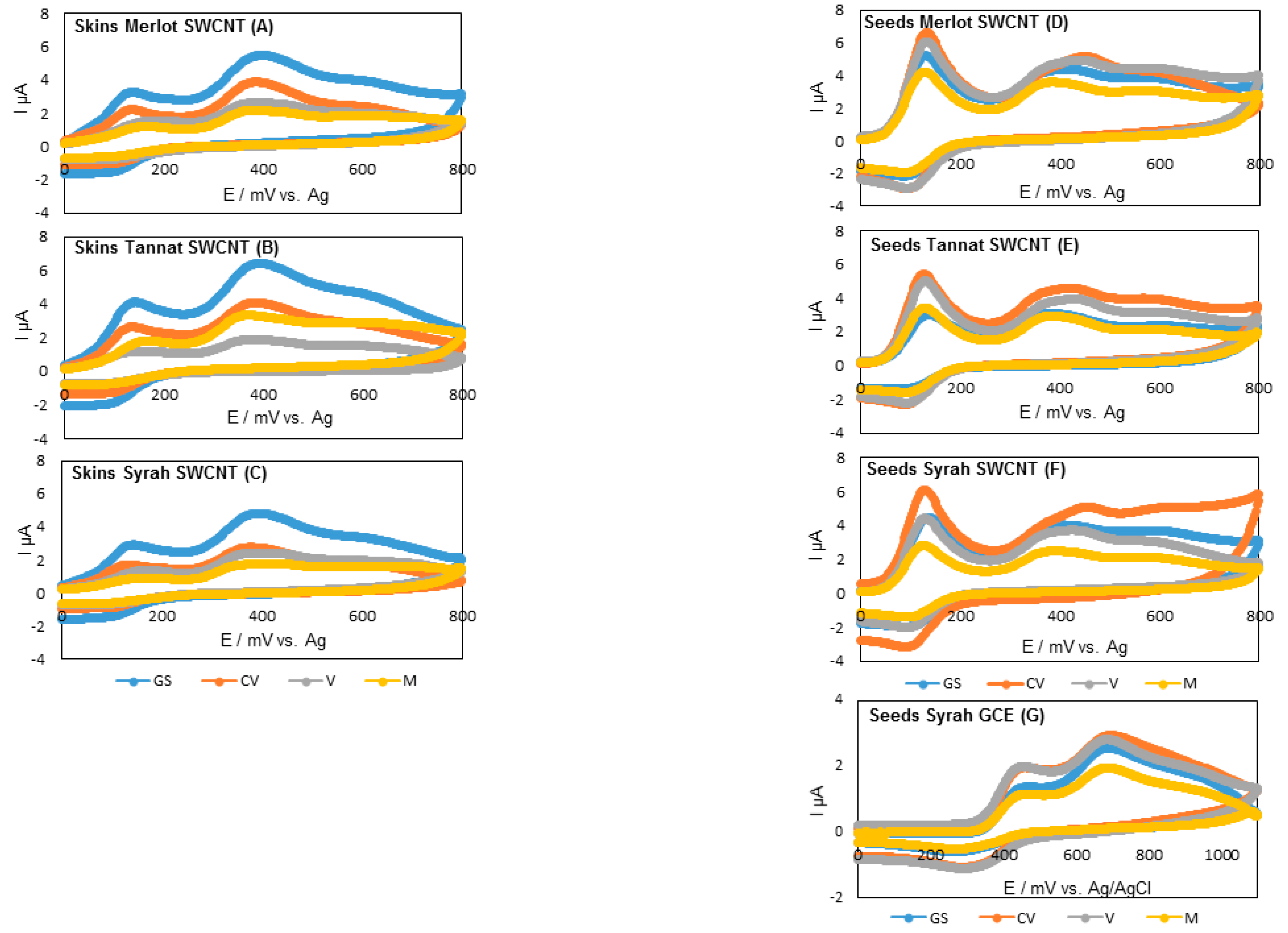
3.2. Electrochemical Behavior of Polyphenol Standards and Skin and Seed Extracts for Various Cultivars at Different Stages of Ripening
3.2.1. Electrochemical Behavior of Standard Polyphenols
3.2.2. Electrochemical Characterization of Skins and Seeds
3.3. Total Phenolic Content and Total Antioxidant Capacity by Spectrophotometric and Electrochemical Assays
3.3.1. Total Phenolic Content and Total Antioxidant Capacity by Spectrophotometric Assays
3.3.2. Antioxidant Capacity by Electrochemical Method of Skin and Seed Grape Extracts
3.4. Correlation between TPC, Antioxidant Capacity, and Phenolic Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, Z.; Owens, C.L.; Zhong, G.-Y.; Cheng, L. Polyphenolic profiles detected in the ripe berries of Vitis vinifera germplasm. Food Chem. 2011, 129, 940–950. [Google Scholar] [CrossRef]
- Cavaliere, C.; Foglia, P.; Gubbiotti, R.; Sacchetti, P.; Samperi, R.; Laganà, A. Rapid-resolution liquid chromatography/mass spectrometry for determination and quantitation of polyphenols in grape berries. Rapid Commun. Mass Spectrom. 2008, 22, 3089–3099. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Göksel, Z.; Erdoğan, S.S.; Öztürk, A.; Atak, A.; Özer, C. Antioxidant Activity and Phenolic Content of Seed, Skin and Pulp Parts of 22 Grape (Vitis vinifera L.) Cultivars (4 Common and 18 Registered or Candidate for Registration): Antioxidant Activity of Grapes. J. Food Process. Preserv. 2015, 39, 1682–1691. [Google Scholar] [CrossRef]
- Nawaz, H.; Shi, J.; Mittal, G.S.; Kakuda, Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep. Purif. Technol. 2006, 48, 176–181. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, Á.; López-Solís, R.; Zamora-Marín, F.; Ricardo-da Silva, J.M.; Laureano, O. Comparative Study of the Phenolic Composition of Seeds and Skins from Carménère and Cabernet Sauvignon Grape Varieties (Vitis vinifera L.) during Ripening. J. Agric. Food Chem. 2010, 58, 3591–3599. [Google Scholar] [CrossRef]
- Fanzone, M.; Zamora, F.; Jofré, V.; Assof, M.; Peña-Neira, Á. Phenolic Composition of Malbec Grape Skins and Seeds from Valle de Uco (Mendoza, Argentina) during Ripening. Effect of Cluster Thinning. J. Agric. Food Chem. 2011, 59, 6120–6136. [Google Scholar] [CrossRef]
- Hubner, A.; Sobreira, F.; Vetore Neto, A.; Pinto, C.A.S.D.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Crespo-Villegas, O.; Garde-Cerdán, T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef]
