Calmangafodipir Reduces Sensory Alterations and Prevents Intraepidermal Nerve Fibers Loss in a Mouse Model of Oxaliplatin Induced Peripheral Neurotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Drugs
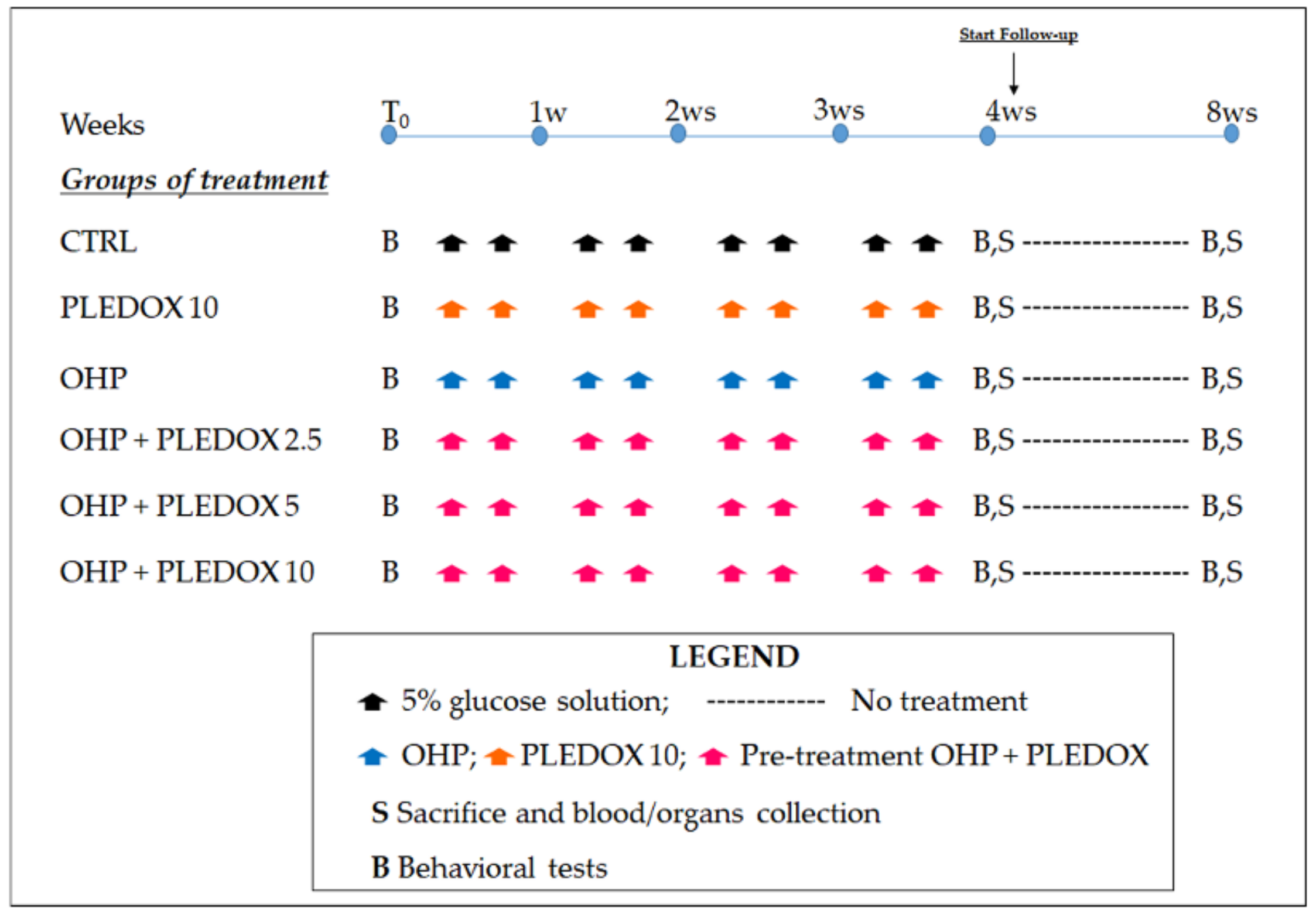
2.2. Study Design
2.3. Behavioral Tests
2.3.1. Dynamic Aesthesiometer Test
2.3.2. Cold Plate Test
2.4. Sampling and Processing of Organs/Tissues
2.5. Data Acquisition
2.6. Statistical Evaluation
3. Results
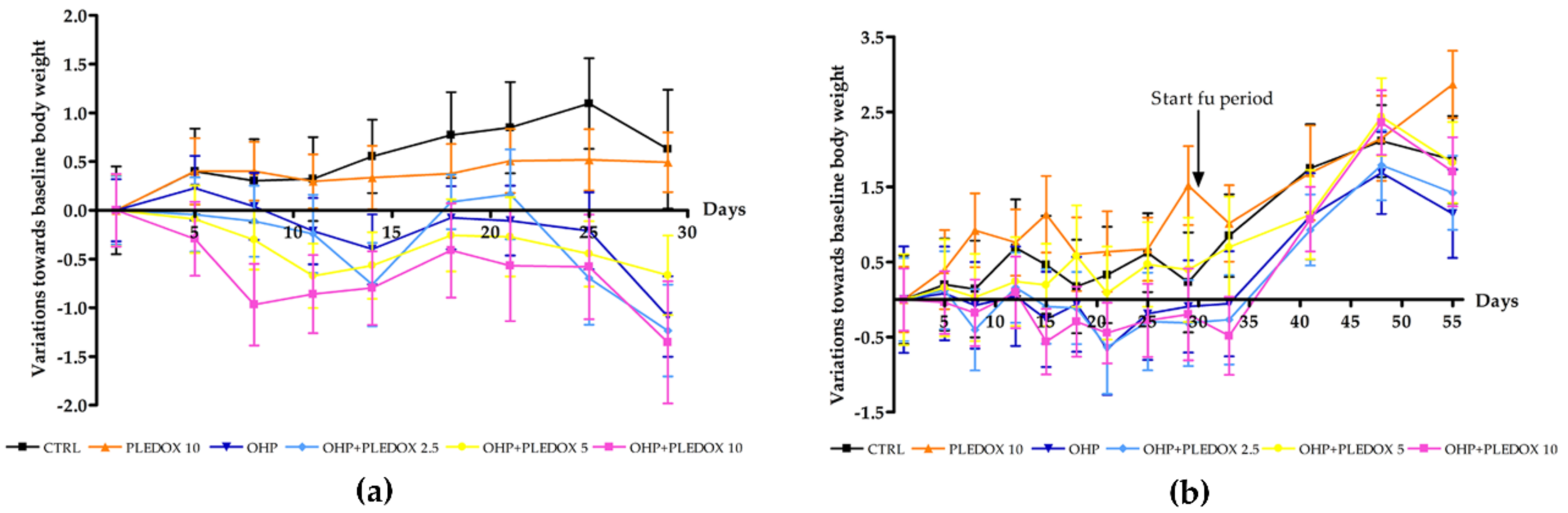
3.1. Clinical Observations and Body Weight Changes
3.2. Dynamic Aesthesiometer Test
3.3. Cold Plate Test
3.4. Blood Cell Count
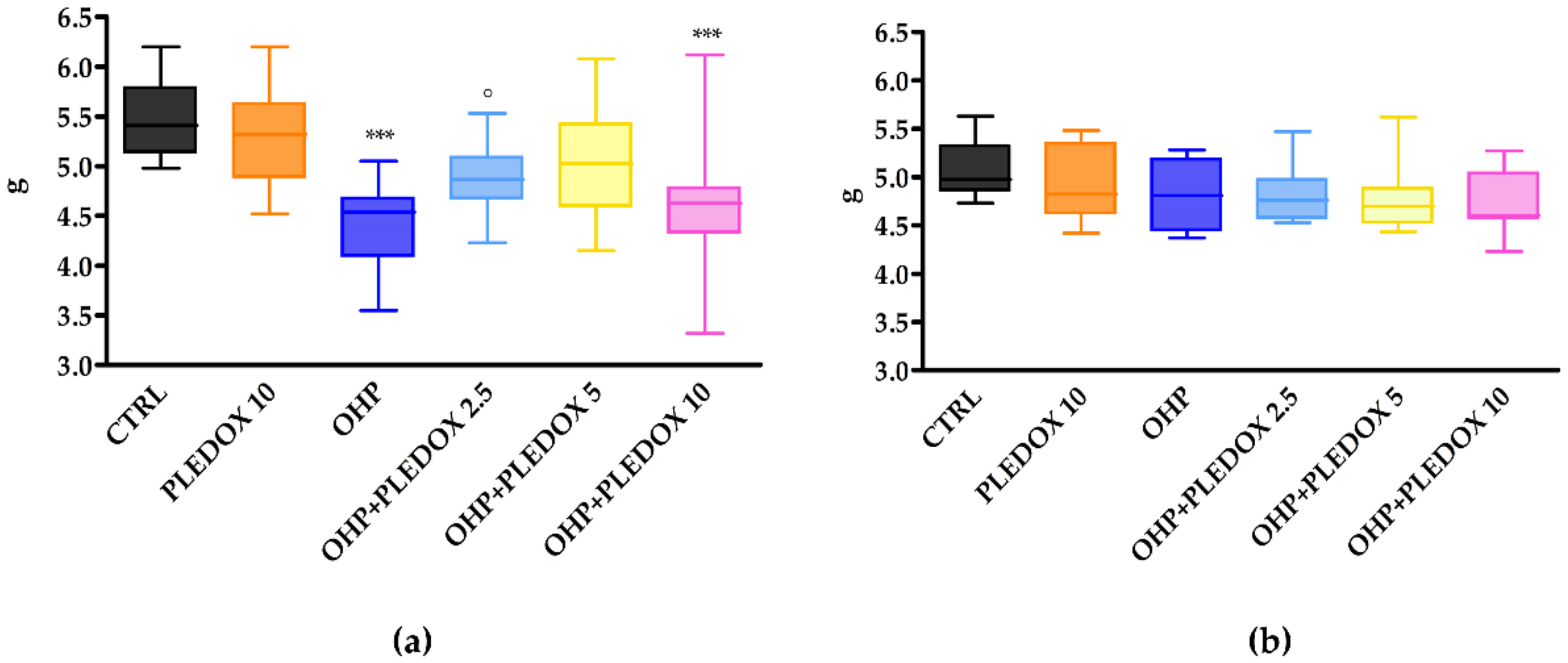
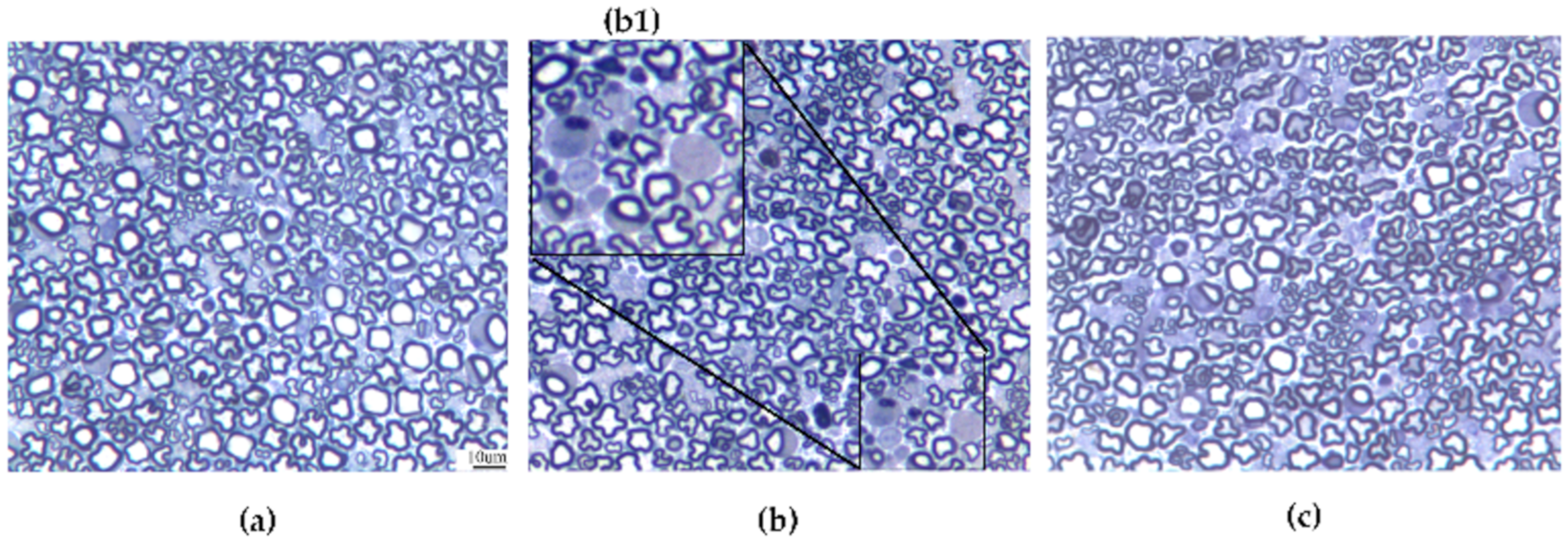
3.5. Caudal Nerve Morphological Analysis
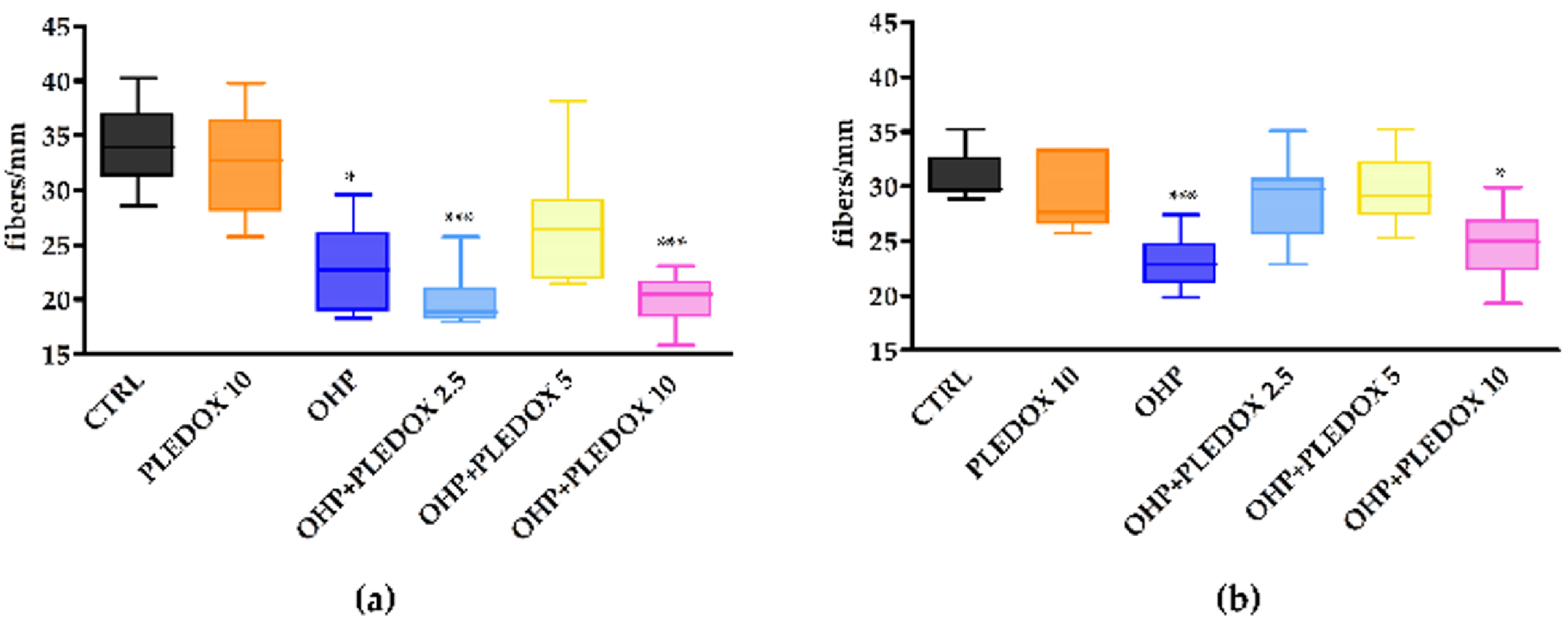
3.6. IENF Density Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.W.; Chaudhry, V.; Cavaletti, G.; Donehower, R.C. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst. Rev. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Doyle, T.; Salvemini, D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat. Rev. Neurol. 2014, 10, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Asplund, A.; Grant, D.; Karlsson, J.O. Mangafodipir (MnDPDP)-and MnCl2-induced endothelium-dependent relaxation in bovine mesenteric arteries. J. Pharmacol. Exp. Ther. 1994, 271, 609–614. [Google Scholar] [PubMed]
- Brurok, H.; Ardenkjær-Larsen, J.H.; Hansson, G.; Skarra, S.; Berg, K.; Karlsson, J.O.; Laursen, I.; Jynge, P. Manganese dipyridoxyl diphosphate: MRI contrast agent with antioxidative and cardioprotective properties? Biochem. Biophys. Res. Commun. 1999, 254, 768–772. [Google Scholar] [CrossRef]
