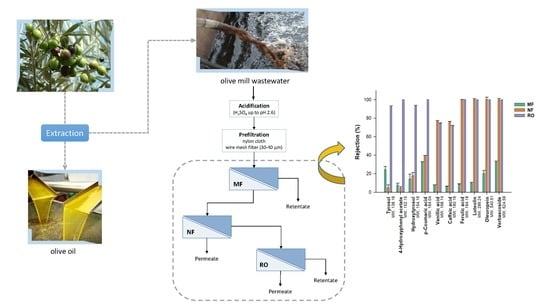
Olive Mill Wastewater Polyphenol-Enriched Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Olive Mill Wastewaters (OMWs)
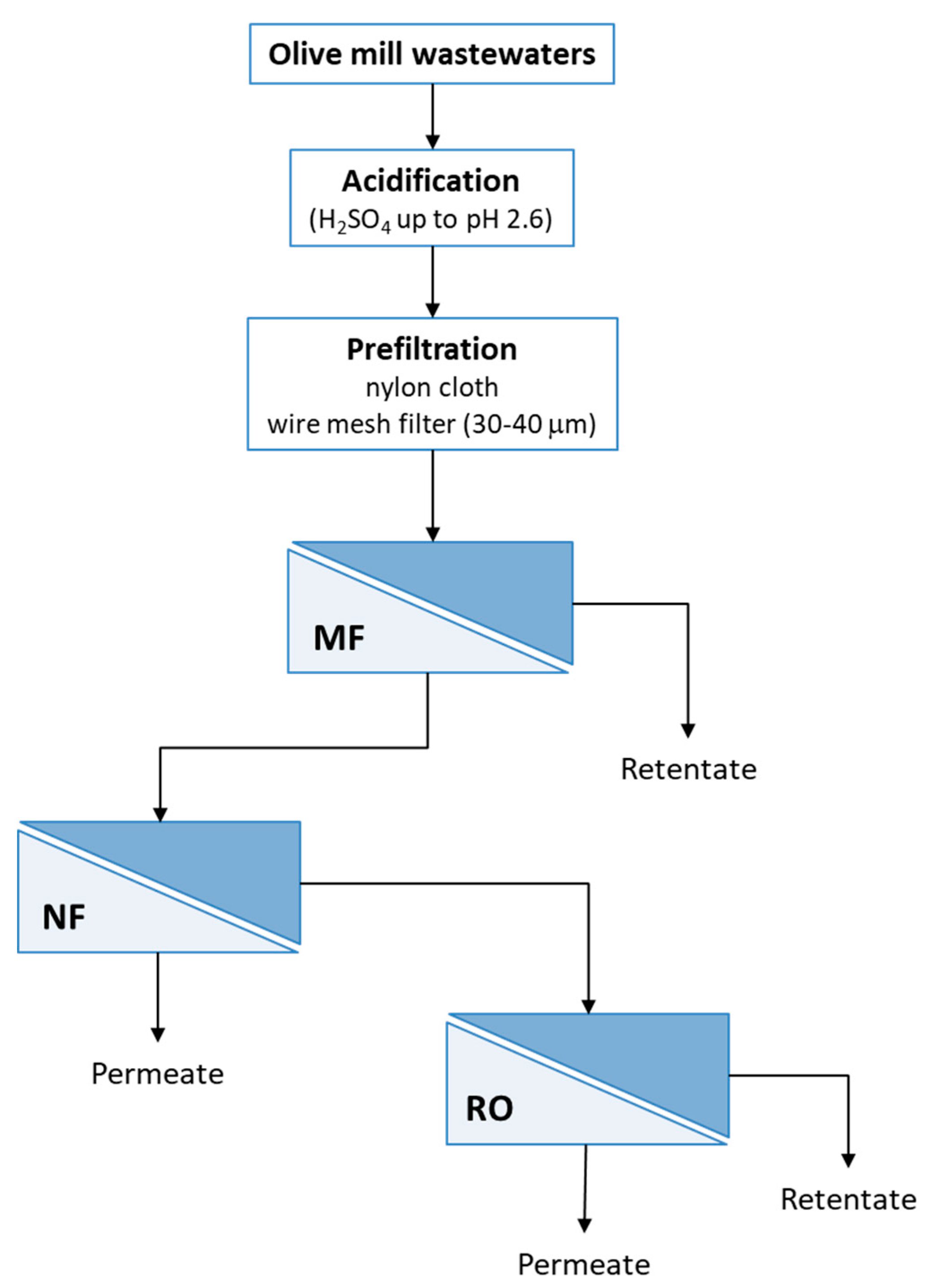
2.3. Membrane Processing: Experimental Set-Up
2.4. Ultra-High Performance Liquid Chromatography (UHPLC) Analyses
2.5. Pancreatic Lipase Inhibition Assay
2.6. α-Amylase Inhibitory Activity Test
2.7. α-Glucosidase Inhibitory Activity Test
2.8. Antioxidant Activity
2.9. Statistical Analysis
3. Results
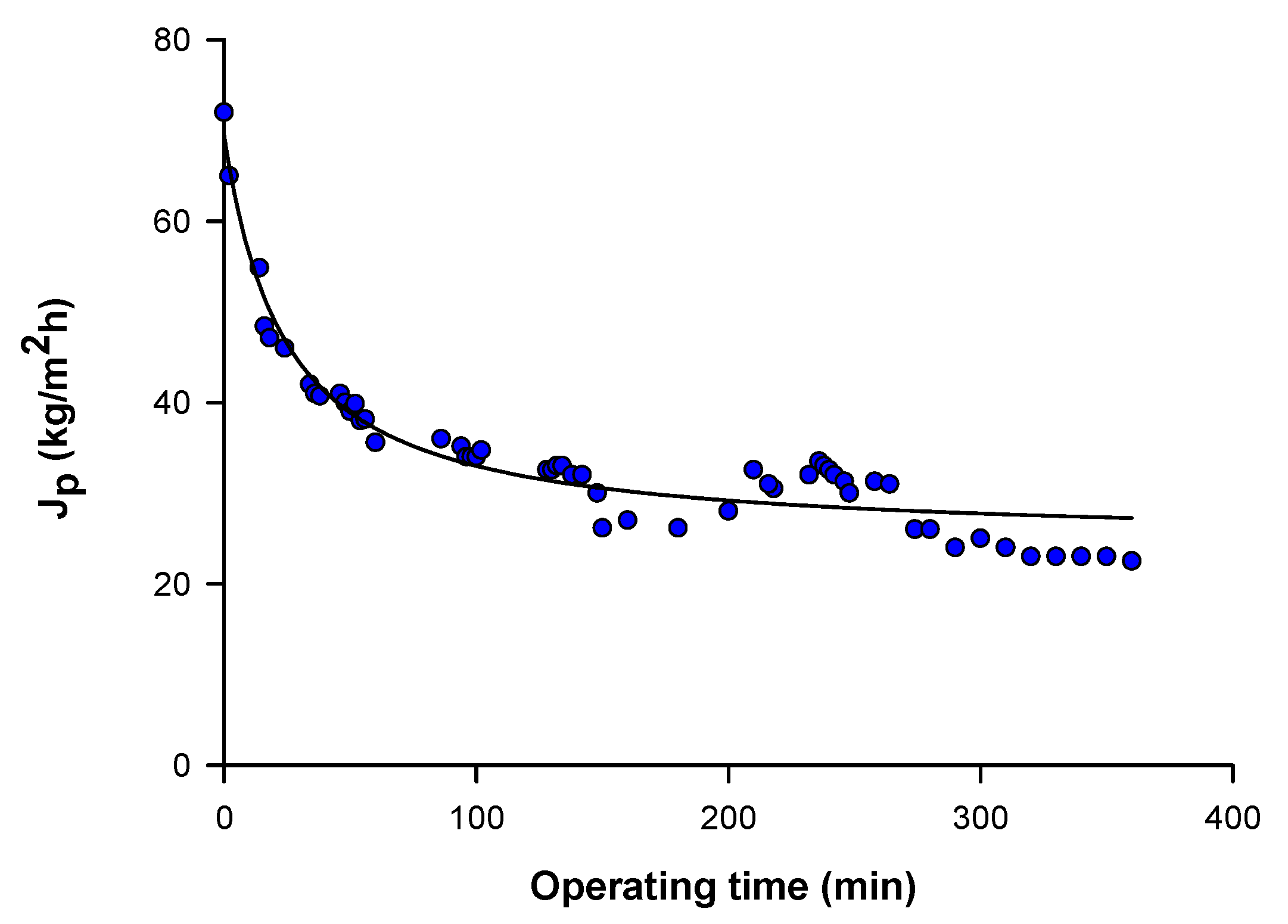
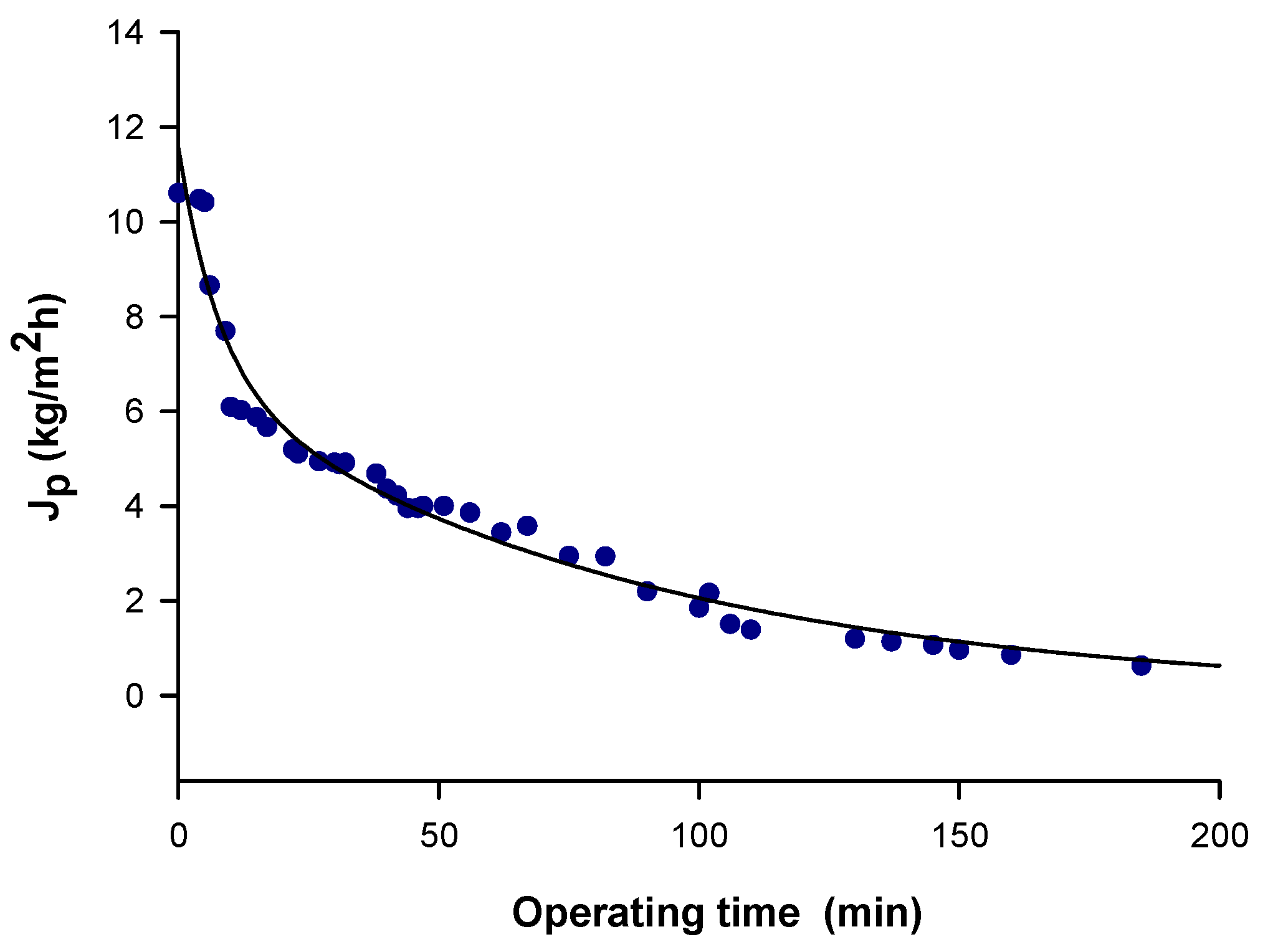
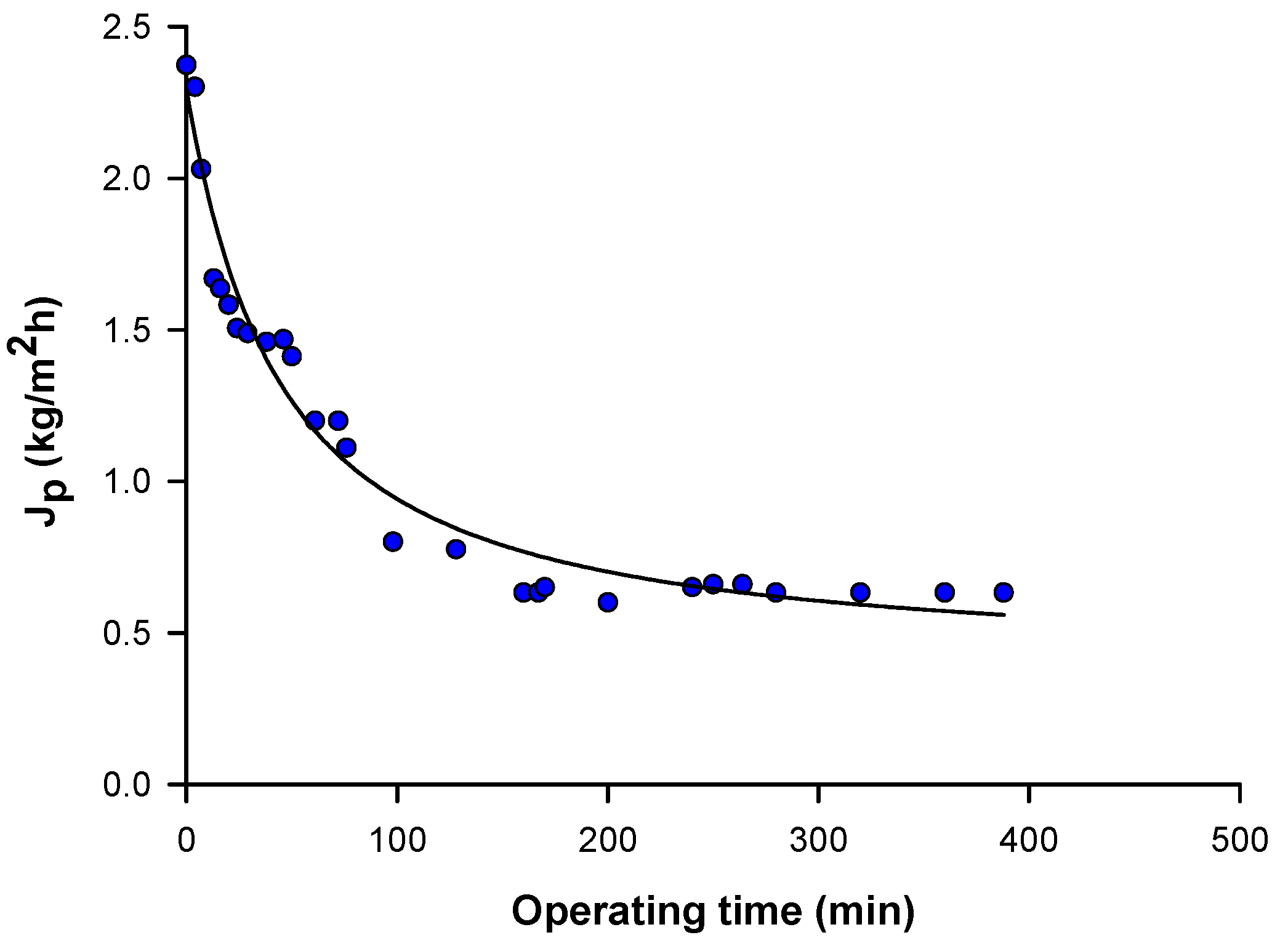
3.1. Membranes Productivity
3.2. Chemical Profile
3.3. In Vitro Biological Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. The green, blue and grey water footprint of crops and derived crop products. Hydrol. Earth Syst. Sci. 2011, 15, 1577–1600. [Google Scholar] [CrossRef]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 1–28. [Google Scholar]
- Angelidaki, I.; Ahring, B.K.; Deng, H.; Schmidt, J.E. Anaerobic digestion of olive mill effluents together with swine manure in UASB reactors. Water Sci. Technol. 2002, 45, 213–218. [Google Scholar] [CrossRef]
- Ammary, B.Y. Treatment of olivemill wastewater using an anaerobic sequencing batch reactor. Desalination 2005, 177, 157–165. [Google Scholar] [CrossRef]
- Lafi, W.K.; Shannak, B.; Al-Shannag, M.; Al-Anber, Z.; Al-Hasan, M. Treatment of olive mill wastewater by combined advanced oxidation and biodegradation. Sep. Purif. Technol. 2009, 70, 141–146. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on natural polyphenols as anticancer agents for skin cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Soleti, R.; Andriantsitohaina, R.; Martinez, M.C. Impact of polyphenols on extracellular vesicle levels and effects and their properties as tools for drug delivery for nutrition and health. Arch. Biochem. Biophys. 