Antiarrhythmic Effects of Melatonin and Omega-3 Are Linked with Protection of Myocardial Cx43 Topology and Suppression of Fibrosis in Catecholamine Stressed Normotensive and Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
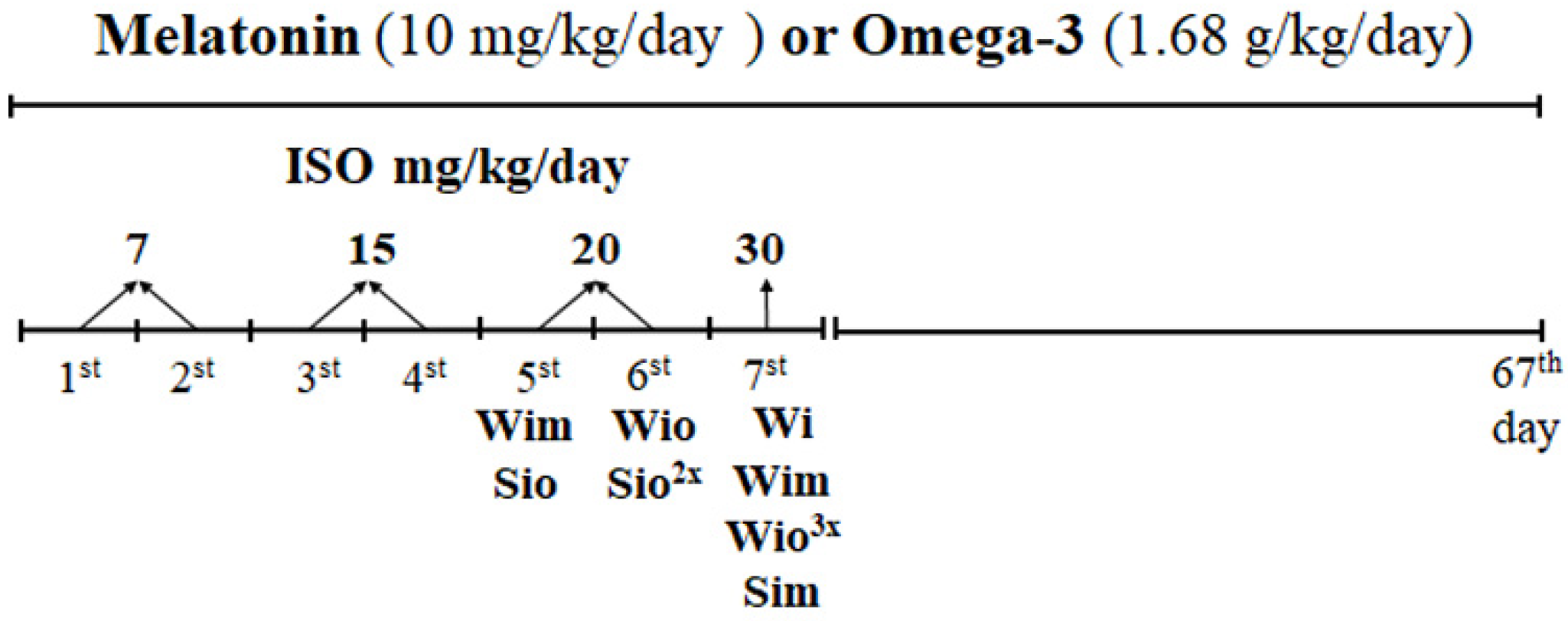
2.1. Experimental Design
2.2. Examination of Heart Function and Ventricular Fibrillation in Langendorff-Perfused Hearts
2.3. SDS-PAGE and Western Blot Analysis
2.4. Zymographic Detection of MMP-2 Activity
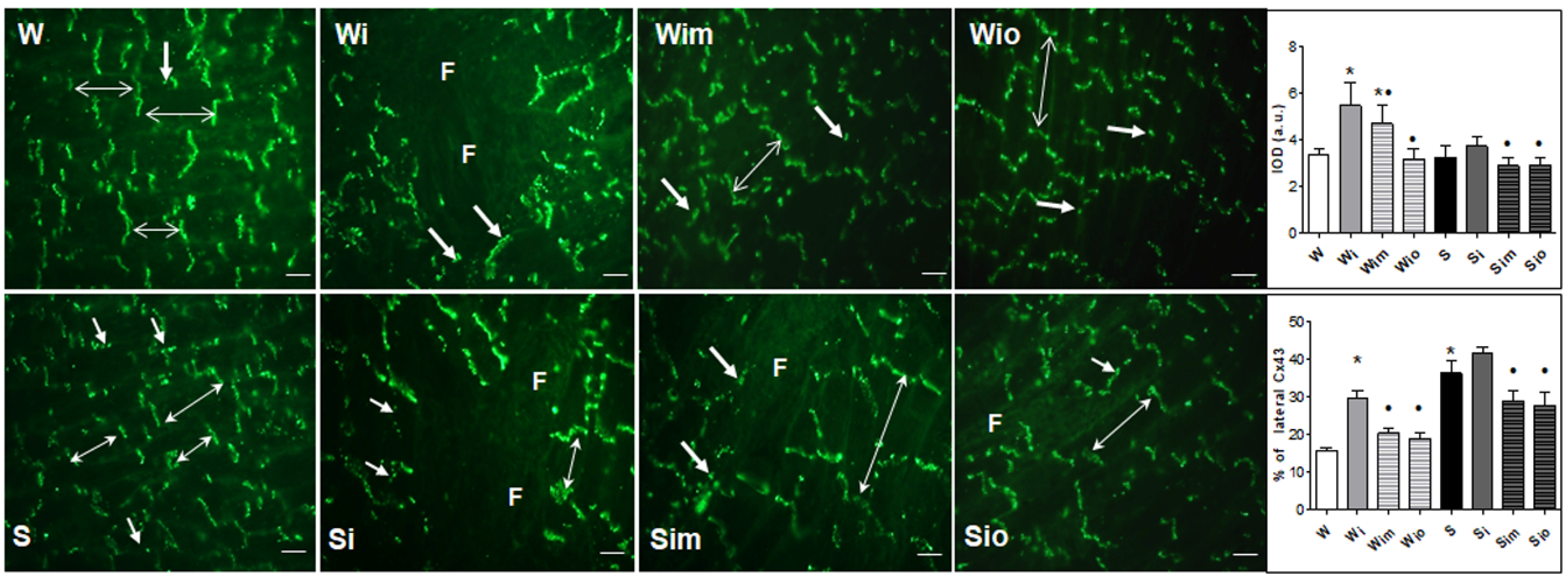
2.5. Immunofluorescence of Cx43 and Quantitative Image Analysis
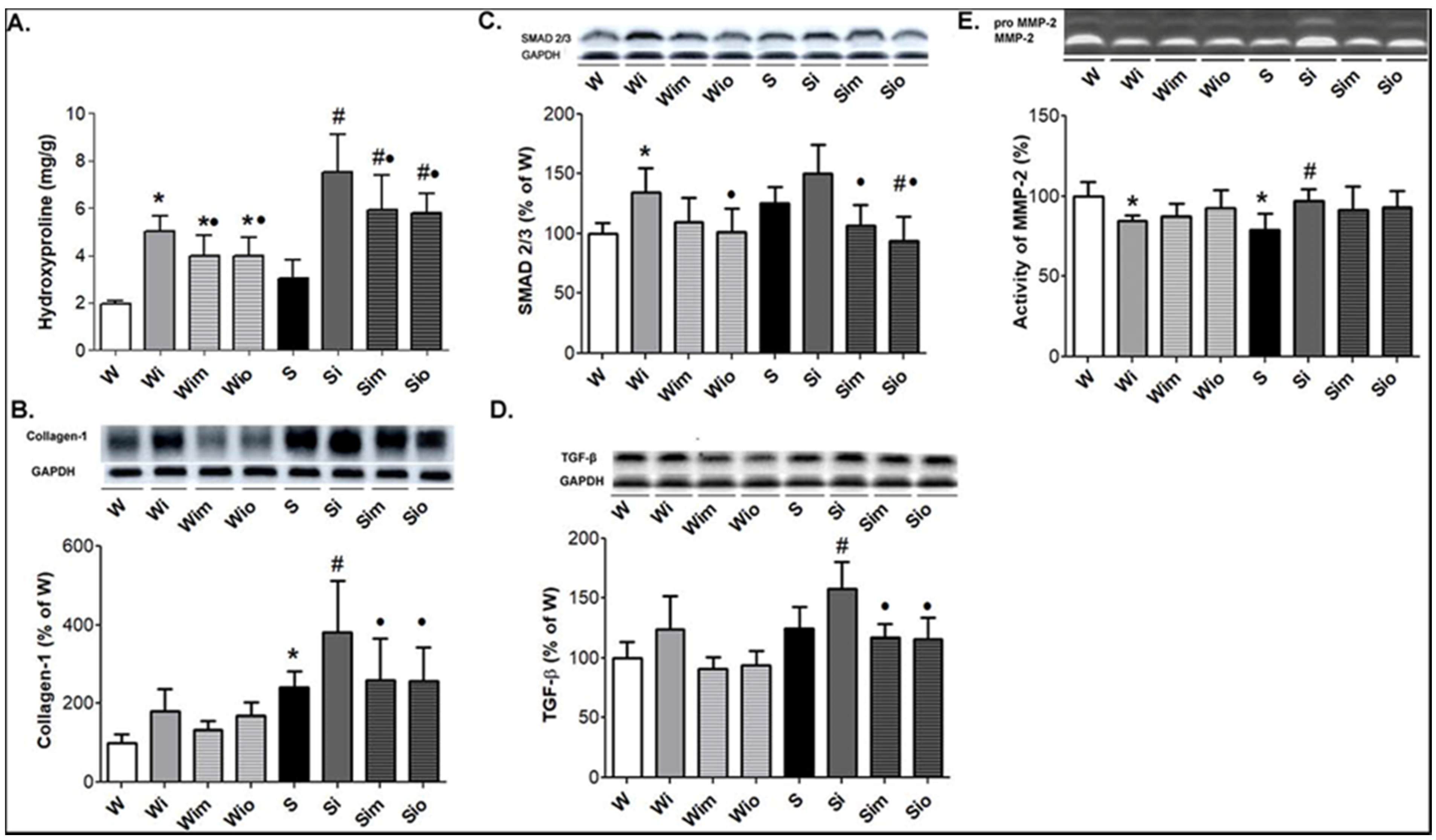
2.6. Determination of Collagen Content by Hydroxyproline Measurement
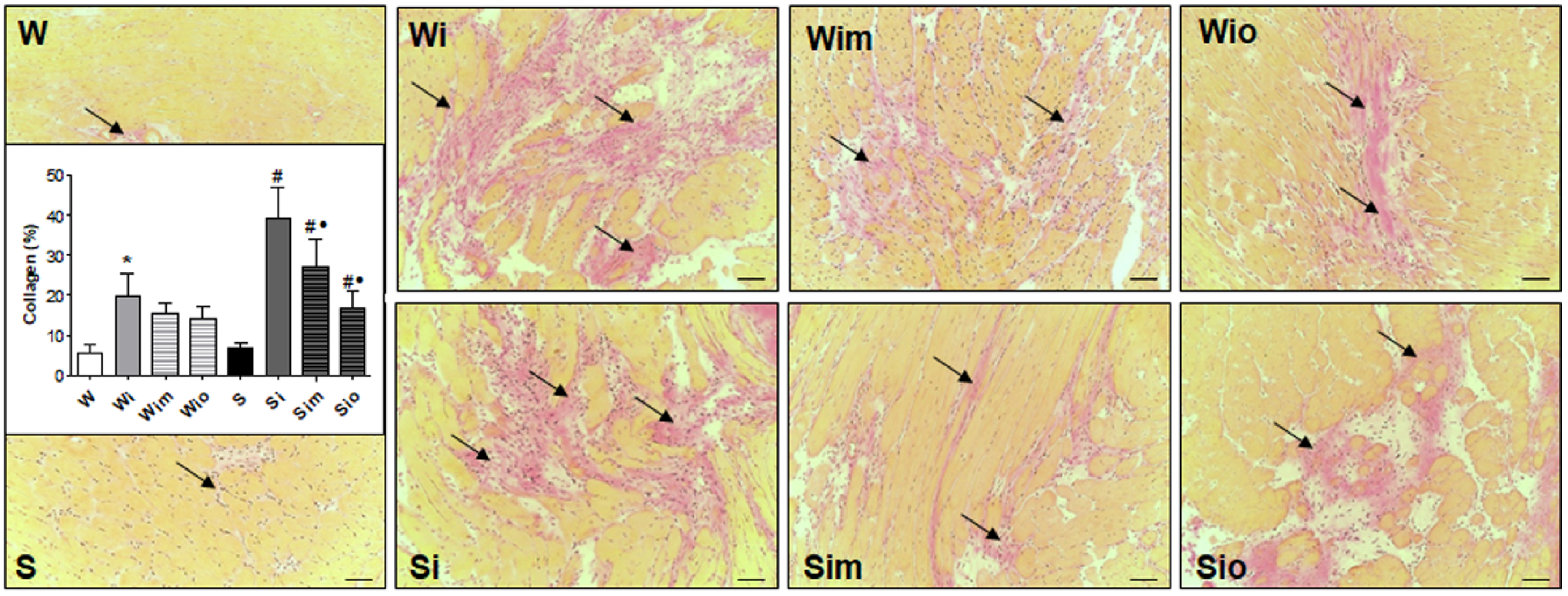
2.7. Determination of Collagen Deposition by van Gieson Technique and Quantitative Image Analysis