- Rocklage, S.M.; Cacheris, W.P.; Quay, S.C.; Ekkehardt Hahn, F.; Raymond, K.N. Manganese(II) N,N′-dipyridoxylethylenediamine-N,N′-diacetate 5,5′-bis(phosphate). Synthesis and characterization of a paramagnetic chelate for magnetic resonance imaging enhancement. Inorg. Chem. 1989, 28, 477–485. [Google Scholar] [CrossRef]
- Bedda, S.; Laurent, A.; Conti, F.; Chéreau, C.; Tran, A.; Tran-Van Nhieu, J.; Jaffray, P.; Soubrane, O.; Goulvestre, C.; Calmus, Y.; et al. Mangafodipir prevents liver injury induced by acetaminophen in the mouse. J. Hepatol. 2003, 39, 765–772. [Google Scholar] [CrossRef]
- Coriat, R.; Alexandre, J.; Nicco, C.; Quinquis, L.; Benoit, E.; Chéreau, C.; Lemaréchal, H.; Mir, O.; Borderie, D.; Tréluyer, J.M.; et al. Treatment of oxaliplatin-induced peripheral neuropathy by intravenous mangafodipir. J. Clin. Investig. 2014, 124, 262–272. [Google Scholar] [CrossRef]
- Karlsson, J.O.G.; Ignarro, L.J.; Lundström, I.; Jynge, P.; Almén, T. Calmangafodipir [Ca4Mn(DPDP)5], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug. Discov. Today 2015, 20, 411–421. [Google Scholar] [CrossRef]
- Schmidt, P.P.; Toft, K.G.; Skotland, T.; Andersson, K. Stability and transmetallation of the magnetic resonance contrast agent MnDPDP measured by EPR. J. Biol. Inorg. Chem. 2002, 7, 241–248. [Google Scholar] [CrossRef]