2018, 644, 57–63. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Sicari, V.; Ursino, C.; Manfredi, I.; Conidi, C.; Figoli, A.; Cassano, A. Concentration of bioactive compounds from elderberry (Sambucus nigra L.) juice by nanofiltration membranes. Plant Food Hum. Nutr. 2018, 73, 336–343. [Google Scholar] [CrossRef]
- Victor-Ortega, M.; Ochando-Pulido, J.; Martínez-Ferez, A. Performance and modeling of continuous ion exchange processes for phenolic recovery from olive mill wastewaters. Process Saf. Environ. Protect. 2016, 100, 242–251. [Google Scholar] [CrossRef]
- Mukherjeea, P.K.; Maitya, N.; Nemaa, N.K.; Sarkarb, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antiox. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Statti, G.A.; Menichini, F. Influence of ripening stage on health benefits properties of Capsicum annuum var. acuminatum L.: In vitro studies. J. Med. Food 2008, 11, 184–189. [Google Scholar] [PubMed]
- Rodriguez-Perez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 58, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, W.; Zhang, H.; Li, Y.; Liu, Z.; He, Z. Acteoside: A lipase inhibitor from the Chinese tea Ligustrum purpurascens kudingcha. Food Chem. 2014, 142, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kalogerakis, N.; Politi, M.; Foteinis, S.; Chatzisymeon, E.; Mantzavinos, D. Recovery of antioxidants from olive mill wastewaters: A viable solution that promotes their overall sustainable management. J. Environ. Manag. 2013, 128, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Klen, T.J.; Vodopivec, B.M. Ultrasonic extraction of phenols from olive mill wastewater: Comparison with conventional methods. J. Agric. Food Chem. 2011, 59, 12725–12731. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and removal of phenolic compounds from olive mill wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Cloud point extraction of phenolic compounds from pretreated olive mill wastewater. J. Environ. Chem. Eng. 2014, 2, 1480–1486. [Google Scholar] [CrossRef]
