Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. ASE Preparation
2.3. Films Preparation
2.4. Films Characterization
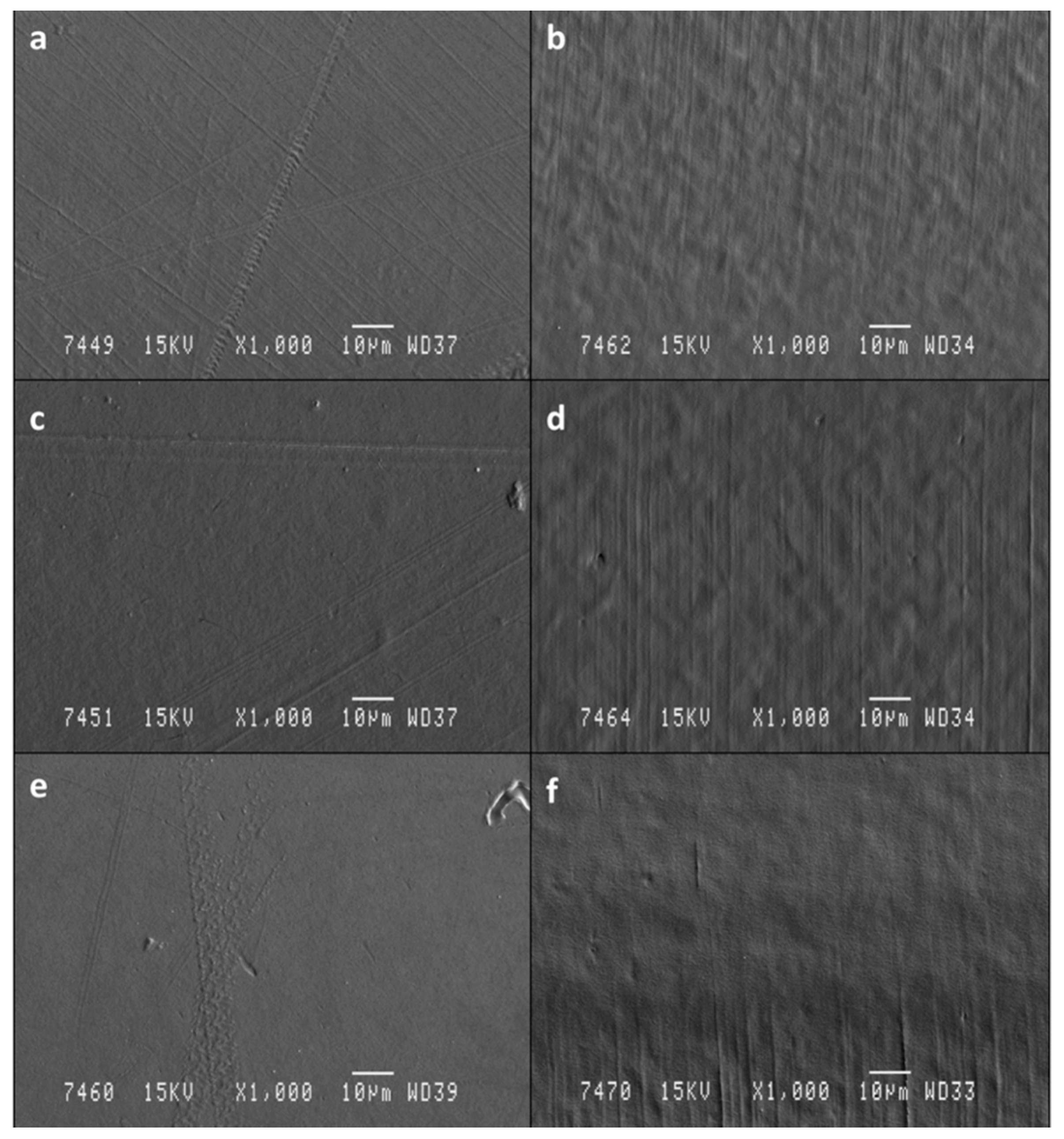
2.4.1. Scanning Electron Microscopy (SEM)
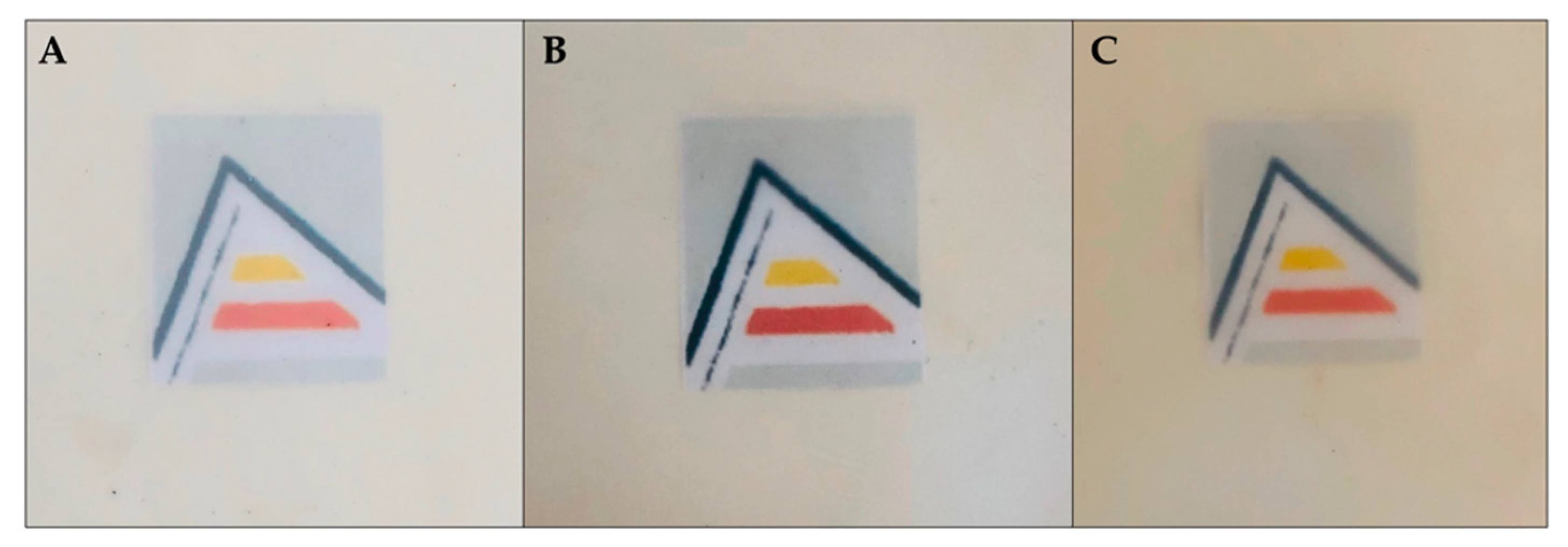
2.4.2. Colour
2.4.3. Barrier Properties
2.4.4. Mechanical Properties
2.4.5. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
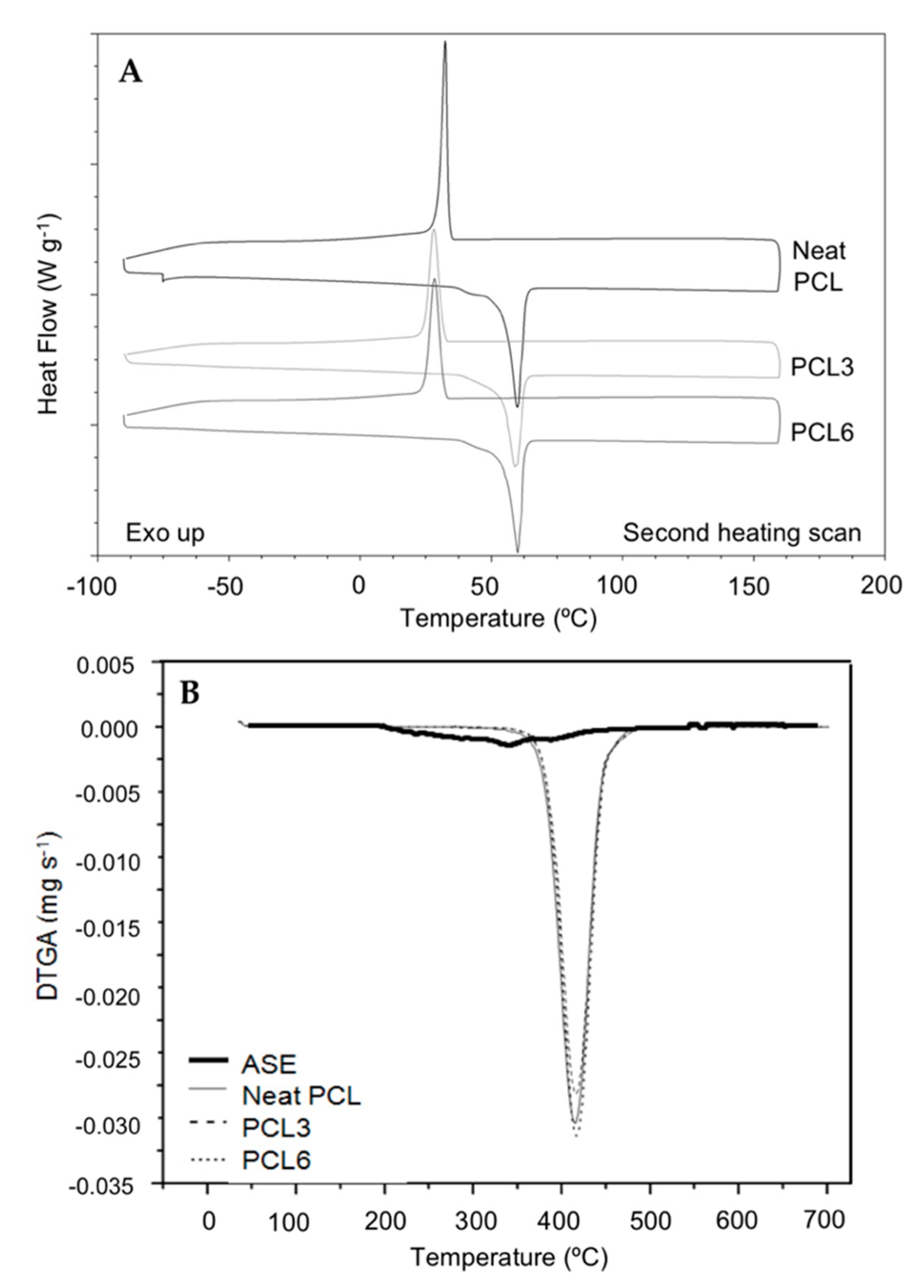
2.4.6. Thermal Analysis
2.4.7. Antioxidant Activity
2.5. Packaging of Fried Almonds
2.6. Oxidative Stability Study of Packaged Fried Almonds
2.6.1. Peroxide and p-Anisidine Values
2.6.2. Hexanal Content
2.6.3. Fatty Acid Profile
2.6.4. ATR-FTIR
2.7. Statistical Analysis
3. Results and Discussion