- Glimelius, B.; Manojlovic, N.; Pfeiffer, P.; Mosidze, B.; Kurteva, G.; Karlberg, M.; Mahalingam, D.; Buhl Jensen, P.; Kowalski, J.; Bengtson, M.; et al. Persistent prevention of oxaliplatin-induced peripheral neuropathy using calmangafodipir (PledOx®): A placebo-controlled randomised phase II study (PLIANT). Acta Oncol. 2018, 57, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.O.G.; Kurz, T.; Flechsig, S.; Näsström, J.; Andersson, R.G. Superior therapeutic index of calmangafodipir in comparison to mangafodipir as a chemotherapy adjunct. Transl. Oncol. 2012, 5, 492–502. [Google Scholar] [CrossRef]
- Lustberg, M.B.; Pfeiffer, P.; Qvortrup, C.; Mura, K.; Bengtson, M.H.; Nittve, M.; Sonesson, C.; Nagahama, F.; Sonehara, Y.; Carlsson, C.S. The Global POLAR program: Two pivotal placebo-controlled studies of calmangafodipir used on top of modified FOLFOX6 to prevent chemotherapy-induced peripheral neuropathy (CIPN). J. Clin. Oncol. 2019, 37 (Suppl. S15). [Google Scholar] [CrossRef]
- Renn, C.L.; Carozzi, V.A.; Rhee, P.; Gallop, D.; Dorsey, S.G.; Cavaletti, G. Multimodal assessment of painful peripheral neuropathy induced by chronic oxaliplatin-based chemotherapy in mice. Mol. Pain 2011, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Marmiroli, P.; Riva, B.; Pozzi, E.; Ballarini, E.; Lim, D.; Chiorazzi, A.; Meregalli, C.; Distasi, C.; Renn, C.L.; Semperboni, S.; et al. Susceptibility of different mouse strains to oxaliplatin peripheral neurotoxicity: Phenotypic and genotypic insights. PLoS ONE 2017, 12, e0186250. [Google Scholar] [CrossRef]
- Cavaletti, G.; Tredici, G.; Marmiroli, P.; Petruccioli, M.G.; Barajon, I.; Fabbrica, D. Morphometric study of the sensory neuron and peripheral nerve changes induced by chronic cisplatin (DDP) administration in rats. Acta Neuropathol. 1992, 84, 364–371. [Google Scholar] [CrossRef]
- Cavaletti, G.; Gilardini, A.; Canta, A.; Rigamonti, L.; Rodriguez-Menendez, V.; Ceresa, C.; Marmiroli, P.; Bossi, M.; Oggioni, N.; D’Incalci, M.; et al. Bortezomib-induced peripheral neurotoxicity: A neurophysiological and pathological study in the rat. Exp. Neurol. 2007, 204, 317–325. [Google Scholar] [CrossRef]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Carozzi, V.A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Sandelius, Å.; Blennow, K.; et al. Neurofilament light chain as disease biomarker in a rodent model of chemotherapy induced peripheral neuropathy. Exp. Neurol. 2018, 307, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef]
- Ewertz, M.; Qvortrup, C.; Eckhoff, L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 2015, 54, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Marmiroli, P.; Cavaletti, G. Drugs for the treatment of peripheral neuropathies. Expert. Opin. Pharmacol. 2016, 17, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015, 28, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-induced oxidative stress in nervous system-derived cellular models: Could it correlate with in vivo neuropathy? Free Radic. Biol. Med. 2013, 61, 143–150. [Google Scholar] [CrossRef]
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.; Wong, D.V.T.; Lima-Júnior, R.C.; De Albuquerque Ribeiro, R.; Vale, M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 2013, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.K.; Chen, X.; Bogen, O.; Levine, J.D. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J. Pain 2008, 9, 463–472. [Google Scholar] [CrossRef]
- Mangus, L.M.; Rao, D.B.; Ebenezer, G.J. Intraepidermal Nerve Fiber Analysis in Human Patients and Animal Models of Peripheral Neuropathy: A Comparative Review. Toxicol. Pathol. 2020, 48, 59–70. [Google Scholar] [CrossRef]
- Anand, P.; Elsafa, E.; Privitera, R.; Naidoo, K.; Yiangou, Y.; Donatien, P.; Gabra, H.; Wasan, H.; Kenny, L.; Rahemtulla, A.; et al. Rational treatment of chemotherapy-induced peripheral neuropathy with capsaicin 8% patch: From pain relief towards disease modification. J. Pain Res. 2019, 12, 2039–2052. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Riley, D.P.; Caputi, A.P.; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001, 53, 135–159. [Google Scholar]
- Bonetta, R. Potential Therapeutic Applications of MnSODs and SOD-Mimetics. Chemistry 2018, 24, 5032–5041. [Google Scholar] [CrossRef]
- McCord, J.M. Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Dose Response 2008, 6, 223–238. [Google Scholar] [CrossRef]
- Mao, G.D.; Thomas, P.D.; Lopaschuk, G.D.; Poznansky, M.J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J. Biol. Chem. 1993, 268, 416–420. [Google Scholar] [PubMed]
- Towart, R.; Karlsson, J.O.G.; Jynge, P. Reduction of Cardiotoxicity of an Antitumor Agent Using Manganese Compound. U.S. Patent 6,147,094, 24 June 1997. [Google Scholar]