- Frascari, D.; Molina Bacca, A.E.; Wardenaar, T.; Oertlé, E.; Pinelli, D. Continuous flow adsorption of phenolic compounds from olive mill wastewater with resin XAD16N: Life cycle assessment, cost–benefit analysis and process optimization. J. Chem. Technol. Biotechnol. 2019, 94, 1968–1981. [Google Scholar] [CrossRef]
- Mudimu, O.A.; Peters, M.; Braun, F.B.A.G. Overview of membrane processes for the recovery of polyphenols from olive mill wastewater. Am. J. Environ. Sci. 2012, 8, 195–201. [Google Scholar]
- Takaç, S.; Karakaya, A. Recovery of phenolic antioxidants from olive mill wastewater. Recent Pat. Chem. Eng. 2009, 2, 230–237. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Mazzei, R.; Giorno, L. Trends and current practices of olive mill wastewater treatment: Application of integrated membrane process and its future perspective. Sep. Purif. Technol. 2016, 162, 45–60. [Google Scholar] [CrossRef]
- Paraskeva, C.A.; Papadakis, V.G.; Tsarouchi, E.; Kanellopoulou, D.G.; Koutsoukos, P.G. Membrane processing for olive mill wastewater fractionation. Desalination 2007, 213, 218–229. [Google Scholar] [CrossRef]
- Russo, C. A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW). J. Membr. Sci. 2007, 288, 239–246. [Google Scholar] [CrossRef]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248, 185–193. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Romani, A.; Mulinacci, N.; Innocenti, M. Recovery and stability over time of phenolic fractions by an industrial filtration system of olive mill wastewaters: A three-year study. J. Sci. Food Agric. 2018, 98, 2761–2769. [Google Scholar] [CrossRef]