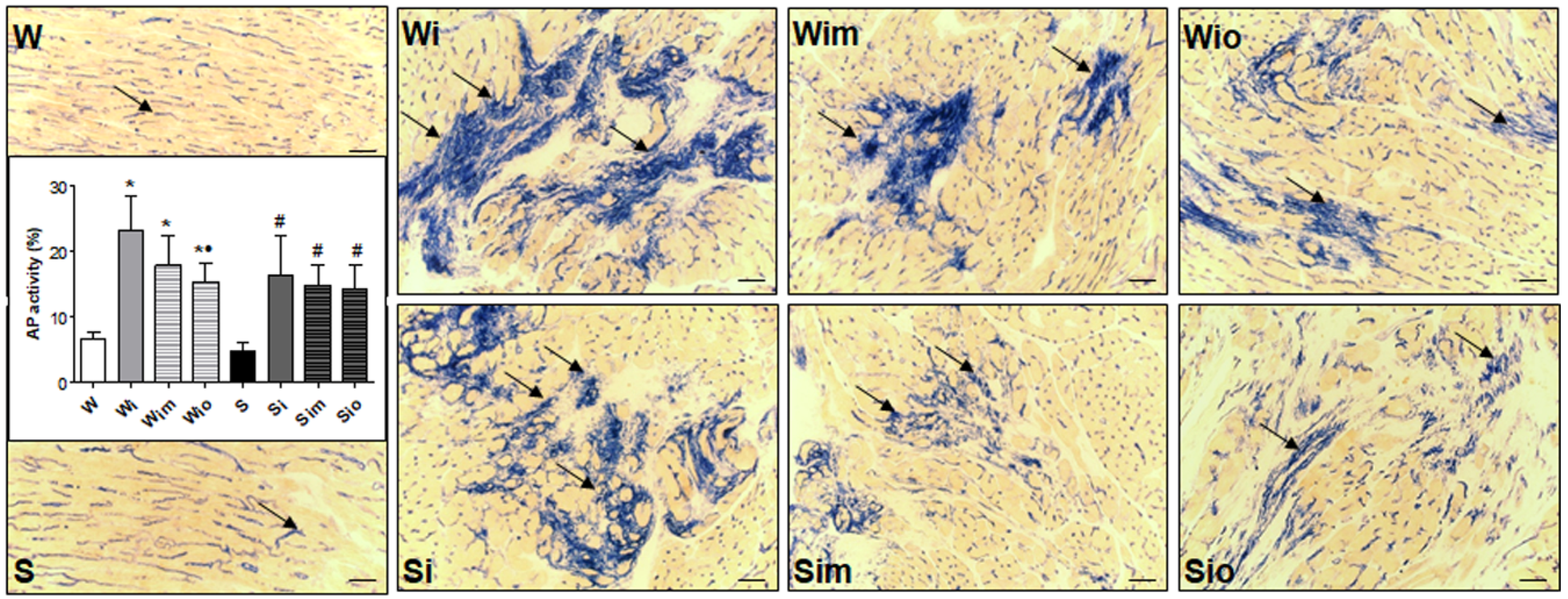
2.8. Determination of Alkaline Phosphatase Activity and Quantitative Image
2.9. Determination of Succinate Dehydrogenase and β-Hydroxybutyrate Dehydrogenase Activities
2.10. Measurement of Lipoperoxidation by Thiobarbituric Acid Reactive Substances (TBARS) Assay
2.11. Statistical Analysis
3. Results
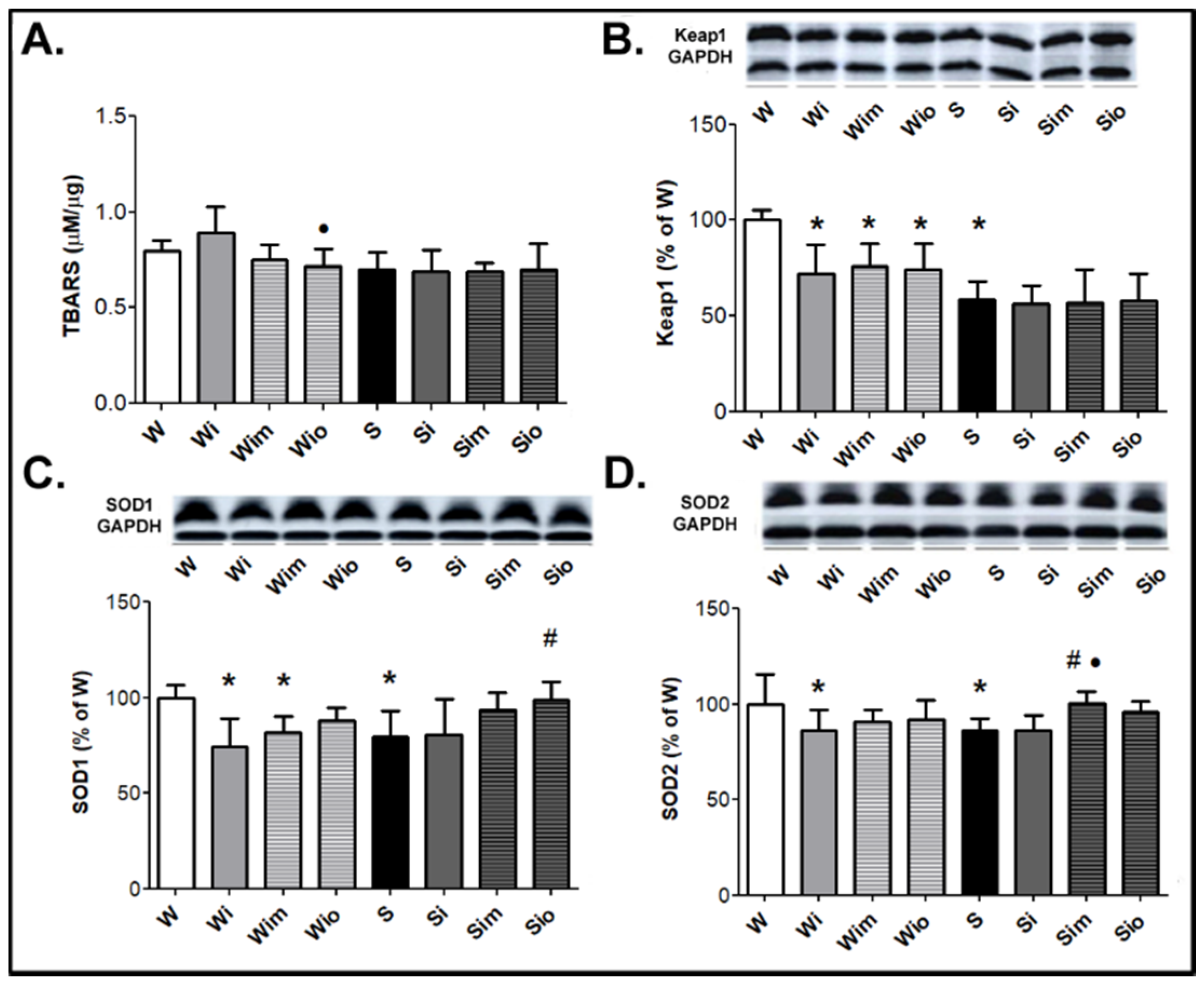
3.1. Survival of Experimental Rats and Registered Biomarkers
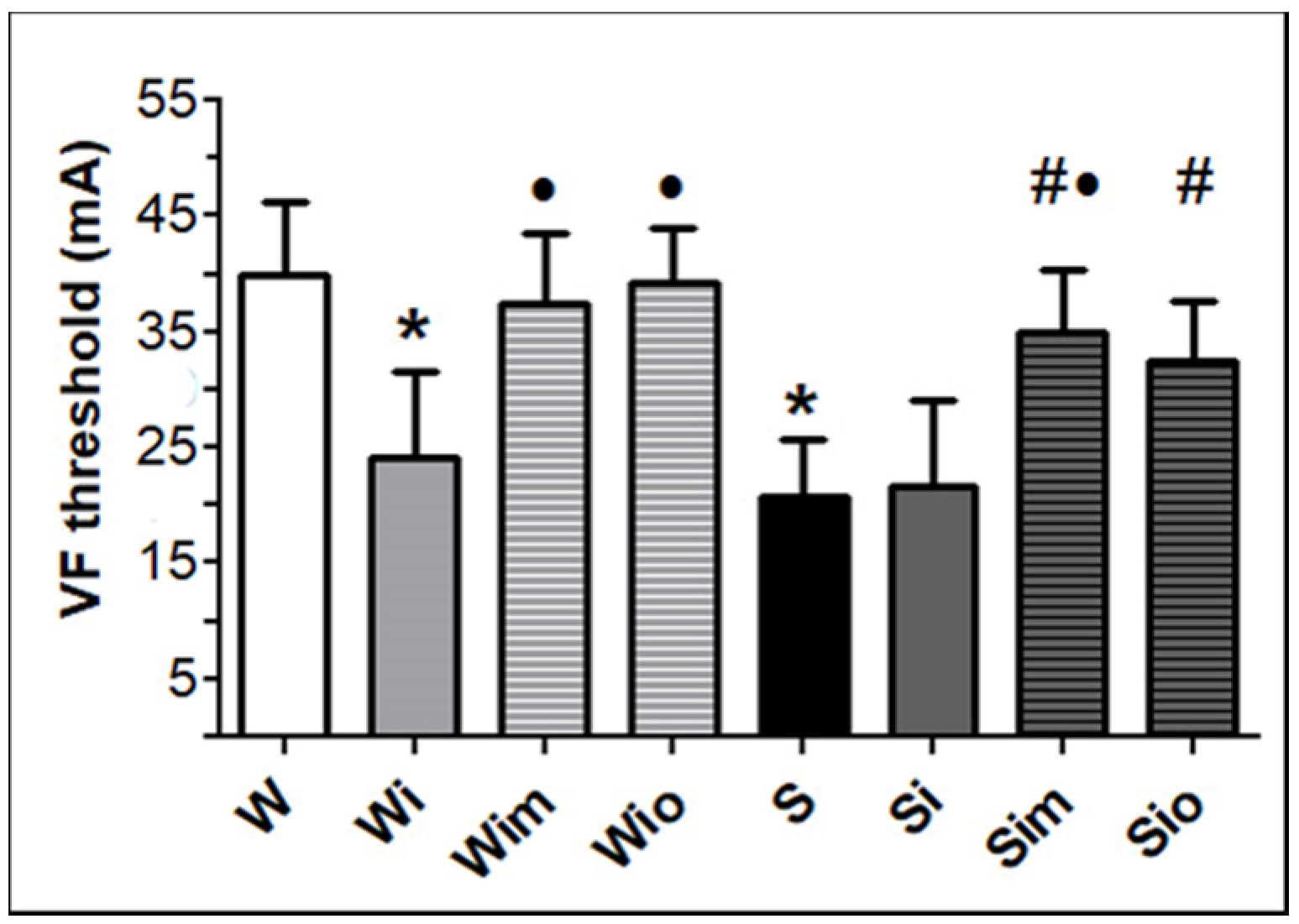
3.2. Heart Function and VF Threshold Registered Ex Vivo
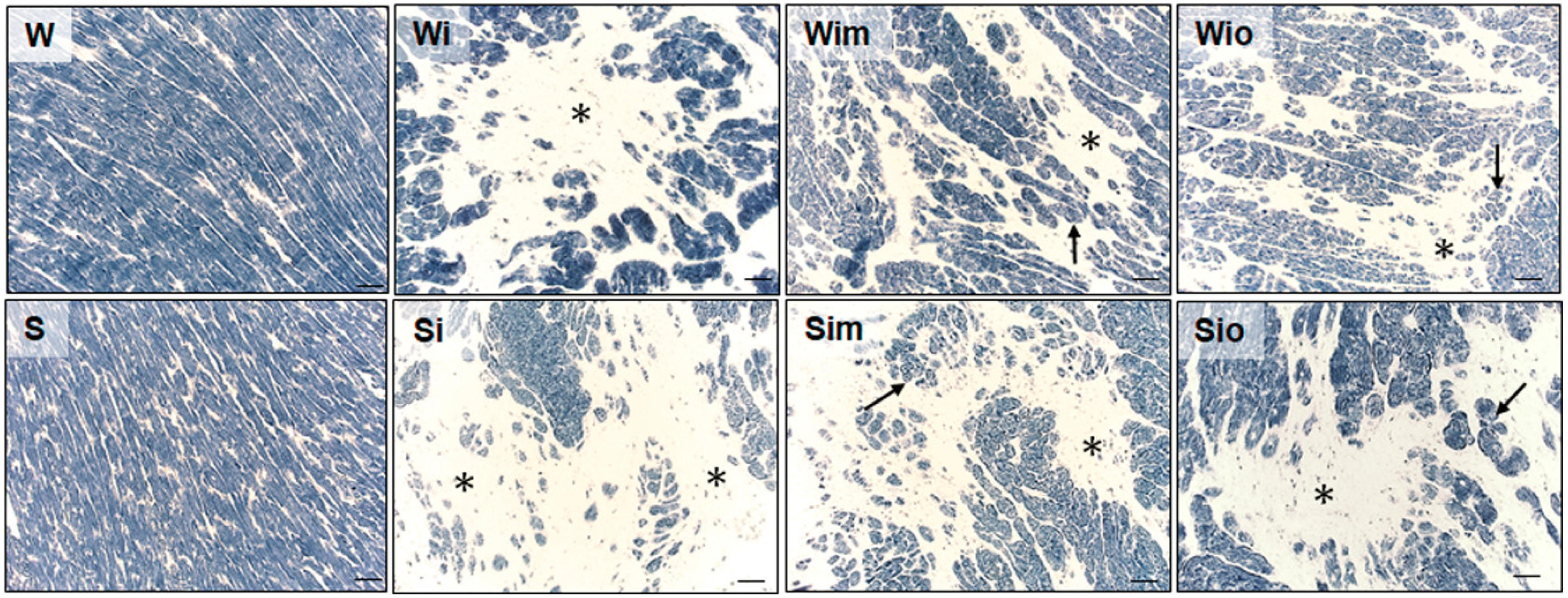
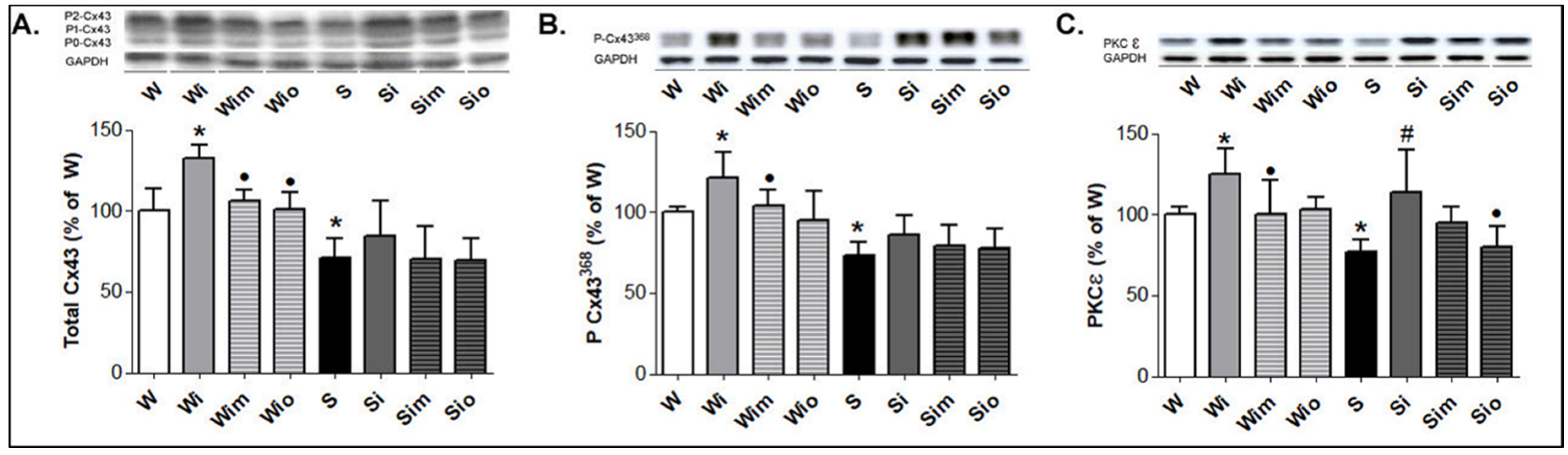
3.3. Cardiomyocyte Alterations in Left Heart Ventricle
3.4. Extracellular Matrix Alterations in Left Heart Ventricle
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhalla, N.S.; Adameova, A.; Kaur, M. Role of catecholamine oxidation in sudden cardiac death. Fundam. Clin. Pharmacol. 2010, 24, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.M.; Carvalho, F.; Bastos, M.L.; Carvalho, R.A.; Carvalho, M.; Remiao, F. Contribution of Catecholamine Reactive Intermediates and Oxidative Stress to the Pathologic Features of Heart Diseases. Curr. Med. Chem. 2011, 18, 2272–2314. [Google Scholar] [CrossRef]
- Tribulova, N.; Knezl, V.; Okruhlicova, L.; Slezak, J. Myocardial gap junctions: targets for novel approaches in the prevention of life-threatening cardiac arrhythmias. