Phenolic Composition and Skin-related Properties of the Aerial Parts Extract of Different Hemerocallis Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Preparation of the Extracts
2.4. Total Flavonoid, Phenolic and Phenolic Acids Content
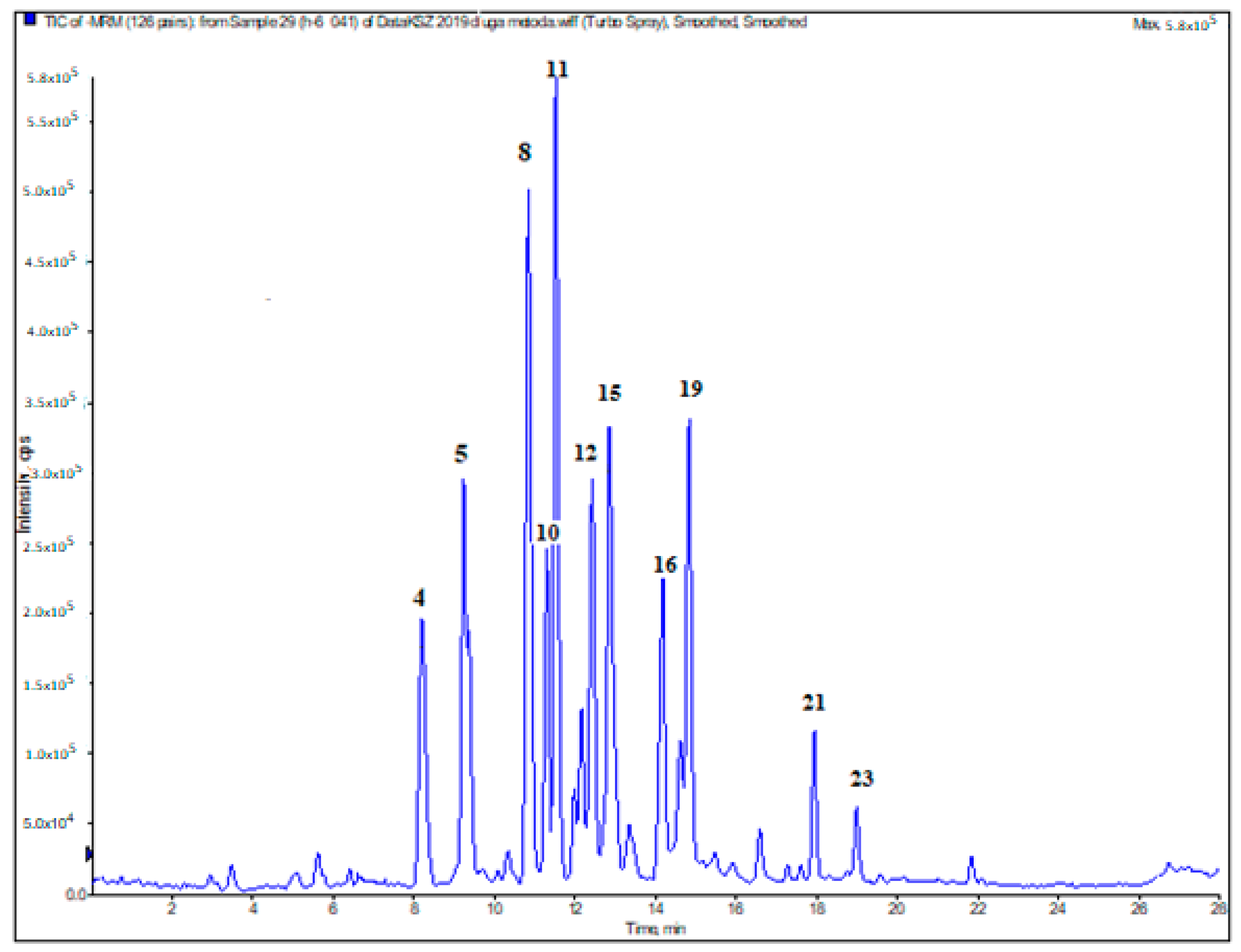
2.5. LC-ESI-MS/MS Analysis
2.6. Antioxidant Activity
2.6.1. DPPH Assay
2.6.2. Metal Chelating Activity (CHEL)
2.7. Enzyme Inhibitory Activity
2.7.1. Anti-Tyrosinase Activity (TYR)
2.7.2. Anti-Elastase Activity (ELA)
2.7.3. Anti-Collagenase Activity (COL)
2.8. Antibacterial Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Analysis
3.2. Skin-Related Activities
3.2.1. DPPH Radical Scavenging Activity
3.2.2. Metal Chelating Activity (CHEL)
3.2.3. Anti-Collagenase Activity (COL)
3.2.4. Anti-Elastase Activity (ELA)
3.2.5. Anti-Tyrosinase Activity (TYR)
3.2.6. Antibacterial Activity
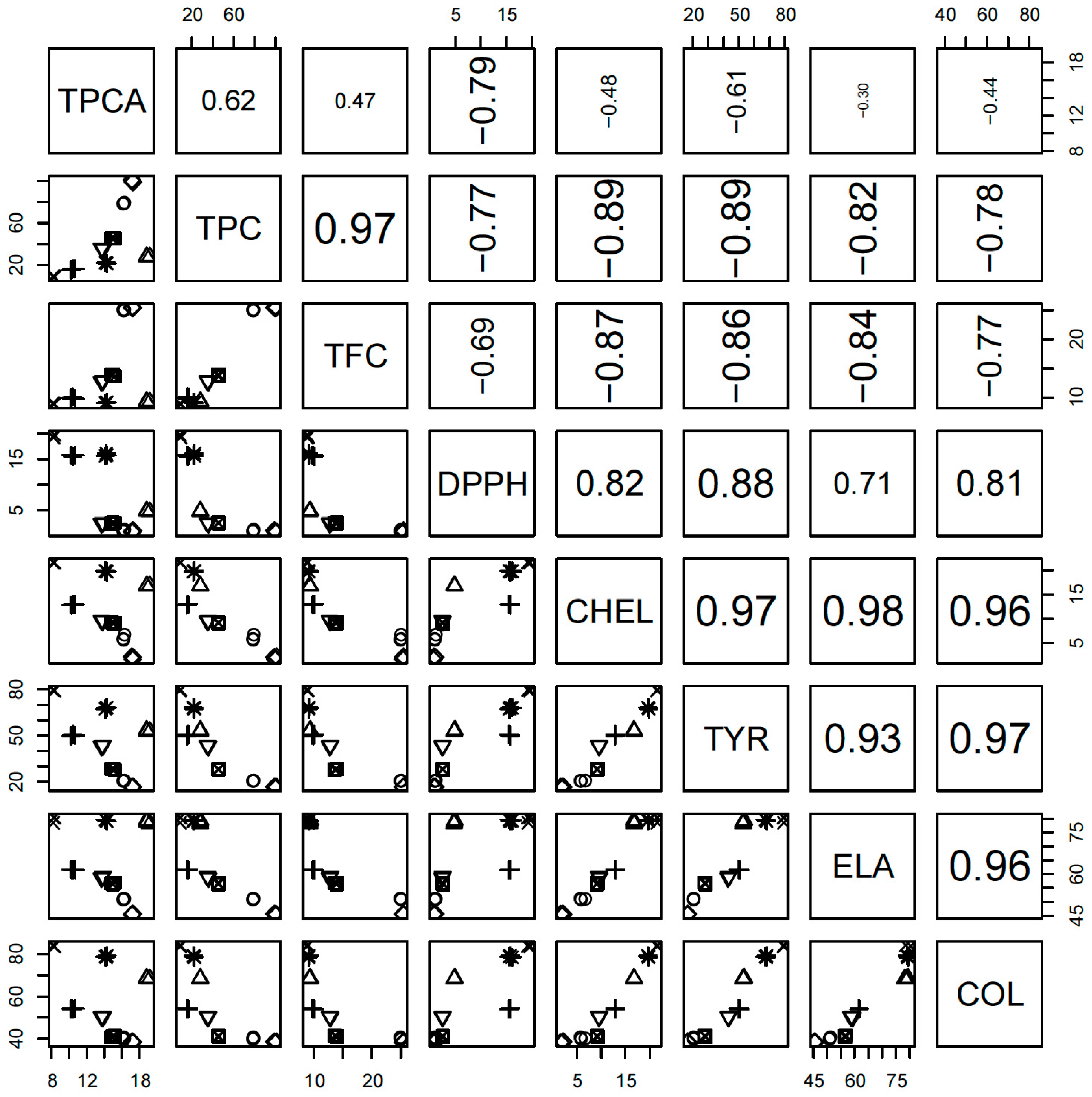
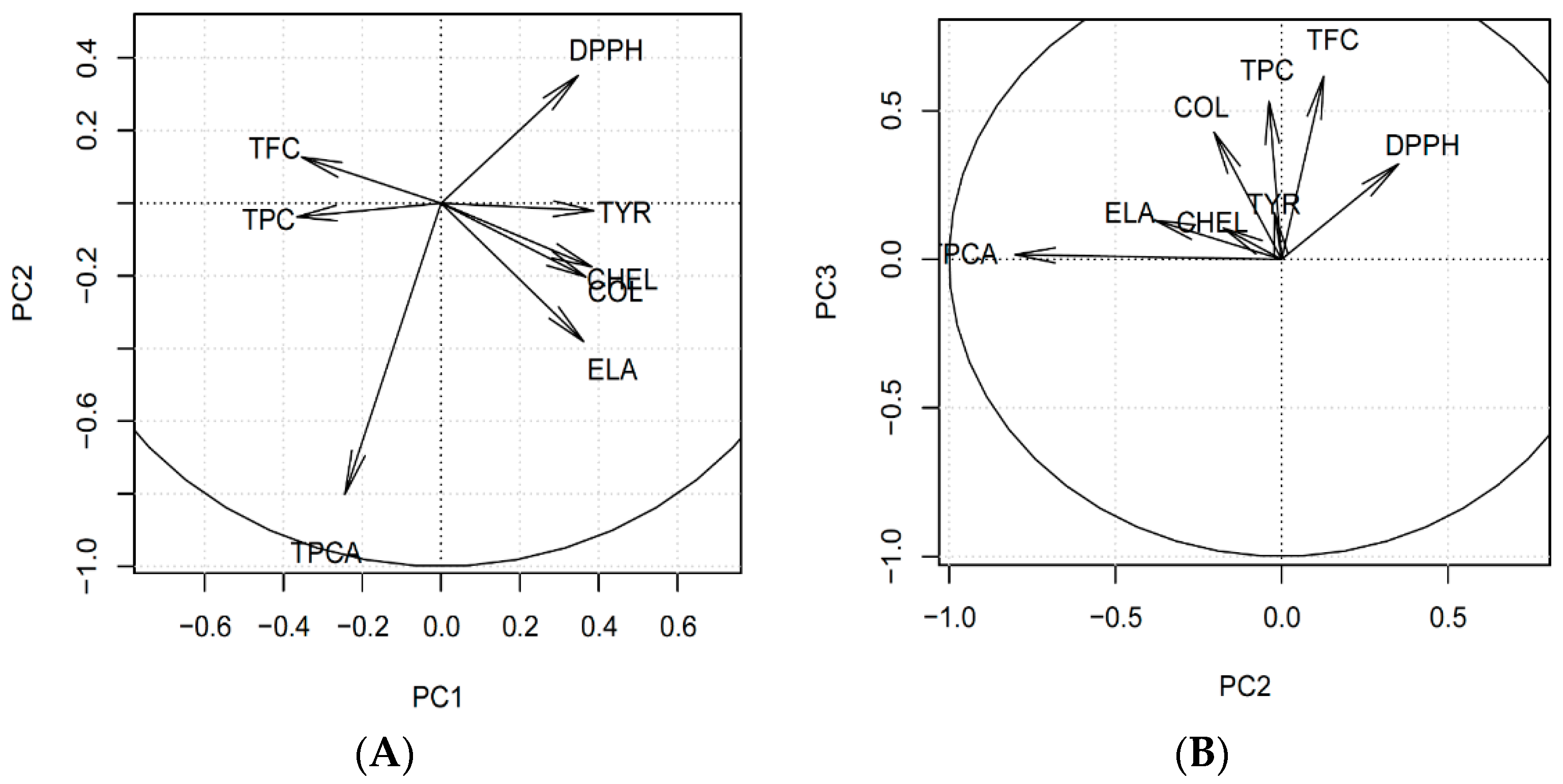
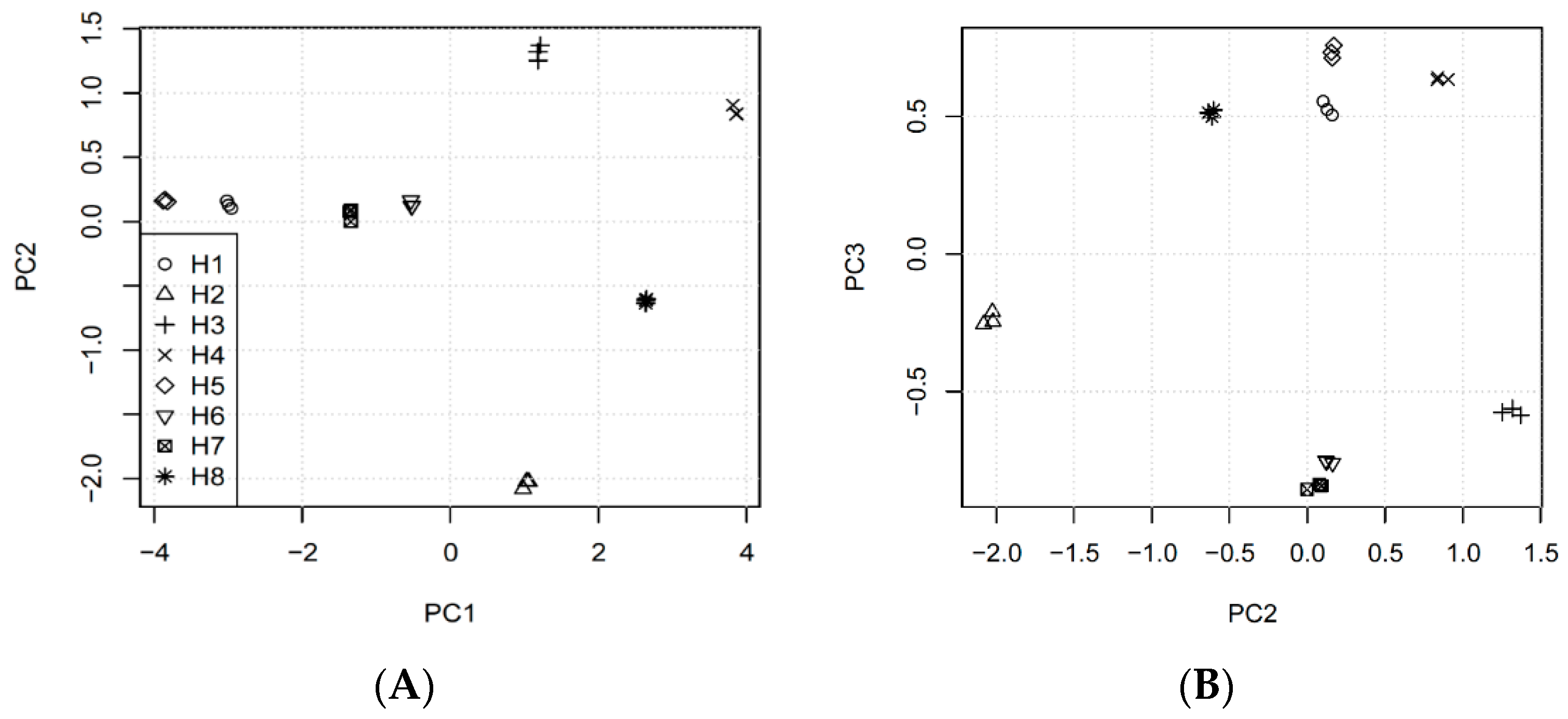
3.3. Multivariate Analysis of the Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nizioł-Łukaszewska, Z.; Bujak, T. Ocena właściwości kosmetyków myjących zawierających ekstrakt wodny z czarnuszki siewnej (Nigella sativa L.). In Rośliny w Nowoczesnej Kosmetologii; Kiełtyka-Dadasiewicz, A., Ed.; Wydawnictwo Akademickie Wyższej Szkoły Społeczno-Przyrodniczej im. Wincentego Pola w Lublinie: Lublin, Poland, 2016; pp. 93–105. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, H.I.; Chang, C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar] [CrossRef]
- Sytar, O. Phenolic acids in the inflorescences of different varieties of buckwheat and their antioxidant activity. J. King Saud Univ. Sci. 2015, 27, 136–142. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. J. Linn. Soc. Bot. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Cichewicz, R.; Nair, M. Isolation and characterization of stelladerol, a new antioxidant naphthalene glycoside, and other antioxidant glycosides from edible daylily (Hemerocallis) flowers. J. Agric. Food Chem. 2002, 50, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Carter, J.; Singh, B.P.; Park, Y.W. Mineral nutrient composition of edible parts of the daylily plant. HortScience 1999, 34, 503. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Lim, K.C.; McKerrow, J.H.; Nair, M.G. Kwanzoquinones A-G and other constituents of Hemerocallis fulva ‘Kwanzo’ roots and their activity against the human pathogenic trematode Schistosoma mansoni. Tetrahedron 2002, 58, 8597–8606. [Google Scholar] [CrossRef]
- Fu, M.R.; He, Z.; Zhao, Y.; Yang, J.; Mao, L. Antioxidant properties and involved compounds of daylily flowers in relation to maturity. Food Chem. 2009, 114, 1192–1197. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamasaki, K.; Maesaki, H.; Tokuno, M.; Okazaki, S.; Moriuchi, H.; Takeshita, K.; Otagiri, M.; Seo, H. An evaluation of novel biological activity in a crude extract from Hemerocallis fulva L. var. sempervirens M. Hotta. Nat. Prod. Res. 2014, 28, 2211–2213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, T.; Fan, B.; Zhang, L.; Lu, C.; Wang, D.; Liu, X.; Wang, F. Advances in researches on chemical composition and functions of Hemerocallis plants. Med. Plant 2018, 9, 16–21. [Google Scholar]
- Namsa, N.D.; Tag, H.; Mandal, M.; Kalita, P.; Das, A.K. An ethnobotanical study of traditional anti-inflammatory plants used by the Lohit community of Arunachal Pradesh, India. J. Ethnopharmacol. 2009, 125, 234–245. [Google Scholar] [CrossRef]
- Gu, L.; Liu, Y.-J.; Wang, Y.-B.; Yi, L.-T. Role of monoaminergic systems in the antidepressant-like effect of ethanol extracts from Hemerocallis citrina. J. Ethnopharmacol. 2012, 139, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, Y.; Sun, J.; Li, G.; Shan, Y.; Chen, P. Study the effects of dying processes on chemical compositions in daylily flowers using flow injection mass spectrometric fingerprinting method and chemometrics. Food Res. Int. 2017, 102, 493–503. [Google Scholar] [CrossRef]
- Konishi, T.; Fujiwara, Y.; Konoshima, T.; Kiyosawa, S.; Nishi, M.; Miyahara, K. Steroidal saponins from Hemerocallis fulva var. kwanso. Chem. Pharm. Bull. 2001, 49, 318–320. [Google Scholar] [CrossRef][Green Version]
- Griesbach, R.J.; Batdorf, L. Flower pigments within Hemerocallis fulva, H. rosea and H. disticha. HortScience 1995, 30, 353–354. [Google Scholar] [CrossRef]
- Zhang, Y.; Cichewicz, R.H.; Nair, M.G. Lipid peroxidation inhibitory compounds from daylily (Hemerocallis fulva) leaves. Life Sci. 2004, 75, 753–763. [Google Scholar] [CrossRef]
- Tai, C.Y.; Chen, B.H. Analysis and stability of carotenoids in the flowers of daylily (Hemorocallis disticha) as affected by various treatments. J. Agric. Food Chem. 2000, 48, 5962–5968. [Google Scholar] [CrossRef]
- Lin, P.; Cai, J.; Li, J.; Sang, W.; Su, Q. Constituents of the essential oil of Hemerocallis flava day lily. Flavour Fragr. J. 2003, 18, 539–541. [Google Scholar] [CrossRef]
- Szewczyk, K.; Kalemba, D.; Miazga-Karska, M.; Krzemińska, B.; Dąbrowska, A.; Nowak, R. The essential oil composition of selected Hemerocallis cultivars and their biological activity. Open Chem. 2019, 17, 1412–1422. [Google Scholar] [CrossRef]
- Inoue, T.; Iwagoe, K.; Konishi, T.; Kiyosawa, S.; Fujiwara, Y. Novel 2,5-dihydrofuryl-γ-lactam derivatives from Hemerocallis fulva L. var. kwanzo Regel. Chem. Pharm. Bull. 1990, 38, 3187–3189. [Google Scholar] [CrossRef]
- Inoue, T.; Konishi, T.; Kiyosawa, S.; Fujiwara, Y. 2,5-Dihydrofuryl-γ-lactam derivatives from Hemerocallis fulva L. var. kwanso Regel. II. Chem. Pharm. Bull. 1994, 42, 154–155. [Google Scholar] [CrossRef]
- Cichewicz, R.; Zhang, Y.; Seeram, N.; Nair, M. Inhibition of human tumor cell proliferation by novel anthraquinones from daylilies. Life Sci. 2004, 74, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Hui-Zi, J.; Wu, L.; Zhang, Y.; Ye, R.; Zhang, W.; Zhang, Y. An exploration of Traditional Chinese Medicinal plants with anti-inflammatory activities. Evid. Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef]
- Kao, F.; Chiang, W.; Liu, H. Inhibitory effect of daylily buds at various stages of maturity on nitric oxide production and the involved phenolic compounds. LWT Food Sci. Technol. 2015, 61, 130–137. [Google Scholar] [CrossRef]
- Du, B.; Tang, X.; Liu, F.; Zhang, C.; Zhao, G.; Ren, F.; Leng, X. Antidepressant-like effects of the hydroalcoholic extracts of Hemerocallis citrina and its potential active components. BMC Complement. Altern. Med. 2014. [Google Scholar] [CrossRef]
- Lin, S.; Chang, H.; Chen, P.; Hsieh, C.; Su, K.; Sheen, L. The antidepressant—Like effect of ethanol extract of daylily flowers in rats. J. Tradit. Complement. Med. 2013, 3, 53–61. [Google Scholar] [CrossRef]
- Chen, H.Y.; Bor, J.Y.; Huang, W.H.; Yen, G.C. Effect of sulfite-treated daylily (Hemerocallis fulva L.) flower on production of nitric oxide and DNA damage in macrophages. J. Food Drug Anal. 2007, 15, 63–70. [Google Scholar] [CrossRef]
- Mao, L.-C.; Pan, X.; Que, F.; Fang, X.-H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur. Food Res. Technol. 2006, 222, 236–241. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, C.; Huang, Y.; Chen, H. Antioxidative caffeoylquinic acids and flavonoids from Hemerocallis fulva flowers. J. Agric. Food Chem. 2011, 59, 8789–8795. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Mao, L.C.; Zheng, X.J. In vitro and in vivo antioxidant activities of daylily flowers and the involvement of phenolic compounds. Asia Pac. J. Clin. Nutr. 2007, 16, 196–203. [Google Scholar] [PubMed]
- Uezu, E. Effects of Hemerocallis on sleep in mice. Psychiatry Clin. Neurosci. 1998, 52, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Guofang, Y.; Na, L.; Lin, Z. Chinese Medicinal Antibacterial Disinfectant and Its Preparation Method. CN 105638780 A, 8 June 2016. [Google Scholar]
- Leconte, N.; Rossignol-Castera, A. Use of an Oily Composition Comprising an Hemerocallis Extract for Improving Firmness of the Skin. EP3052199B1, 13 December 2017. [Google Scholar]
- Lin, X. A Refreshing Whitening Acne Removal Formula. CN 109925269 A, 25 June 2019. [Google Scholar]
- Qinhua, K. Hemerocallis-Rose Whitening Mask and Its Preparation Method. CN 108498441 A, 7 September 2018. [Google Scholar]
- Szewczyk, K.; Bogucka-Kocka, A.; Vorobets, N.; Grzywa-Celińska, A.; Granica, S. Phenolic composition of the leaves of Pyrola rotundifolia L. and their antioxidant and cytotoxic activity. Molecules 2020, 25, 1749. [Google Scholar] [CrossRef] [PubMed]
- Polish Pharmacopoeia IX; PTFarm; Polish Pharmaceutical Society: Warsaw, Poland, 2011; p. 150.
- Bogucka-Kocka, A.; Vorobets, N.; Chrząszcz, M.; Pietrzak, W.; Szewczyk, K. Polyphenol composition of extracts of the fruits of Laserpitium krapffii Crantz and their antioxidant and cytotoxic activity. Antioxidants 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Szewczyk, K.; Gawlik-Dziki, U.; Rzymowska, J.; Komsta, Ł. Antioxidative and cytotoxic potential of some Chenopodium L. species growing in Poland. Saudi J. Biol. Sci. 2016, 23, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crop. Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crop. Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti0collagenase activity of Hypericum hircinum L. Ind. Crop. Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Lim, J.A.; Chung, T.Y.; Cho, E.J. Total phenol content and antioxidative activity of fractions from Hemerocallis fulva leaves. Cancer Prev. Res. 2012, 17, 257–263. [Google Scholar]
- Fu, M.; Mao, L. In vitro antioxidant activities of five cultivars of daylily flowers from China. Nat. Prod. Res. 2008, 22, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.; Grzeszczuk, M. Nutritional and biological value of five edible flower species. Not. Bot. Horti Agrobot. 2019, 47, 128–134. [Google Scholar] [CrossRef]
- Clifford, M.N.; Wu, W.; Kuhnert, N. The chlorogenic acids of Hemerocallis. Food Chem. 2006, 95, 574–578. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Ismail, I.S. Cosmetic potential of Southeast Asian herbs: An overview. Phytochem. Rev. 2015, 14, 419–428. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Ferrali, M.; Signori, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Uitto, J. Connective tissue biochemistry of the aging dermis: Age-associated alternations in collagen and elastin. Clin. Geriatr. Med. 1989, 5, 127–148. [Google Scholar] [CrossRef]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-aging potential and anti-tyrosinase activity of three Hypericum species with focus on phytochemical composition by LC-MS/MS. Ind. Crop. Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef]
- Brás, N.F.; Gonçalves, R.; Mateus, N.; Fernandes, P.A.; Ramos, M.J.; de Freitas, V. Inhibition of pancreatic elastase by polyphenolic compounds. J. Agric. Food Chem. 2010, 58, 10668–10676. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Uyama, H. Tyrosinase, inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A.; el Gayyar, M.A. A new type of tyrosinase inhibitors from natural products as potential treatments for hyperpigmentation. Boll. Chim. Farm. 2001, 140, 267–271. [Google Scholar] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Cushine, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Marzouk, B.; Marzouk, Z.; Décor, R.; Mhadhebi, L.; Fenina, N.; Aouni, M. Antibacterial and antifungal activities of several populations of Tunisian Citrullus colocynthis Schrad immature fruits and seeds. J. Mycol. Med. 2010, 20, 179–184. [Google Scholar] [CrossRef]
| Compound | LOD [ng/mL] | LOQ [ng/mL] | R2 | Linearity Range [ng/mL] |
|---|---|---|---|---|
| gallic acid | 33.3 | 95.0 | 0.9987 | 167–3300 |
| myricetin | 5.0 | 10.0 | 0.9985 | 10–3600 |
| kaempferol | 20.0 | 33.0 | 0.9989 | 33–20,000 |
| protocatechuic acid | 17.0 | 34.0 | 0.9997 | 34–3470 |
| chlorogenic acid | 72.0 | 180.0 | 0.9991 | 180–18,000 |
| cis-sinapic acid | 17.4 | 69.4 | 0.9999 | 69.4–3470 |
| rosmarinic acid | 7.1 | 17.9 | 0.9994 | 17.9–7140 |
| 4-hydroxybenzoic acid | 17.4 | 34.7 | 0.9993 | 69.4–3470 |
| syringic acid | 167.0 | 666.0 | 0.9993 | 666–11,100 |
| cis-caffeic acid | 60.0 | 160.0 | 0.9990 | 175–3500 |
| gentisic acid | 1.7 | 3.3 | 0.9997 | 3.3–330 |
| vanillic acid | 100.0 | 250.0 | 0.9997 | 330–33,000 |
| caffeic acid | 60.0 | 160.0 | 0.9990 | 175–3500 |
| quercetin-3-O-rutinoside (rutin) | 120.0 | 300.0 | 0.9985 | 2000–25,000 |
| quercetin-3-O-galactoside (hyperoside) | 150.0 | 200.0 | 0.9987 | 1000–25,000 |
| quercetin-3-O-glucoside (isoquercetin) | 150.0 | 300.0 | 0.9987 | 2000–25,000 |
| kaempferol-3-O-rutinoside (nicotiflorin) | 60.0 | 120.0 | 0.9991 | 120–50,000 |
| isorhamnetin-3-O-rutinoside (narcissoside) | 100.0 | 150.0 | 0.9985 | 200–2500 |
| p-coumaric acid | 7.3 | 18.1 | 0.9996 | 18.1–1820 |
| kaempferol-3-O-glucoside (astragalin) | 100.0 | 200.0 | 0.9978 | 1200–24,000 |
| isorhamnetin-3-O-glucoside | 100.0 | 250.0 | 0.9985 | 2000–20,000 |
| quercetin 3-O-rhamnoside (quercitrin) | 50.0 | 100.0 | 0.9986 | 1000–25,000 |
| o-coumaric acid | 7.3 | 18.1 | 0.9996 | 18.1–1820 |
| quercetin | 5.0 | 10.0 | 0.9980 | 20–3000 |
| salicylic acid | 3.3 | 16.5 | 0.9989 | 16.5–1650 |
| isorhamnetin | 15.0 | 30.0 | 0.9984 | 50–60,000 |
| Sample | Total Phenolic Content [mg GAE/g DE] | Total phenolic Acids [mg CAE/g DE] | Total Flavonoid Content [mg QE/g DE] |
|---|---|---|---|
| H1 | 78.8 ± 0.4 | 16.2 ± 0.1 | 25.0 ± 0.1 |
| H2 | 28.0 ± 0.1 | 18.9 ± 0.2 | 9.3 ± 0.1 |
| H3 | 16.1 ± 0.5 | 10.4 ± 0.2 | 9.9 ± 0.1 |
| H4 | 9.0 ± 0.4 | 8.3 ± 0.1 | 8.9 ± 0.1 |
| H5 | 99.8 ± 1.1 | 17.2 ± 0.1 | 25.4 ± 0.0 |
| H6 | 35.3 ± 0.5 | 13.8 ± 0.1 | 12.8 ± 0.1 |
| H7 | 45.5 ± 0.0 | 15.0 ± 0.2 | 13.8 ± 0.2 |
| H8 | 22.1 ± 0.4 | 14.2 ± 0.1 | 9.1 ± 0.0 |
| No | Compound | RT [min] | [M–H]−[m/z] | Fragment Ions [m/z] | CE [eV] | Amounts [µg/g DE] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | ||||||
| 1 | gallic acid | 5.07 | 168.7 | 78.9 124.9 | −36 −14 | <LOQ | <LOQ | nd | nd | <LOQ | <LOQ | nd | <LOQ |
| 2 | myricetin | 5.95 | 316.7 | 136.9 150.9 | −32 −26 | nd | <LOQ | nd | nd | nd | <LOQ | nd | nd |
| 3 | kaempferol | 7.56 | 284.7 | 116.8 93.0 | −46 −52 | nd | nd | nd | nd | <LOQ | <LOQ | nd | nd |
| 4 | protocatechuic acid | 8.23 | 152.9 | 80.9 107.8 | −26 −38 | 45.9 ± 0.6 | 74.5 ± 2.8 | 86.4 ± 1.1 | 63.2 ± 0.2 | 94.0 ± 2.0 | 116.8 ± 0.5 | 61.5 ± 1.5 | 577.5 ± 12.5 |
| 5 | chlorogenic acid | 9.30 | 352.9 | 190.8 84.9 | −24 −60 | 982.5 ± 5.0 | 1010.0 ± 10.0 | 461.3 ± 11.3 | 356.3 ± 6.3 | 986.3 ± 8.8 | 209.8 ± 1.0 | 945.0 ± 5.0 | 291.3 ± 1.3 |
| 6 | cis-sinapic acid | 9.78 | 22.8 | 121.0 148.9 | −36 −20 | <LOQ | <LOQ | <LOQ | nd | nd | <LOQ | nd | nd |
| 7 | rosmarinic acid | 10.23 | 358.7 | 132.6 160.8 | −44 −20 | nd | 12.0 ± 0.2 | nd | nd | nd | nd | nd | nd |
| 8 | 4-hydroxybenzoic acid | 11.27 | 136.8 | 92.9 107.9 | −18 −18 | <LOQ | 20.6 ± 0.3 | 9.6 ± 0.2 | 46.8 ± 0.4 | 5.1 ± 0.1 | 373.8 ± 1.3 | <LOQ | <LOQ |
| 9 | syringic acid | 11.41 | 196.9 | 122.8 181.9 | −24 −12 | <LOQ | 4.9 ± 0.2 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 10 | cis-caffeic acid | 11.68 | 178.7 | 88.9 134.9 | −46 −16 | 45.4 ± 1.1 | 24.4 ± 0.9 | 13.3 ± 0.4 | 10.0 ± 0.1 | 21.2 ± 0.1 | 72.4 ± 1.9 | 10.8 ± 0.3 | 98.0 ± 1.0 |
| 11 | quercetin-3-O-rutinoside (rutin) | 11.99 | 608.7 | 299.6 270.9 | −46 −60 | 927.9 ± 22.9 | 213.6 ± 5.3 | 251.6 ± 6.6 | 139.0 ± 2.5 | 1403.2 ± 43.7 | 114.7 ± 0.3 | 1049.7 ± 32.8 | 223.4 ± 1.9 |
| 12 | quercetin-3-O-galactoside (hyperoside) | 12.80 | 462.7 | 299.7 254.7 | −28 −42 | <LOQ | 193.8 ± 2.2 | 214.7 ± 5.9 | 128.8 ± 3.9 | <LOQ | 319.6 ± 12.7 | <LOQ | <LOQ |
| 13 | quercetin-3-O-glucoside (isoquercetin) | 13.00 | 462.7 | 299.7 270.7 | −30 −44 | 1727.9 ± 13.9 | <LOQ | <LOQ | <LOQ | 366.1 ± 2.1 | <LOQ | 1699.4 ± 51.6 | 238.8 ± 2.8 |
| 14 | kaempferol-3-O-rutinoside (nicotiflorin) | 13.31 | 592.7 | 284.8 226.7 | −38 −68 | 183.4 ± 3.8 | <LOQ | 17.2 ± 0.4 | 127.2 ± 2.3 | 269.4 ± 9.6 | <LOQ | 105.5 ± 2.4 | <LOQ |
| 15 | isorhamnetin-3-O-rutinoside (narcissoside) | 13.52 | 622.8 | 314.9 298.8 | −40 −52 | 151.4 ± 85.0 | 15.1 ± 0.1 | 29.7 ± 1.0 | 48.4 ± 0.6 | 1160.0 ± 19.5 | 49.7 ± 0.7 | 57.6 ± 2.1 | <LOQ |
| 16 | p-coumaric acid | 14.28 | 162.8 | 93.0 119.0 | −44 −14 | 34.6 ± 0.4 | 53.1 ± 0.9 | 13.1 ± 0.2 | 11.8 ± 0.0 | 20.1 ± 0.6 | 65.0 ± 0.8 | 36.9 ± 1.2 | 19.1 ± 0.3 |
| 17 | kaempferol-3-O-glucoside (astragalin) | 14.66 | 446.7 | 226.8 254.8 | −54 −40 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | nd | <LOQ | <LOQ |
| 18 | isorhamnetin-3-O-glucoside | 14.76 | 476.8 | 313.9 270.9 | −30 −44 | <LOQ | <LOQ | <LOQ | <LOQ | 245.1 ± 1.2 | <LOQ | <LOQ | <LOQ |
| 19 | quercetin 3-O-rhamnoside (quercitrin) | 14.83 | 446.7 | 299.7 270.7 | −30 −40 | 76.6 ± 1.9 | 40.0 ± 0.3 | 51.3 ± 1.2 | <LOQ | 238.5 ± 7.7 | 376.3 ± 0.0 | 81.3 ± 1.2 | 123.9 ± 4.2 |
| 20 | o-coumaric acid | 17.17 | 162.8 | 119.0 93.0 | −14 −46 | <LOQ | nd | nd | nd | nd | nd | nd | nd |
| 21 | quercetin | 17.91 | 300.7 | 150.9 178.8 | −26 −20 | 10.7 ± 0.1 | <LOQ | <LOQ | <LOQ | 12.1 ± 0.1 | 50.9 ± 1.1 | 1.5± 0.1 | 42.3 ± 1.2 |
| 22 | salicylic acid | 18.06 | 136.9 | 75.0 93.0 | −48 −16 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 23 | isorhamnetin | 19.09 | 314.7 | 299.7 150.9 | −20 −30 | <LOQ | <LOQ | <LOQ | <LOQ | 0.3 ± 0.0 | 6.0 ± 0.1 | <LOQ | <LOQ |
| Sample | IC50 | |
|---|---|---|
| DPPH [mg/mL] | CHEL [μg/mL] | |
| H1 | 1.1 ± 0.1 * | 6.1 ± 0.6# |
| H2 | 4.8 ± 0.1 * | 16.8 ± 0.0# |
| H3 | 15.7 ± 0.1 * | 12.9 ± 0.1# |
| H4 | 19.4 ± 0.3 * | 21.6 ± 0.0# |
| H5 | 0.9 ± 0.2 | 1.9 ± 0.3# |
| H6 | 2.5 ± 0.1 * | 9.6 ± 0.1 |
| H7 | 2.6 ± 0.0 * | 9.2 ± 0.1# |
| H8 | 15.8 ± 0.3 * | 19.2 ± 0.1# |
| AA | 0.5 ± 0.0 | nt |
| Na2EDTA*2H2O | nt | 9.8 ± 0.0 |
| Sample | IC50 [µg/mL] | ||
|---|---|---|---|
| Collagenase Inhibition | Elastase Inhibition | Tyrosinase Inhibition | |
| H1 | 40.3 ± 0.4 * | 51.0 ± 0.2 * | 20.6 ± 0.1# |
| H2 | 68.5 ± 0.2 * | 78.9 ± 0.6 * | 53.1 ± 0.4# |
| H3 | 54.1 ± 0.1 * | 61.6 ± 0.1 | 50.2 ± 0.4# |
| H4 | 83.8 ± 0.2 * | 79.8 ± 1.1 * | 79.3 ± 0.2# |
| H5 | 38.5 ± 0.2 * | 45.5 ± 0.1 * | 16.6 ± 0.1# |
| H6 | 50.4 ± 0.2 * | 59.0 ± 0.4 * | 43.2 ± 0.2# |
| H7 | 41.2 ± 0.3 * | 56.7 ± 0.2 * | 28.0 ± 0.2# |
| H8 | 78.8 ± 0.4 * | 79.4 ± 0.3 * | 67.8 ± 0.4# |
| EGCG | 34.9 ± 0.4 | 62.4 ± 0.1 | nt |
| Kojic acid | nt | nt | 17.6 ± 0.1 |
| Bacteria | Inhibition Zone Diameter (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | |
| Staphylococcus aureus ATCC 25923 | 4.4 ± 0.3 | 8.0 ± 2.3 | 8.5 ± 1.9 | 14.0 ± 1.9 | 27.3 ± 3.1 | 11.8 ± 2.3 | 19.5 ± 2.1 | 9.9 ± 1.8 |
| Staphylococcus epidermidis ATCC 1222 | 5.5 ± 2.2 | 4.0 ± 1.1 | 5.3± 2.2 | 9.5 ± 1.3 | 19.0 ± 0.1 | 14.3 ± 3.0 | 17.0 ± 0.4 | 11.7 ± 1.1 |
| Staphylococcus aureus from wound | 4.6 ± 1.4 | 5.5 ± 0.8 | 7.2 ±1.8 | 12.4 ± 3.5 | 19.5 ± 2.3 | 9.1 ± 1.0 | 14.0 ± 1.3 | 10.0 ± 0.9 |
| Staphylococcus epidermidis from wound | 4.7 ± 1.3 | 7.5 ± 1.2 | 6.1 ± 2.4 | 8.0 ± 0.9 | 16.5 ± 2.1 | 12.2 ± 1.6 | 15.0 ± 0.6 | 9.5 ± 1.8 |
| Escherichia coli ATCC 25992 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Escherichia coli from wound | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk, K.; Miazga-Karska, M.; Pietrzak, W.; Komsta, Ł.; Krzemińska, B.; Grzywa-Celińska, A. Phenolic Composition and Skin-related Properties of the Aerial Parts Extract of Different Hemerocallis Cultivars. Antioxidants 2020, 9, 690. https://doi.org/10.3390/antiox9080690
Szewczyk K, Miazga-Karska M, Pietrzak W, Komsta Ł, Krzemińska B, Grzywa-Celińska A. Phenolic Composition and Skin-related Properties of the Aerial Parts Extract of Different Hemerocallis Cultivars. Antioxidants. 2020; 9(8):690. https://doi.org/10.3390/antiox9080690
Chicago/Turabian StyleSzewczyk, Katarzyna, Małgorzata Miazga-Karska, Wioleta Pietrzak, Łukasz Komsta, Barbara Krzemińska, and Anna Grzywa-Celińska. 2020. "Phenolic Composition and Skin-related Properties of the Aerial Parts Extract of Different Hemerocallis Cultivars" Antioxidants 9, no. 8: 690. https://doi.org/10.3390/antiox9080690
APA StyleSzewczyk, K., Miazga-Karska, M., Pietrzak, W., Komsta, Ł., Krzemińska, B., & Grzywa-Celińska, A. (2020). Phenolic Composition and Skin-related Properties of the Aerial Parts Extract of Different Hemerocallis Cultivars. Antioxidants, 9(8), 690. https://doi.org/10.3390/antiox9080690