- Romani, A.; Scardigli, A.; Pinelli, P. An environmentally friendly process for the production of extracts rich in phenolic antioxidants from Olea europaea L. and Cynara scolymus L. matrices. Eur. Food Res. Technol. 2017, 243, 1229–1238. [Google Scholar] [CrossRef]
- Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valorization of olive mill wastewater by membrane processes to recover natural antioxidant compounds for cosmeceutical and nutraceutical applications or functional foods. Antioxidants 2018, 7, 72. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Hafidi, A.; García-Payo, M.C.; Khayet, M. Concentration of olive mill wastewater by membrane distillation for polyphenols recovery. Desalination 2009, 245, 670–674. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A.; García-Payo, M.C.; Khayet, M. Treatment of olive mill wastewater by membrane distillation using polytetrafluoroethylene membranes. Sep. Purif. Technol. 2012, 98, 55–61. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Curcio, E.; Poerio, T.; Mazzei, R.; Di Profio, G.; Drioli, E.; Giorno, L. Treatment of olive mill wastewater by forward osmosis. Sep. Purif. Technol. 2015, 147, 292–302. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Khayet, M.; Kiai, H.; Hafidi, A.; García-Payo, M.C. Treatment of crude olive mill wastewaters by osmotic distillation and osmotic membrane distillation. Sep. Purif. Technol. 2013, 104, 327–332. [Google Scholar] [CrossRef]
- Bazzarelli, F.; Piacentini, E.; Poerio, T.; Mazzei, R.; Cassano, A.; Giorno, L. Advances in membrane operations for water purification and biophenols recovery/valorization from OMWWs. J. Membr. Sci. 2016, 497, 402–409. [Google Scholar] [CrossRef]