- Archibald, F.S.; Fridovich, I. The scavenging of superoxide radical by manganous complexes: In vitro. Arch. Biochem. Biophys. 1982, 214, 452–463. [Google Scholar] [CrossRef]
- Yri, O.E.; Vig, J.; Hegstad, E.; Hovde, Ø.; Pignon, I.; Jynge, P. Mangafodipir as a cytoprotective adjunct to chemotherapy—A case report. Acta Oncol. 2009, 48, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Iveson, T.J.; Kerr, R.S.; Saunders, M.P.; Cassidy, J.; Hollander, N.H.; Tabernero, J.; Haydon, A.; Glimelius, B.; Harkin, A.; Allan, K.; et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): An international, randomized, phase 3, non-inferiority trial. Lancet Oncol. 2018, 19, 562–578. [Google Scholar] [CrossRef]

| Group | WBC | RBC | Hgb | Hct | MCV | MCH |
| CTRL | 6.2 ± 1.7 | 9.5 ± 1 | 15.6 ± 1.8 | 53.2 ± 6 | 56.3 ± 0.8 | 16.4 ± 0.2 |
| PLEDOX 10 | 8.1 ± 4.1 | 8.8 ± 1.1 | 14.5 ± 1.7 | 49.4 ± 6.6 | 55.8 ± 1.2 | 16.5 ± 0.4 |
| OHP | 2.5 ± 0.8 | 4.7 ± 0.7 | 7 ± 1.4 | 24.3 ± 4.6 | 51 ± 2.2 | 14.6 ± 0.8 |
| OHP + PLEDOX 2.5 | 2.5 ± 1 | 4.8 ± 0.3 | 7.1 ± 0.5 | 24.5 ± 2.1 | 51.2 ± 1.3 | 14.8 ± 0.6 |
| OHP + PLEDOX 5 | 3.4 ± 0.9 | 5.1 ± 0.5 | 8 ± 1.1 | 27.9 ± 3.9 | 54.3 ± 2.6 | 15.5 ± 0.7 |
| OHP + PLEDOX 10 | 3.2 ± 1 | 5.2 ± 1 | 8 ± 2 | 27.7 ± 7 | 52.5 ± 3.1 | 15.1 ± 0.9 |
| Group | MCHC | RDW | Plt | MPV | Pct | PDW |
| CTRL | 29.3 ± 0.3 | 11.9 ± 0.1 | 566.7 ± 211.2 | 10.3 ± 0.8 | 0.6 ± 0.2 | 12.1 ± 1.9 |
| PLEDOX 10 | 29.5 ± 0.8 | 11.7 ± 0.2 | 507.3 ± 311 | 10.5 ± 1.1 | 0.5 ± 0.3 | 10.6 ± 5.5 |
| OHP | 28.7 ± 0.7 | 12.2 ± 0.5 | 324 ± 152.7 | 10.7 ± 0.3 | 0.3 ± 0.2 | 15.6 ± 0.4 |
| OHP + PLEDOX 2.5 | 28.9 ± 1 | 12.6 ± 0.4 | 379.5 ± 107.3 | 10.5 ± 0.3 | 0.4 ± 0.1 | 15.7 ± 0.1 |
| OHP + PLEDOX 5 | 28.5 ± 0.2 | 13 ± 1.1 | 496.5 ± 138.2 | 10.4 ± 0.4 | 0.5 ± 0.1 | 15.1 ± 0.7 |
| OHP + PLEDOX 10 | 28.8 ± 0.4 | 13.1 ± 1.8 | 448.8 ± 148 | 10.4 ± 0.5 | 0.5 ± 0.1 | 15.8 ± 0.3 |
| Group | WBC | RBC | Hgb | Hct | MCV | MCH |
| CTRL | 7.5 ± 2.3 | 10.1 ± 1.6 | 17.1 ± 3.3 | 55.3 ± 9.9 | 54.5 ± 1.4 | 16.8 ± 0.6 |
| PLEDOX 10 | 5.2 ± 2.7 | 9.2 ± 0.4 | 15.2 ± 0.7 | 49.4 ± 2.3 | 53.7 ± 1 | 16.4 ± 0.3 |
| OHP | 5.3 ± 1.4 | 8.5 ± 0.9 | 13.7 ± 1.7 | 46.1 ± 5.3 | 54.5 ± 1 | 16.2 ± 0.5 |
| OHP + PLEDOX 2.5 | 5.2 ± 1.4 | 8.7 ± 0.9 | 14.5 ± 1.9 | 47.6 ± 5.6 | 54.3 ± 1 | 16.6 ± 0.4 |
| OHP + PLEDOX 5 | 5.2 ± 1.3 | 7.2 ± 1.9 | 11.6 ± 3.7 | 38.9 ± 11.2 | 54 ± 1.4 | 15.9 ± 0.9 |
| OHP + PLEDOX 10 | 5.5 ± 2 | 8.6 ± 0.3 | 14.2 ± 0.6 | 46.9 ± 1.9 | 54.5 ± 0.5 | 16.5 ± 0.2 |
| Group | MCHC | RDW | Plt | MPV | Pct | PDW |
| CTRL | 30.7 ± 0.5 | 13.6 ± 0.6 | 684.2 ± 358.3 | 10.2 ± 1.4 | 0.6 ± 0.3 | 7.1 ± 5.5 |
| PLEDOX 10 | 30.6 ± 0.2 | 13.8 ± 0.6 | 605.7 ± 277.8 | 9.4 ± 0.4 | 0.6 ± 0.3 | 11.9 ± 1.9 |
| OHP | 29.8 ± 0.5 | 13.2 ± 0.4 | 492.7 ± 239.6 | 10.5 ± 1 | 0.5 ± 0.2 | 11.3 ± 6 |
| OHP + PLEDOX 2.5 | 30.4 ± 0.5 | 13.3 ± 0.6 | 469 ± 176.5 | 10.4 ± 0.7 | 0.5 ± 0.2 | 14.8 ± 1.7 |
| OHP + PLEDOX 5 | 29.5 ± 1 | 13.3 ± 1 | 316 ± 203.5 | 10.4 ± 0.8 | 0.3 ± 0.2 | 12.6 ± 6.5 |
| OHP + PLEDOX 10 | 30.3 ± 0.2 | 13.2 ± 0.5 | 487.2 ± 194.4 | 10.2 ± 0.4 | 0.5 ± 0.2 | 14.4 ± 2.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canta, A.; Chiorazzi, A.; Pozzi, E.; Fumagalli, G.; Monza, L.; Meregalli, C.; Carozzi, V.A.; Rodriguez-Menendez, V.; Oggioni, N.; Näsström, J.; et al. Calmangafodipir Reduces Sensory Alterations and Prevents Intraepidermal Nerve Fibers Loss in a Mouse Model of Oxaliplatin Induced Peripheral Neurotoxicity. Antioxidants 2020, 9, 594. https://doi.org/10.3390/antiox9070594
Canta A, Chiorazzi A, Pozzi E, Fumagalli G, Monza L, Meregalli C, Carozzi VA, Rodriguez-Menendez V, Oggioni N, Näsström J, et al. Calmangafodipir Reduces Sensory Alterations and Prevents Intraepidermal Nerve Fibers Loss in a Mouse Model of Oxaliplatin Induced Peripheral Neurotoxicity. Antioxidants. 2020; 9(7):594. https://doi.org/10.3390/antiox9070594
Chicago/Turabian StyleCanta, Annalisa, Alessia Chiorazzi, Eleonora Pozzi, Giulia Fumagalli, Laura Monza, Cristina Meregalli, Valentina A. Carozzi, Virginia Rodriguez-Menendez, Norberto Oggioni, Jacques Näsström, and et al. 2020. "Calmangafodipir Reduces Sensory Alterations and Prevents Intraepidermal Nerve Fibers Loss in a Mouse Model of Oxaliplatin Induced Peripheral Neurotoxicity" Antioxidants 9, no. 7: 594. https://doi.org/10.3390/antiox9070594
APA StyleCanta, A., Chiorazzi, A., Pozzi, E., Fumagalli, G., Monza, L., Meregalli, C., Carozzi, V. A., Rodriguez-Menendez, V., Oggioni, N., Näsström, J., Marmiroli, P., & Cavaletti, G. (2020). Calmangafodipir Reduces Sensory Alterations and Prevents Intraepidermal Nerve Fibers Loss in a Mouse Model of Oxaliplatin Induced Peripheral Neurotoxicity. Antioxidants, 9(7), 594. https://doi.org/10.3390/antiox9070594