- Balík, J.; Kyseláková, M.; Vrchotová, N.; Tříska, J.; Kumšta, M.; Veverka, J.; Híc, P.; Totušek, J.; Lefnerová, D. Relations between polyphenols content and antioxidant activity in vine grapes and leaves. Czech. J. Food Sci. 2009, 26, S25–S32. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, M.J.; Chen, Y.Y.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Torres, S. Electrochemical behaviour and antioxidant capacity of anthocyanins from Chilean red wine, grape and raspberry. Food Chem. 2010, 121, 44–48. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.D.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Bozan, B.; Tosun, G.; Özcan, D. Study of polyphenol content in the seeds of red grape (Vitis vinifera L.) varieties cultivated in Turkey and their antiradical activity. Food Chem. 2008, 109, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Coklar, H. Antioxidant capacity and phenolic profile of berry, seed, and skin of Ekşikara (Vitis vinifera L.) grape: Influence of harvest year and altitude. Int. J. Food Prop. 2017, 20, 2071–2087. [Google Scholar] [CrossRef] [Green Version]
- Guendez, R. Determination of low molecular weight polyphenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chem. 2005, 89, 1–9. [Google Scholar] [CrossRef]
- Hosu, A.D.; Cimpoiu, C.; Miclaus, V.; Jantschi, L. Antioxidant Content of Three Different Varieties of Wine Grapes. Biotechnol. Biotechnol. Equip. 2011, 25, 2217–2221. [Google Scholar] [CrossRef] [Green Version]
- Ky, I.; Teissedre, P.-L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From grape to wine: Changes in phenolic composition and its influence on antioxidant activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.I.; Al-Mahasneh, M.A.; Ismaeal, K.; Hidar, A.-G.; Yang, W. Total Phenolics, Antioxidant Activities, and Anthocyanins of Different Grape Seed Cultivars Grown in Jordan. Int. J. Food Prop. 2008, 11, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Kadouh, H.C.; Sun, S.; Zhu, W.; Zhou, K. α-Glucosidase inhibiting activity and bioactive compounds of six red wine grape pomace extracts. J. Funct. Foods 2016, 26, 577–584. [Google Scholar] [CrossRef]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase Inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J. Pharm. Pharm. Sci. 2012, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Kwon, O.; Kim, J.Y. Supplementation of a polyphenol extract from Ecklonia cava reduces body fat, oxidative and inflammatory stress in overweight healthy subjects with abdominal obesity: A randomized, placebo-controlled, double-blind trial. J. Funct. Foods 2018, 46, 356–364. [Google Scholar] [CrossRef]
- Jack, B.U.; Malherbe, C.J.; Mamushi, M.; Muller, C.J.F.; Joubert, E.; Louw, J.; Pheiffer, C. Adipose tissue as a possible therapeutic target for polyphenols: A case for Cyclopia extracts as anti-obesity nutraceuticals. Biomed. Pharmacother. 2019, 120, 109439. [Google Scholar] [CrossRef] [PubMed]
- Callcott, E.T.; Santhakumar, A.B.; Luo, J.; Blanchard, C.L. Therapeutic potential of rice-derived polyphenols on obesity-related oxidative stress and inflammation. J. Appl Biomed. 2018, 16, 255–262. [Google Scholar] [CrossRef]
- Khurana, S.; Piche, M.; Hollingsworth, A.; Venkataraman, K.; Tai, T.C. Oxidative stress and cardiovascular health: Therapeutic potential of polyphenols. Can. J. Physiol. Pharmacol. 2013, 91, 198–212. [Google Scholar] [CrossRef]
- Amiot, M.-J.; Riollet, C.; Landrier, J.-F. Polyphénols et syndrome métabolique. Médecine Mal. Métaboliques 2009, 3, 476–482. [Google Scholar] [CrossRef]
- Richard, T.; Temsamani, H.; Delaunay, J.-C.; Krisa, S.; Mérillon, J.-M. Stilbènes: De la chimie à la neuroprotection. Cah. Nutr. Diététique 2014, 49, 173–180. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.-L. Evolution of Analysis of Polyhenols from Grapes, Wines, and Extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Dai, Y.-Q.; Shiu, K.-K. Glucose Biosensor Based on Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode. Electroanalysis 2004, 16, 1697–1703. [Google Scholar] [CrossRef]
- Chowdhry, A.; Kaur, J.; Khatri, M.; Puri, V.; Tuli, R.; Puri, S. Characterization of functionalized multiwalled carbon nanotubes and comparison of their cellular toxicity between HEK 293 cells and zebra fish in vivo. Heliyon 2019, 5, e02605. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Xiang, J.; Zhou, J.; Ding, H. A disposable amperometric sensor for rapid detection of serotonin in the blood and brain of the depressed mice based on Nafion membrane-coated colloidal gold screen-printed electrode. J. Electroanal. Chem. 2010, 640, 1–7. [Google Scholar] [CrossRef]
- Giné Bordonaba, J.; Terry, L.A. Electrochemical behaviour of polyphenol rich fruit juices using disposable screen-printed carbon electrodes: Towards a rapid sensor for antioxidant capacity and individual antioxidants. Talanta 2012, 90, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Pasakon, P.; Mensing, J.P.; Phokaratkul, D.; Karuwan, C.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. A high-performance, disposable screen-printed carbon electrode modified with multi-walled carbon nanotubes/graphene for ultratrace level electrochemical sensors. J. Appl. Electrochem. 2019, 49, 217–227. [Google Scholar] [CrossRef]
- Zerbib, M.; Mazauric, J.-P.; Meudec, E.; Le Guernevé, C.; Lepak, A.; Nidetzky, B.; Cheynier, V.; Terrier, N.; Saucier, C. New flavanol O-glycosides in grape and wine. Food Chem. 2018, 266, 441–448. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Evolution of Flavanols, Anthocyanins, and Their Derivatives during the Aging of Red Wines Elaborated from Grapes Harvested at Different Stages of Ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Mattioli, A.U.; Teslić, N.; Kilmartin, P.A.; Versari, A. Antioxidant activity of commercial food grade tannins exemplified in a wine model. Food Addit. Contam. Part A 2016, 33, 1761–1774. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Torchio, F.; Cagnasso, E.; Gerbi, V.; Rolle, L. Mechanical properties, phenolic composition and extractability indices of Barbera grapes of different soluble solids contents from several growing areas. Anal. Chim. Acta 2010, 660, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Rolle, L.; Río Segade, S.; Torchio, F.; Giacosa, S.; Cagnasso, E.; Marengo, F.; Gerbi, V. Influence of Grape Density and Harvest Date on Changes in Phenolic Composition, Phenol Extractability Indices, and Instrumental Texture Properties during Ripening. J. Agric. Food Chem. 2011, 59, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 1. Grape and wine chemistry. Food Chem. 2013, 138, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Mulero, J.; Pardo, F.; Zafrilla, P. Antioxidant activity and phenolic composition of organic and conventional grapes and wines. J. Food Compos. Anal. 2010, 23, 569–574. [Google Scholar] [CrossRef]
- Delgado, R.; Martín, P.; del Álamo, M.; González, M.-R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Niu, S.; Hao, F.; Mo, H.; Jiang, J.; Wang, H.; Liu, C.; Fan, X.; Zhang, Y. Phenol profiles and antioxidant properties of white skinned grapes and their coloured genotypes during growth. Biotechnol. Biotechnol. Equip. 2017, 31, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.-M.; Revilla, E. Anthocyanin Composition of Cabernet Sauvignon and Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2003, 51, 3372–3378. [Google Scholar] [CrossRef]
- Robinson, S.P.; Davies, C. Molecular biology of grape berry ripening. Aust. J. Grape Wine Res. 2000, 6, 175–188. [Google Scholar] [CrossRef]
- Boido, E.; García-Marino, M.; Dellacassa, E.; Carrau, F.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterisation and evolution of grape polyphenol profiles of Vitis vinifera L. cv. Tannat during ripening and vinification: Polyphenolic profiles of Tannat. Aust. J. Grape Wine Res. 2011, 17, 383–393. [Google Scholar] [CrossRef]
- Haselgrove, L.; Botting, D.; Heeswijck, R.; Høj, P.B.; Dry, P.R.; Ford, C.; Land, P.G.I. Canopy microclimate and berry composition: The effect of bunch exposure on the phenolic composition of Vitis vinifera L. cv. Shiraz grape berries. Aust. J. Grape Wine Res. 2000, 6, 141–149. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. Correlation of Wine Phenolic Composition versus Cyclic Voltammetry Response. Am. J. Enol. Vitic. 2002, 53, 294–302. [Google Scholar]
- Newair, E.F.; Kilmartin, P.A.; Garcia, F. Square wave voltammetric analysis of polyphenol content and antioxidant capacity of red wines using glassy carbon and disposable carbon nanotubes modified screen-printed electrodes. Eur. Food Res. Technol. 2018, 244, 1225–1237. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Folin-Ciocalteu, FRAP, and DPPH* Assays for Measuring Polyphenol Concentration in White Wine. Am. J. Enol. Vitic. 2015, 66, 463–471. [Google Scholar] [CrossRef]
- Vilas-Boas, Â.; Valderrama, P.; Fontes, N.; Geraldo, D.; Bento, F. Evaluation of total polyphenol content of wines by means of voltammetric techniques: Cyclic voltammetry vs. differential pulse voltammetry. Food Chem. 2019, 276, 719–725. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic Voltammetry Method Suitable for Characterizing Antioxidant Properties of Wine and Wine Phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine by-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [Green Version]
- El Rayess, Y.; Barbar, R.; Wilson, E.A.; Bouajila, J. Analytical Methods for Wine Polyphenols Analysis and for Their Antioxidant Activity Evaluation; Nova Science Publishers: New York, NY, USA, 2014. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Nowicka, P.; Turkiewicz, I.; Golis, T. Characterization in vitro potency of biological active fractions of seeds, skins and flesh from selected Vitis vinifera L. cultivars and interspecific hybrids. J. Funct. Foods 2019, 56, 353–363. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- José Jara-Palacios, M.; Luisa Escudero-Gilete, M.; Miguel Hernández-Hierro, J.; Heredia, F.J.; Hernanz, D. Cyclic voltammetry to evaluate the antioxidant potential in winemaking by-products. Talanta 2017, 165, 211–215. [Google Scholar] [CrossRef]
- Selcuk, A.R.; Demiray, E.; Yilmaz, Y. Antioxidant Activity of Grape Seeds Obtained from Molasses (Pekmez) and Winery Production. Acad. Food J. 2011, 9, 39–43. [Google Scholar]
- Doshi, P.; Adsule, P.; Banerjee, K. Phenolic composition and antioxidant activity in grapevine parts and berries (Vitis vinifera L.) cv. Kishmish Chornyi (Sharad Seedless) during maturation. Int. J. Food Sci. Technol. 2006, 41, 1–9. [Google Scholar] [CrossRef]

| Skins | Seed | ||
|---|---|---|---|
| Flavanols (mg/g DW) | Anthocyanins (mg M3GE/g DW) | Flavanols (mg/g DW) | |
| Merlot | |||
| Green stage | 199 ± 19 a | ND | 545 ± 9 a |
| Close to veraison | 124 ± 16 b | 2 ± 1 c | 598 ± 30 a |
| Veraison | 45 ± 9 c | 17 ± 1 b | 437 ± 17 b |
| Maturity | 42 ± 2 c | 22 ± 1 a | 329 ± 24 c |
| Tannat | |||
| Green stage | 224 ± 40 a | ND | 424 ± 27 bc |
| Close to veraison | 166 ± 74 a | ND | 530 ± 16 a |
| Veraison | 31 ± 5 b | 10 ± 3 b | 469 ± 21 b |
| Maturity | 19 ± 2 b | 36 ± 2 a | 382 ± 23 c |
| Syrah | |||
| Green stage | 198 ± 34 a | ND | 496 ± 19 ab |
| Close to veraison | 100 ± 26 b | 3 ± 1 c | 532 ± 21 a |
| Veraison | 40 ± 7 c | 28 ± 1 a | 439 ± 32 b |
| Maturity | 18 ± 1 c | 14 ± 1.0 b | 201 ± 41 c |
| Standards | Potential (mV) | |||
|---|---|---|---|---|
| SWCNT-SPCE (vs. Ag) | GCE (vs. Ag/AgCl-KCl 3M) | |||
| Ep,a1 | Ep,a2 | Ep,a1 | Ep,a2 | |
| Catechin | 132 | 468 | 483 | 826 |
| Caffeic acid | 139 | / | 445 | / |
| Gallic acid | 122 | 465 | 472 | 766 |
| Oenin chloride | 377 | 669 | 652 | 987 |
| Rutin | 201 | 460 | 260 | 898 |
| Skins | ||||
|---|---|---|---|---|
| TPC (mg GAE/g DW) | DPPH (µmol TE/g DW) | ABTS (µmol TE/g DW) | FRAP (mmol Fe+2E/g DW) | |
| Merlot | ||||
| Green stage | 280 ± 39 a | 763 ± 67 a | 804 ± 37 a | 2781 ± 186 a |
| Close to veraison | 138 ± 12 b | 575 ± 46 b | 748 ± 41 a | 1925 ± 81 b |
| Veraison | 82 ± 11 b | 349 ± 24 c | 424 ± 11 b | 1036 ± 114 c |
| Maturity | 76 ± 9 b | 403 ± 28 c | 527 ± 80 b | 1180 ± 16 c |
| Tannat | ||||
| Green stage | 258 ± 21 a | 932 ± 120 a | 1211 ± 120 a | 2704 ± 431 a |
| Close to veraison | 188 ± 44 b | 647 ± 123 b | 1109 ± 188 a | 2322 ± 537 a |
| Veraison | 72 ± 14 c | 409 ± 54 b | 696 ± 79 b | 805 ± 8 b |
| Maturity | 111 ± 9 c | 528 ± 48 b | 612 ± 36 b | 1219 ± 39 b |
| Syrah | ||||
| Green stage | 212 ± 39 a | 853 ± 94 a | 843 ± 124 a | 2159 ± 432 a |
| Close to veraison | 103 ± 21 b | 674 ± 10 ab | 687 ± 141 ab | 1239 ± 251 b |
| Veraison | 85 ± 8 b | 639 ± 22 b | 529 ± 88 ab | 851 ± 29 b |
| Maturity | 63 ± 8 b | 557 ± 29 b | 357 ± 14 b | 780 ± 62 b |
| Seeds | ||||
| Merlot | ||||
| Green stage | 867 ± 60 a | 3855 ± 413 a | 1681 ± 302 ab | 6047 ± 612 a |
| Close to veraison | 834 ± 7 a | 3998 ± 317 a | 1846 ± 123 a | 6006 ± 9928 a |
| Veraison | 805 ± 92 b | 3675 ± 172 a | 1663.92 ± 89 ab | 5436 ± 391 a |
| Maturity | 571 ± 23 b | 2876 ± 300 b | 1340.8 ± 67 b | 4683 ± 492 a |
| Tannat | ||||
| Green stage | 586 ± 57 ab | 3608 ± 201 ab | 1467 ± 266 ab | 4651 ± 726 ab |
| Close to veraison | 712 ± 69 a | 3875 ± 118 a | 1697 ± 45 a | 5557 ± 503 a |
| Veraison | 676 ± 18 a | 3706 ± 302 a | 1656 ± 137 a | 4201 ± 903 ab |
| Maturity | 489 ± 55 b | 3114 ± 127 b | 1240.95 ± 47 b | 3266 ± 300 b |
| Syrah | ||||
| Green stage | 556 ± 52 ab | 2677 ± 216 ab | 1171 ± 91 a | 3979 ± 2115 a |
| Close to veraison | 615 ± 21 a | 2915 ± 467 a | 1325 ± 46 a | 5386 ± 742 a |
| Veraison | 467 ± 6 b | 2366 ± 105 ab | 1079 ± 159 a | 4719 ± 639 a |
| Maturity | 454 ± 96 b | 1991 ± 211 b | 590 ± 186 b | 3460 ± 1065 a |
| Skins | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ep,a1 (mV) | Ep,a2 (mV) | Q240mV (µC/g DW) | Q520mV (µC/g DW) | Q520mv-Q240mv (µC/g DW) | Q800mV (µC/g DW) | Q240mV/Q800mV (%) | ||
| Merlot | Green stage | 137 ± 3 b | 391 ± 4 a | 44 ± 6 a | 153 ± 26 a | 110 ± 19 a | 262 ± 55 a | 17 ± 1 ab |
| Close to veraison | 134 ± 2 b | 383 ± 4 a | 39 ± 3 a | 126 ± 14 a | 87 ± 11 a | 166 ± 1 b | 22 ± 3 a | |
| Veraison | 159 ± 5 a | 363 ± 2 b | 15 ± 1 b | 57 ± 2 b | 42 ± 1 b | 154 ± 4 b | 13 ± 1 b | |
| Maturity | 157 ± 3 a | 370 ± 1 b | 22 ± 1 b | 75 ± 5 b | 53 ± 4 b | 118 ± 7 b | 19 ± 1 a | |
| Tannat | Green stage | 139 ± 5 b | 392 ± 5 a | 65 ± 8 a | 211 ± 20 a | 145 ± 13 a | 315 ± 36 a | 21 ± 1 a |
| Close to veraison | 133 ± 1 b | 383 ± 2 b | 42 ± 10 b | 134 ± 37 b | 92 ± 27 b | 215 ± 5 b | 18 ± 6 a | |
| Veraison | 130 ± 9 b | 356 ± 3 c | 21 ± 6 c | 64 ± 18 c | 43 ± 12 c | 154 ± 24 b | 16 ± 8 a | |
| Maturity | 164 ± 2 a | 362 ± 1 c | 27 ± 5 bc | 105 ± 16 bc | 78 ± 11 bc | 174 ± 3 b | 18 ± 5 a | |
| Syrah | Green stage | 137 ± 1 b | 383 ± 1 a | 36 ± 5 a | 119 ± 21 a | 83 ± 16 a | 172 ± 27 a | 21 ± 1 a |
| Close to veraison | 126 ± 2 c | 377 ± 3 ab | 29 ± 5 ab | 91 ± 18 ab | 62 ± 13 ab | 152 ± 11 a | 18 ± 3 ab | |
| Veraison | 160 ± 5 a | 362 ± 3 bc | 21 ± 1 b | 77 ± 4 ab | 56 ± 3 ab | 141 ± 6 a | 16 ± 1 ab | |
| Maturity | 141 ± 3 b | 359 ± 2 c | 16 ± 3 b | 59 ± 5 b | 42 ± 3 b | 129 ± 1 a | 14 ± 2 b | |
| Seeds | ||||||||
| Merlot | Green stage | 129 ± 4 a | 390 ± 8 bc | 358 ± 36 a | 905 ± 90 a | 547 ± 54 a | 1232 ± 152 bc | 30 ± 3.64 a |
| Close to veraison | 133 ± 3 a | 449 ± 4 a | 393 ± 24 a | 958 ± 53 a | 565 ± 29 a | 1407 ± 35 ab | 28 ± 1 ab | |
| Veraison | 132 ± 2 a | 397 ± 1 b | 379 ± 8 a | 944 ± 23 a | 564 ± 15 a | 1471 ± 65 a | 26 ± 0.63 ab | |
| Maturity | 128 ± 1 a | 380 ± 3 c | 252 ± 19 b | 639 ± 56 b | 387 ± 39 b | 1036 ± 94 c | 24 ± 0.47 b | |
| Tannat | Green stage | 135 ± 3 a | 377 ± 6 b | 206 ± 29 b | 555 ± 64 b | 349 ± 35 b | 808 ± 112 b | 26 ± 1.63 a |
| Close to veraison | 128 ± 1 a | 392 ± 6 ab | 319 ± 22 a | 827 ± 49 a | 508 ± 28 a | 1313 ± 52 a | 24 ± 1.08 a | |
| Veraison | 129 ± 3 a | 418 ± 20 a | 302 ± 16 a | 746 ± 49 a | 444 ± 33 a | 1112 ± 162 ab | 27 ± 2.45 a | |
| Maturity | 129 ± 4 a | 379 ± 2 b | 216 ± 7 b | 532 ± 20 b | 316 ± 16 b | 813 ± 61 b | 27 ± 1.14 a | |
| Syrah | Green stage | 136 ± 3 a | 397 ± 5 c | 302 ± 18 b | 724 ± 36 b | 516 ± 179 ab | 1165 ± 26 ab | 26 ± 2.24 a |
| Close to veraison | 127 ± 2 b | 438 ± 6 a | 388 ± 24 a | 937 ± 75 a | 549 ± 50 a | 1497 ± 15 a | 23 ± 0.33 a | |
| Veraison | 129 ± 1 b | 409 ± 4 b | 268 ± 20 b | 691 ± 80 b | 424 ± 30 ab | 1082 ± 44 ab | 25 ± 0.84 a | |
| Maturity | 126 ± 4 b | 391 ± 4 c | 177 ± 33 c | 463 ± 93 c | 286 ± 60 b | 818 ± 42 b | 24 ± 2.6 a | |
| Skins | ||||||||||
| Folin | DPPH | ABTS | FRAP | Q240mV | Q520mV | Q520mV-Q240mV | Q800mV | Flavanols | Anthocyanins | |
| Folin | 1 | 0.83 | 0.80 | 0.94 | 0.88 | 0.90 | 0.90 | 0.84 | 0.93 | −0.62 |
| DPPH | 0.83 | 1 | 0.75 | 0.79 | 0.81 | 0.82 | 0.81 | 0.69 | 0.86 | −0.55 |
| ABTS | 0.80 | 0.75 | 1 | 0.84 | 0.89 | 0.86 | 0.85 | 0.69 | 0.86 | −0.62 |
| FRAP | 0.94 | 0.79 | 0.84 | 1 | 0.86 | 0.87 | 0.87 | 0.75 | 0.94 | −0.68 |
| Q240mV | 0.88 | 0.81 | 0.89 | 0.86 | 1 | 0.99 | 0.98 | 0.84 | 0.87 | −0.60 |
| Q520mV | 0.90 | 0.82 | 0.86 | 0.87 | 0.99 | 1 | 1.00 | 0.85 | 0.86 | −0.53 |
| Q520mV-Q240mV | 0.90 | 0.81 | 0.85 | 0.87 | 0.98 | 1.00 | 1 | 0.85 | 0.85 | −0.49 |
| Q800mV | 0.84 | 0.69 | 0.69 | 0.75 | 0.84 | 0.85 | 0.85 | 1 | 0.72 | −0.50 |
| Flavanols | 0.93 | 0.86 | 0.86 | 0.94 | 0.87 | 0.86 | 0.85 | 0.72 | 1 | −0.77 |
| Anthocyanins | −0.62 | −0.55 | −0.62 | −0.68 | −0.60 | −0.53 | −0.49 | −0.50 | −0.77 | 1 |
| Seeds | ||||||||||
| Folin | DPPH | ABTS | FRAP | Q240mV | Q520mV | Q520mV-Q240mV | Q800mV | Flavanols | ||
| Folin | 1 | 0.78 | 0.77 | 0.67 | 0.76 | 0.79 | 0.66 | 0.60 | 0.67 | |
| DPPH | 0.78 | 1 | 0.92 | 0.44 | 0.56 | 0.59 | 0.56 | 0.41 | 0.66 | |
| ABTS | 0.77 | 0.92 | 1 | 0.56 | 0.66 | 0.69 | 0.62 | 0.49 | 0.71 | |
| FRAP | 0.67 | 0.44 | 0.56 | 1 | 0.62 | 0.66 | 0.41 | 0.51 | 0.58 | |
| Q240mV | 0.76 | 0.56 | 0.66 | 0.62 | 1 | 0.99 | 0.89 | 0.88 | 0.80 | |
| Q520mV | 0.79 | 0.59 | 0.69 | 0.66 | 0.99 | 1 | 0.90 | 0.88 | 0.80 | |
| Q520mV-Q240mV | 0.66 | 0.56 | 0.62 | 0.41 | 0.89 | 0.90 | 1 | 0.79 | 0.74 | |
| Q800mV | 0.60 | 0.41 | 0.49 | 0.51 | 0.88 | 0.88 | 0.79 | 1 | 0.64 | |
| Flavanols | 0.67 | 0.66 | 0.71 | 0.58 | 0.80 | 0.80 | 0.74 | 0.64 | 1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benbouguerra, N.; Richard, T.; Saucier, C.; Garcia, F. Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis vinifera var. Merlot, Tannat, and Syrah). Antioxidants 2020, 9, 800. https://doi.org/10.3390/antiox9090800
Benbouguerra N, Richard T, Saucier C, Garcia F. Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis vinifera var. Merlot, Tannat, and Syrah). Antioxidants. 2020; 9(9):800. https://doi.org/10.3390/antiox9090800
Chicago/Turabian StyleBenbouguerra, Nawel, Tristan Richard, Cédric Saucier, and François Garcia. 2020. "Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis vinifera var. Merlot, Tannat, and Syrah)" Antioxidants 9, no. 9: 800. https://doi.org/10.3390/antiox9090800
APA StyleBenbouguerra, N., Richard, T., Saucier, C., & Garcia, F. (2020). Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis vinifera var. Merlot, Tannat, and Syrah). Antioxidants, 9(9), 800. https://doi.org/10.3390/antiox9090800