3.1. Films Characterization
3.1.1. SEM Analysis
3.1.2. Colour
3.1.3. Barrier Properties
3.1.4. Mechanical Properties
3.1.5. ATR-FTIR Analysis
3.1.6. Thermal Characterization
3.1.7. Antioxidant Activity
3.2. Oxidative Stability Study of Packaged Fried Almonds
3.2.1. PV, AV and Hexanal Content
3.2.2. Fatty Acid Profile Determination
3.2.3. ATR-FTIR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beltrán, A.; Prats, M.S.; Maestre, S.E.; Grané, N.; Martín, M.L. Classification of four almond cultivars using oil degradation parameters based on FTIR and GC data. J. Am. Oil Chem. Soc. 2009, 86, 51–58. [Google Scholar] [CrossRef]
- Beltrán, A.; Ramos, M.; Grané, N.; Martín, M.L.; Garrigós, M.C. Monitoring the oxidation of almond oils by HS-SPME–GC–MS and ATR-FTIR: Application of volatile compounds determination to cultivar authenticity. Food Chem. 2011, 126, 603–609. [Google Scholar] [CrossRef]
- Valdés, A.; Beltrán, A.; Karabagias, I.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Monitoring the oxidative stability and volatiles in blanched, roasted and fried almonds under normal and accelerated storage conditions by DSC, thermogravimetric analysis and ATR-FTIR. Eur. J. Lipid Sci. Technol. 2015, 117, 1199–1213. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations, (FAO)-Food Loss and Food Waste. 2020. Available online: http://www.fao.org/food-loss-and-food-waste/en/ (accessed on 12 June 2020).
- Rai, S.; Dutta, P.K.; Mehrotra, G.K. Natural antioxidant and antimicrobial agents from agrowastes: An emergent need to food packaging. Waste Biomass Valori. 2020, 11, 1905–1916. [Google Scholar] [CrossRef]
- Hamza, S.; Saad, H.; Charrier, B.; Ayed, N.; Charrier-El Boughtoury, S. Physico-chemical characterization of Tunisian plant fibers and its utilizations as reinforcement for plaster based composites. Ind. Crops Prod. 2013, 49, 357–365. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Prieto, C.; Lagaron, J.M. Nanomaterials to enhance food quality, safety, and health impact. Nanomaterials 2020, 10, 941. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Preparation and characterization of corn starch bio-active edible packaging films based on zein incorporated with orange-peel oil. Antioxidants 2019, 8, 391. [Google Scholar] [CrossRef]
- Fehlberg, J.; Lee, C.L.; Matuana, L.M.; Almenar, E. Orange peel waste from juicing as raw material for plastic composites intended for use in food packaging. J. Appl. Polym. Sci. 2020, 137, 48841. [Google Scholar] [CrossRef]
- Xie, Y.; Niu, X.; Yang, J.; Fan, R.; Shi, J.; Ullah, N.; Feng, X.; Chen, L. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int. J. Biol. Macromol. 2020, 150, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Cejudo, C.; Casas, L.; Fernández, M.T.; Mantell, C.; Martínez de la Ossa, E.J. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Masavang, S.; Mahe, J.; Sommano, S.; Ruksiriwanich, W.; Brachais, C.; Chambin, O.; Jantrawut, P. Mango (cv. Nam Dokmai) peel as a source of pectin and its potential use as a film-forming polymer. Food Hydrocoll. 2020, 102, 105611. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Grau-Atienza, A.; Jordà, A.; Burgos, N.; Jiménez, A.; Serrano, E.; Garrigós, M.C. Biodegradable Poly(ε-Caprolactone) Active Films Loaded with MSU-X Mesoporous Silica for the Release of α-Tocopherol. Polymers 2020, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Beltrán, A.; Peltzer, M.; Valente, A.J.M.; Garrigós, M.C. Release and antioxidant activity of carvacrol and thymol from polypropylene active packaging films. LWT Food Sci. Technol. 2014, 58, 470–477. [Google Scholar] [CrossRef]
- Beltrán, A.; Valente, A.; Jiménez, A.; Garrogós, M.C. Characterization of Poly(ε-caprolactone)-Based nanocomposites containing hydroxytyrosol for active food packaging. J. Agric. Food Chem. 2014, 62, 2244–2252. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of antimicrobial and antioxidant gelatin/curcumin composite films for active food packaging application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Moraes, J.; Fernandes, A.C.; Borges de Almeida, A.; de Lima, T.; Pereira, K.; Fernandes, C.; Alves de Figueiredo, H.; da Silva, E.R.; Henrique, F.; et al. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Foltynowicz, Z.; Bardenshtein, A.; Sängerlaub, S.; Antvorskov, H.; Kozak, W. Nanoscale, zero valent iron particles for application as oxygen scavenger in food packaging. Food Packag. Shelf Life 2017, 11, 74–83. [Google Scholar] [CrossRef]
- Rodríguez, G.M.; Sibaja, J.C.; Espitia, P.J.P.; Otoni, C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food Hydrocoll. 2020, 103, 105630. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Jeong, J.-S. Clearing up the oxygen dip in HPAEC–PAD sugar analysis: Sodium sulfite as an oxygen scavenger. J. Chromatogr. B 2019, 1128, 121759. [Google Scholar] [CrossRef]
- Cecchi, T.; Passamonti, P.; Cecchi, P. Study of the quality of extra virgin olive oil stored in PET bottles with or without an oxygen scavenger. Food Chem. 2010, 120, 730–735. [Google Scholar] [CrossRef]
- Van Hellemond, E.W.; Leferink, N.G.H.; Heuts, D.; Fraaije, M.W.; van Berkel, W. Occurrence and biocatalytic potential of carbohydrate oxidases. Adv. Appl. Microbiol. 2006, 60, 17–54. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Neogi, S. Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Gómez-Cordovés, C.M.; Rybarczyk, A.; Amarowicz, R.; Bartolomé, B. Comparative flavan-3-ol profile and antioxidant capacity of roasted peanut, hazelnut, and almond skins. J. Agric. Food Chem. 2009, 57, 10590–10599. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Ludueña, L.; Vázquez, A.; Alvarez, V. Effect of lignocellulosic filler type and content on the behavior of polycaprolactone based eco-composites for packaging applications. Carbohydr. Polym. 2012, 87, 411–421. [Google Scholar] [CrossRef]
- Bezerra, E.B.; França, D.C.; Morais, D.D.S.; Silva, I.D.S.; Siqueira, D.D.; Araújo, E.M.; Wellen, R.M.R. Compatibility and characterization of Bio-PE/PCL blends. Polímeros Ciência e Tecnologia 2019, 29, e2019022. [Google Scholar] [CrossRef]
- Khalid, S.; Yu, L.; Feng, M.; Meng, L.; Bai, Y.; Ali, A.; Liu, H.; Chen, L.F. Development and characterization of biodegradable antimicrobial packaging films based on polycaprolactone, starch and pomegranate rind hybrids. Food Packag. Shelf Life 2018, 18, 71–79. [Google Scholar] [CrossRef]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag. Shelf Life 2017, 11, 31–39. [Google Scholar] [CrossRef]
- Valdés, A.; Fenollar, O.; Beltrán, A.; Balart, R.; Fortunati, E.; Kenny, J.M.; Garrigós, M.C. Characterization and enzymatic degradation study of poly(ε-caprolactone)-based biocomposites from almond agricultural by-products. Polym. Degrad. Stab. 2016, 132, 181–190. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Garrigós, M.C. Characterization and degradation characteristics of poly(ε-caprolactone)-based composites reinforced with almond skin residues. Polym. Degrad. Stab. 2014, 108, 269–279. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigós, M.C.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133, 42631. [Google Scholar] [CrossRef]
- ISO 62:2008. Plastics-Determination of Water Absorption. Available online: https://www.iso.org/obp/ui/#iso:std:iso:62:ed-3:v1:en (accessed on 16 April 2020).
- UNE. 53097:2002 Sheet Materials-Determination of Water Vapour Transmission Rate-Gravimetric (Dish) Method. 2002. Available online: https://www.en.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0026745 (accessed on 16 April 2020).
- ASTM D882-09. Standard test method for tensile properties of thin plastic sheeting. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 1997. [Google Scholar]
- Paneva, D.; Manolova, N.; Argirova, M.; Rashkov, I. Antibacterial electrospun poly(ɛ-caprolactone)/ascorbyl palmitate nanofibrous materials. Int. J. Pharm. 2011, 416, 346–355. [Google Scholar] [CrossRef]
- Krittika Norajit, K.; Myong Kim, K.; Hyung Ryu, G. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- ISO 3960:2007. Corrected Version 2009-05-15. Animal and Vegetable Fats and Oils-Determination of Peroxide Value-Iodometric (Visual) Endpoint Determination. Available online: https://www.iso.org/standard/39158.html (accessed on 12 June 2020).
- Alden Press. Determination of the p-Anisidine Value, Method 2.504. In IUPAC Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed.; Alden Press: Oxford, UK, 1987; pp. 210–211. [Google Scholar]
- Mexis, S.F.; Badeka, A.V.; Riganakos, K.A.; Karakostas, K.X.; Kontominas, M.G. Effect of packaging and storage conditions on quality of shelled walnuts. Food Control 2009, 20, 743–751. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Antimicrobial and physical-mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Development and characterization of carrageenan/grapefruit seedextract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.T. Fortification of dietary biopolymers-based packaging material with bioactive plant extracts. Food Res. Int. 2012, 49, 80–91. [Google Scholar] [CrossRef]
- Salevíc, A.; Prieta, C.; Cabedo, L.; Nedovíc, V.; Lagarón, J.M. Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract. Nanomaterials 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan-polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- López De Dicastillo, C.; Nerín, C.; Alfaro, P.; Catal, R.; Gavara, R.; Hernández-Muñoz, P. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (EVOH) and green tea extract. J. Agric. Food Chem. 2011, 59, 7832–7840. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Enhancing the functional properties of acetylated hemicellulose films for active food packaging using acetylated nanocellulose reinforcement and polycaprolactone coating. Food Packag. Shelf Life 2020, 24, 100481. [Google Scholar] [CrossRef]
- Salmieri, S.; Lacroix, M. Physicochemical Properties of Alginate/Polycaprolactone-Based Films Containing Essential Oils. J. Agric. Food Chem. 2012, 54, 10205–10214. [Google Scholar] [CrossRef]
- Hoidy, W.H.; Al-Mulla, E.A.J.; Al-Janabi, K.W. Mechanical and thermal properties of PLLA/PCL modified CLAY nanocomposites. J. Polym. Environ. 2010, 18, 608–616. [Google Scholar] [CrossRef]
- López-De-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Sienkiewicz, N.; Członka, S.; Kairyte, A.; Vaitkus, S. Curcumin as a natural compound in the synthesis of rigid polyurethane foams with enhanced mechanical, antibacterial and anti-ageing properties. Polym. Testing 2019, 79, 106046. [Google Scholar] [CrossRef]
- Foujdar, R.; Bera, M.B.; Chopra, H.K. Optimization of process variables of probe ultrasonic-assisted extraction of phenolic compounds from the peel of Punica granatum Var. Bhagwa and it’s chemical and bioactivity characterization. J. Food Proc. Preserv. 2020, 44, e14317. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Sentandreu, E.; Lagaron, J.M. Development of multilayer corn starch-based food packaging structures containing β-carotene by means of the electro-hydrodynamic processing. Starch 2016, 68, 603–610. [Google Scholar] [CrossRef]
- Pereira de Abreu, D.; Maroto, J.; Villalba, K.; Cruz, J. Antioxidants from barley husks impregnated in films of low-density polyethylene and their effect over lipid deterioration of frozen cod (Gadus morhua). J. Sci. Food Agric. 2012, 92, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Bakkalbasi, E.; Yilmaz, O.; Javidipour, I.; Artik, N. Effects of packaging materials, storage conditions and variety on oxidative stability of shelled walnuts. LWT-Food Sci. Technol. 2012, 46, 203–209. [Google Scholar] [CrossRef]
- Lin, X.; Wu, J.; Zhu, R.; Chen, P.; Huangm, G.; Li, Y.; Ye, N.; Huang, B.; Lai, Y.; Zhang, H.; et al. California Almond Shelf Life: Lipid Deterioration during Storage. J. Food Sci. 2012, 77, C583–C593. [Google Scholar] [CrossRef]
- Zajdenwerg, C.; Branco, G.; Alamed, J.; Decker, E.; Castro, I. Correlation between sensory and chemical markers in the evaluation of Brazil nut oxidative shelf-life. Eur. Food Res. Technol. 2011, 233, 109–116. [Google Scholar] [CrossRef]
- Valdés, A.; Beltrán, A.; Garrigós, M.C. Characterization and Classification of Almond Cultivars by Using Spectroscopic and Thermal Techniques. J. Food Sci. 2013, 78, C138–C144. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 108, 1051–1061. [Google Scholar] [CrossRef]
 ), PCL3 (
), PCL3 ( ) and PCL6 (
) and PCL6 ( ) at 0, 7, 17, 33 and 56 days of oxidative treatment at 40 °C. Mean ± SD; n = 3.
) at 0, 7, 17, 33 and 56 days of oxidative treatment at 40 °C. Mean ± SD; n = 3.
 ), PCL3 (
), PCL3 ( ) and PCL6 (
) and PCL6 ( ) at 0, 7, 17, 33 and 56 days of oxidative treatment at 40 °C. Mean ± SD; n = 3.
) at 0, 7, 17, 33 and 56 days of oxidative treatment at 40 °C. Mean ± SD; n = 3.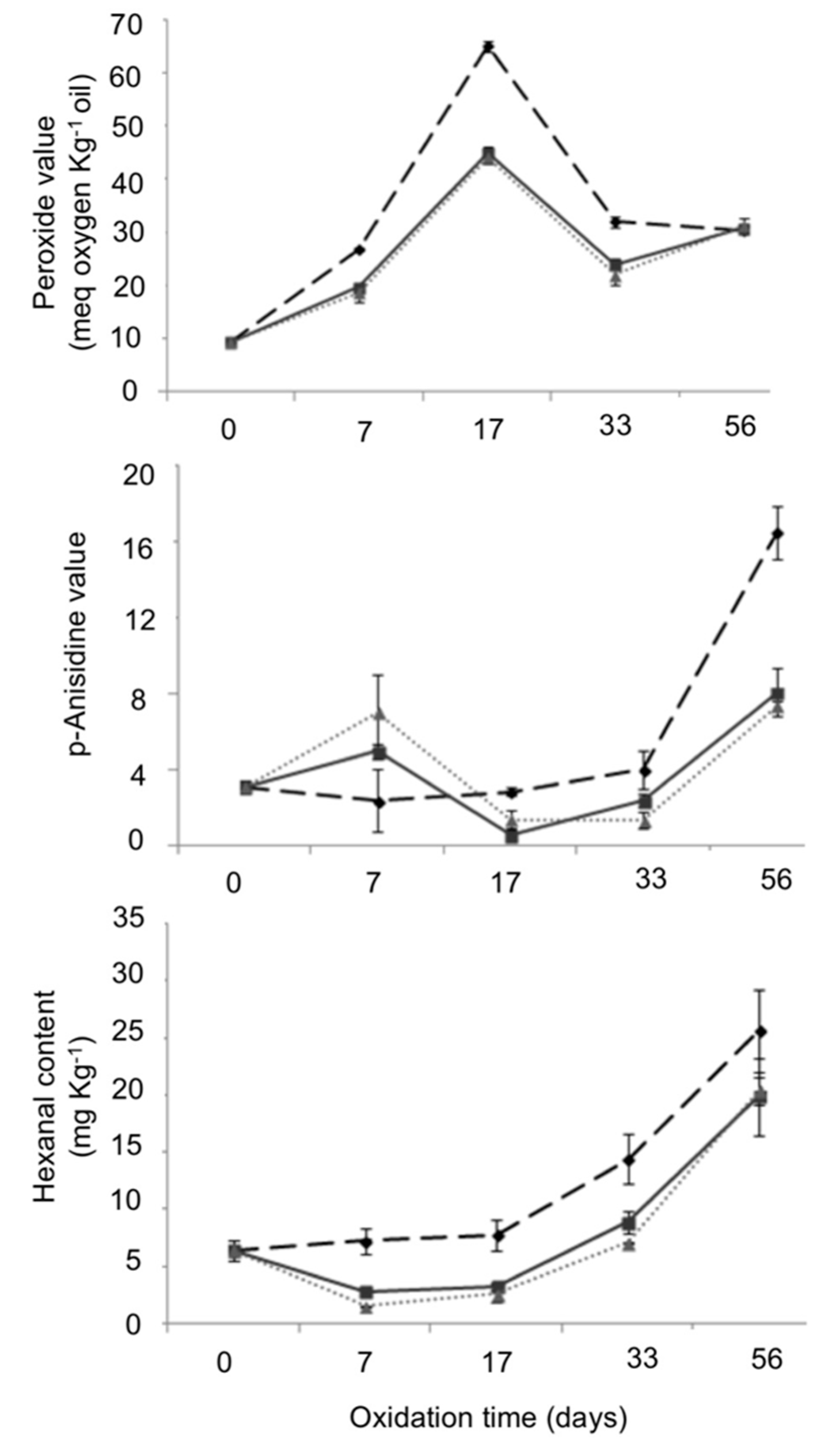
| Property | Neat PCL | PCL3 | PCL6 |
|---|---|---|---|
| L* | 78.0 ± 1.2 a | 72.4 ± 0.3 b | 61.9 ± 0.4 c |
| a* | −0.7 ± 0.4 a | 3.4 ± 0.2 b | 8.5 ± 0.1 c |
| b* | −1.1 ± 0.2 a | 15.9 ± 0.4 b | 21.2 ± 0.4 c |
| ΔE* | - | 18.3 ± 0.6 a | 29.0 ± 0.6 b |
| Water absorption (%, 120 h) | 0.38 ± 0.09 a | 1.05 ± 0.05 b | 0.98 ± 0.21 b |
| OTR (cm3 mm m−2 day) | 95 ± 8 a | 109 ± 5 a | 115 ± 8 a |
| WVP × 10−4 (kg m Pa-1 s−1 m−2) | 1.8 ± 0.1 a | 2.4 ± 0.3 b | 2.5 ± 0.4 b |
| Young’s modulus (MPa) | 352 ± 20 a | 336 ± 20 a | 340 ± 15 a |
| Elongation at break (%) | 77 ± 12 a | 79 ± 14 a | 74 ± 10 a |
| Tensile strength (MPa) | 16 ± 1 a | 13 ± 2 a | 14 ± 3 a |
| Thermal property | Neat PCL | PCL3 | PCL6 |
|---|---|---|---|
| ΔHc (J g−1) | 59 ± 1 a | 62 ± 4 a | 59 ± 1 a |
| Tc (°C) | 30 ± 1 a | 29 ± 1 a | 29 ± 1 a |
| ΔHm (J g−1) | 59 ± 1 a | 58 ± 3 a | 57 ± 2 a |
| Tm (°C) | 55 ± 1 a | 54 ± 1 a | 54 ± 2 a |
| Tg (°C) | −61 ± 2 a | −63 ± 2 a | −64 ± 1 a |
| Tini (°C) | 385 ± 2 a | 383 ± 3 a | 382 ± 3 a |
| Tmax (°C) | 415 ± 1 a | 415 ± 1 a | 415 ± 1 a |
| Time (days) | Fatty Acid | Sample | ||
|---|---|---|---|---|
| Neat PCL | PCL 3 | PCL 6 | ||
| 0 | Palmitic | 7.91 ± 0.17 a | 7.91 ± 0.18 a | 7.91 ± 0.18 a |
| 7 | 8.00 ± 0.65 a | 7.75 ± 0.65 a | 6.68 ± 0.49 b | |
| 17 | 7.63 ± 2.32 a | 6.82 ± 0.14 b | 7.50 ± 0.35 a,b,c | |
| 33 | 7.12 ± 0.27 a | 6.64 ± 0.03 b | 7.40 ± 0.39 a,b,c | |
| 56 | 8.40 ± 0.05 a | 6.4 ± 0.24 b | 7.31 ± 0.10 c | |
| 0 | Stearic | 7.03 ± 0.56 a | 7.03 ± 0.56 a | 7.03 ± 0.56 a |
| 7 | 7.48 ± 0.35 a | 9.86 ± 0.35 b | 12.61 ± 2.93 b,c | |
| 17 | 11.74 ± 1.52 b | 10.93 ± 0.68 b | 12.11 ± 0.53 b | |
| 33 | 11.15 ± 0.16 b | 9.84 ± 0.47 b | 10.08 ± 0.22 c | |
| 56 | 10.77 ± 0.25 b | 9.40 ± 0.32 b | 9.73 ± 0.70 c | |
| 0 | Oleic | 68.65 ± 0.86 a | 68.65 ± 0.86 a | 68.65 ± 0.86 a |
| 7 | 70.56 ± 2.27 a | 71.21 ± 2.27 a | 67.45 ± 1.08 a | |
| 17 | 68.31 ± 0.88 a | 66.92 ± 0.12 b | 64.59 ± 1.23 b | |
| 33 | 68.30 ± 0.77 a | 68.35 ± 0.51 a | 66.28 ± 0.97 a,b | |
| 56 | 69.24 ± 0.06 a | 69.08 ± 1.08 a | 67.01 ± 0.84 a | |
| 0 | Linoleic | 16.29 ± 0.65 a | 16.29 ± 0.65 a | 16.29 ± 0.65 a |
| 7 | 13.86 ± 3.22 a,b | 16.37 ± 3.22 a | 14.65 ± 0.28 b | |
| 17 | 14.20 ± 0.09 b | 15.20 ± 0.44 a | 15.68 ± 0.37 a | |
| 33 | 13.28 ± 1.15 b | 15.04 ± 0.06 a | 16.11 ± 0.43 a | |
| 56 | 11.44 ± 0.08 c | 14.85 ± 0.54 a | 15.83 ± 0.14 a | |
| 0 | Palmitoleic | 0.114 ± 0.002 a | 0.114 ± 0.002 a | 0.114 ± 0.002 a |
| 7 | 0.105 ± 0.010 a | 0.132 ± 0.010 b | 0.116 ± 0.004 a,b | |
| 17 | 0.117± 0.002 a | 0.122 ± 0.001 b | 0.120 ± 0.002 b | |
| 33 | 0.145 ± 0.007 b | 0.124 ± 0.001 b | 0.122 ± 0.004 b | |
| 56 | 0.148 ± 0.004 b | 0.120 ± 0.005 a,b | 0.122 ± 0.003 b | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés García, A.; Juárez Serrano, N.; Beltrán Sanahuja, A.; Garrigós, M.C. Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants 2020, 9, 629. https://doi.org/10.3390/antiox9070629
Valdés García A, Juárez Serrano N, Beltrán Sanahuja A, Garrigós MC. Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants. 2020; 9(7):629. https://doi.org/10.3390/antiox9070629
Chicago/Turabian StyleValdés García, Arantzazu, Nerea Juárez Serrano, Ana Beltrán Sanahuja, and María Carmen Garrigós. 2020. "Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds" Antioxidants 9, no. 7: 629. https://doi.org/10.3390/antiox9070629
APA StyleValdés García, A., Juárez Serrano, N., Beltrán Sanahuja, A., & Garrigós, M. C. (2020). Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants, 9(7), 629. https://doi.org/10.3390/antiox9070629