- Bazzarelli, F.; Poerio, T.; Mazzei, R.; D’Agostino, N.; Giorno, L. Study of OMWWs suspended solids destabilization to improve membrane processes performance. Sep. Purif. Technol. 2015, 149, 183–189. [Google Scholar] [CrossRef]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Process. Preserv. 2019, 43, e14211. [Google Scholar] [CrossRef]
- El-shiekh, R.A.; Al-Mahdy, D.A.; Hifnawy, M.S.; Abdel-Sattar, E.A.; El-Shiekh, R.A.; Al-Mahdy, D.A.; Hifnawy, M.S.; Abdel-Sattar, E.A. In-vitro screening of selected traditional medicinal plants for their anti-obesity and antioxidant activities. S. Afr. J. Bot. 2019, 123, 43–50. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Sicari, V.; Pellicanò, T.M.; Tenuta, M.C.; Leporini, M.; Menichini, F.; Loizzo, M.R. Poncirus trifoliata (L.) Raf.: Chemical composition, antioxidant properties and hypoglycaemic activity via the inhibition of α-amylase and α-glucosidase enzymes. J. Funct. Food. 2016, 25, 477–485. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, C. baccatum, C. chacoense and C. chinense: A comparison between fresh and processed peppers. LWT Food Sci. Technol. 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Cassano, A.; Conidi, C. Membrane-based technologies for meeting the recovery of biologically active compounds from foods and their by-products. Crit. Rev. Food Sci. Nutr. 2019, 59, 2927–2948. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Mazzei, R.; Cassano, A.; Giorno, L. Integrated membrane system for the production of phytotherapics from olive mill wastewaters. J. Membr. Sci. 2014, 454, 322–329. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border to nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Cassano, A.; De Luca, G.; Conidi, C.; Drioli, E. Effect of polyphenols-membrane interactions on the performance of membrane-based processes. A review. Coord. Chem. Rev. 2017, 351, 45–75. [Google Scholar] [CrossRef]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of two-phase olive mill wastewater and recovery of phenolic compounds using membrane technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef]
- Dandona, P.; Ghanim, H.; Chaudhuri, A.; Dhindsa, S.; Kim, S.S. Macronutrient intake induces oxidative and inflammatory stress: Potential relevance to atherosclerosis and insulin resistance. Exp. Mol. Med. 2010, 42, 245–253. [Google Scholar] [CrossRef]
- Serra, D.; Mera, P.; Malandrino, M.I.; Mir, J.F.; Herrero, L. Mitochondrial fatty acid oxidation in obesity. Antioxid. Redox Signal. 2013, 19, 269–284. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol exerts anti-inflammatory and Anti-oxidant activities in a mouse model of systemic inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Siracusa, L.; Spatafora, C.; Renis, M.; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg. Chem. 2007, 35, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; De Stefano, D.; Di Meglio, P.; Irace, C.; Savarese, M.; Sacchi, R.; Cinelli, M.P.; Carnuccio, R. Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Hadrich, F.; Bouallagui, Z.; Junkyu, H.; Isoda, H.; Sayadi, S. The α-glucosidase and α-amylase enzyme inhibitory of hydroxytyrosol and oleuropein. J. Oleo Sci. 2015, 64, 835–843. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Young, M.; Tsiani, E. Antidiabetic effects of hydroxytyrosol: In vitro and in vivo evidence. Antioxidants 2019, 8, 188. [Google Scholar] [CrossRef]
- Zang, Y.; Igarashi, K.; Li, Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A(y) mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, H.J.; Li, P.F.; Yang, Y.B.; Wu, L.H.; Chou, G.X.; Wang, Z.T. Diterpenoids and phenylethanoid glycosides from the roots of Clerodendrum bungei and their inhibitory effects against angiotensin converting enzyme and α-glucosidase. Phytochemistry 2014, 103, 196–202. [Google Scholar] [CrossRef]
- Bedbabis, S.; Ferrara, G. Effects of long-term irrigation with treated wastewater on leaf mineral element contents and oil quality in Olive cv Chemlali. J. Hortic. Sci. Biotechnol. 2018, 93, 216–223. [Google Scholar] [CrossRef]
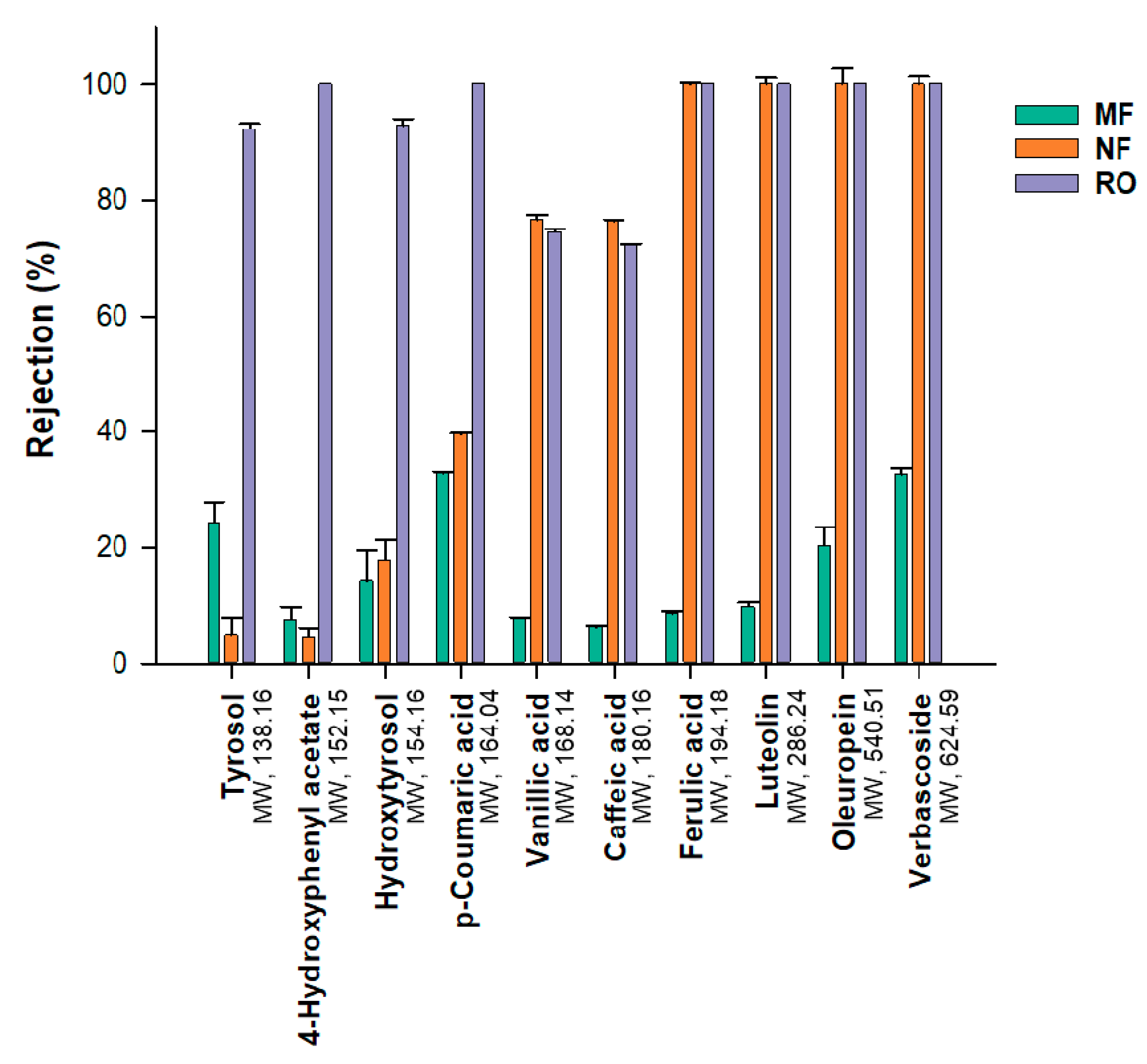
| Phenolic Compounds | Feed | MF-P | NF-R | NF-P | RO-R | RO-P |
|---|---|---|---|---|---|---|
| Caffeic acid | 8.1 ± 0.5 c | 7.6 ± 0.5 d | 27.7 ± 1.4 b | 1.8 ± 0.2 e | 45.7 ± 1.2 a | 0.5 ± 0.03 f |
| p-Coumaric acid | 6.4 ± 0.4 c | 4.3 ± 0.2 d | 12.2 ± 0.8 b | 2.6 ± 0.2 e | 35.9 ± 1.6 a | nd |
| Ferulic acid | 6.9 ± 0.6 c | 6.3 ± 0.3 d | 20.1 ± 1.1 b | nd | 51.3 ± 1.4 a | nd |
| Luteolin | 15.2 ± 0.6 c | 13.7 ± 1.1 d | 71.5 ± 2.7 b | nd | 82.8 ± 3.1 a | nd |
| 4-Hydroxyphenyl acetate | 72.6 ± 1.8 a | 67.0 ± 2.6 b | 29.6 ± 1.2 e | 64.0 ± 3.2 c | 57.1 ± 1.2 d | nd |
| Hydroxytyrosol | 373.3 ± 4.8 c | 320.1 ± 5.8 d | 1017.5 ± 8.8 b | 268.3 ± 1.2 e | 1522.2 ± 7.3 a | 18.8 ± 1.2 f |
| Oleuropein | 106.8 ± 4.0 c | 85.2 ± 2.7 d | 263.2 ± 4.2 b | nd | 510.0 ± 5.5 a | nd |
| Tyrosol | 89.7 ± 2.1 c | 68.1 ± 5.1 d | 157.3 ± 4.3 b | 64.8 ± 1.2 e | 519.0 ± 6.2 a | 5.0 ± 0.6 f |
| Vanillic acid | 29.4 ± 0.3 c | 27.8 ± 1.7 d | 97.0 ± 2.5 b | 6.5 ± 0.9 e | 116.2 ± 3.1 a | 1.4 ± 0.1 f |
| Verbascoside | 26.7 ± 1.1 c | 18.0 ± 1.3 d | 82.8 ± 3.4 b | nd | 130.9 ± 1.2 a | nd |
| Sample | α-Amylase | α-Glucosidase | Lipase |
|---|---|---|---|
| Feed | 287.9 ± 3.7 **** | 167.0 ± 2.8 **** | 245.7 ± 3.7 *** |
| MF-P | 253.7 ± 3.6 **** | 125.4 ± 2.7 **** | 211.0 ± 3.4 *** |
| NF-R | 122.6 ± 2.7 **** | 116.0 ± 2.5 **** | 183.1 ± 2.9 *** |
| NF-P | 470.9 ± 4.7 **** | 710.1 ± 5.7 **** | 826.1 ± 6.0 *** |
| RO-R | 65.3 ± 1.7 *** | 66.2 ± 1.7 *** | 175.6 ± 2.9 *** |
| RO-P | 16.2% | 26.8% | 10.8% |
| Positive control | |||
| Acarbose | 50.1 ± 0.8 | 35.7 ± 1.3 | |
| Orlistat | 37.2 ± 1.2 | ||
| Sample | DPPH Test IC50 (μg/mL) | ABTS Test IC50 (μg/mL) | β-Carotene Bleaching Test IC50 (μg/mL) | FRAP Test (μM Fe (II)/g) | |
|---|---|---|---|---|---|
| t 30 min | t 60 min | ||||
| Feed | 61.3 ± 3.5 **** | 15.2 ± 1.5 *** | 48.8% | 34.0% | 59.4 ± 1.7 ** |
| MF-P | 53.5 ± 3.3 **** | 7.7 ± 0.8 ** | 50.0 ± 2.9 **** | 40.1% | 66.6 ± 1.8 * |
| NF-R | 52.6 ± 3.2 **** | 7.3 ± 1.6 ** | 32.3 ± 1.9 **** | 69.0 ± 3.5 **** | 86.5 ± 1.9 |
| NF-P | 146.9 ± 4.2 **** | 112.8 ± 1.9 **** | 95.4 ± 4.0 **** | 31.2% | 10.7 ± 1.7 **** |
| RO-R | 38.0 ± 1.9 **** | 6.9 ± 1.9 * | 25.1 ± 1.2 **** | 54.7 ± 3.3 **** | 91.9 ± 1.6 |
| RO-P | 310.2 ± 4.7 **** | 17.5% | 23.51% | 19.2% | 3.6% |
| Positive control | |||||
| Ascorbic acid | 5.1 ± 0.9 | 1.7 ± 0.5 | |||
| Propyl gallate | 0.09 ± 0.02 | 0.09 ± 0.01 | |||
| BHT | 63.4 ± 2.5 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Cassano, A. Olive Mill Wastewater Polyphenol-Enriched Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds. Antioxidants 2020, 9, 602. https://doi.org/10.3390/antiox9070602
Tundis R, Conidi C, Loizzo MR, Sicari V, Cassano A. Olive Mill Wastewater Polyphenol-Enriched Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds. Antioxidants. 2020; 9(7):602. https://doi.org/10.3390/antiox9070602
Chicago/Turabian StyleTundis, Rosa, Carmela Conidi, Monica R. Loizzo, Vincenzo Sicari, and Alfredo Cassano. 2020. "Olive Mill Wastewater Polyphenol-Enriched Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds" Antioxidants 9, no. 7: 602. https://doi.org/10.3390/antiox9070602
APA StyleTundis, R., Conidi, C., Loizzo, M. R., Sicari, V., & Cassano, A. (2020). Olive Mill Wastewater Polyphenol-Enriched Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds. Antioxidants, 9(7), 602. https://doi.org/10.3390/antiox9070602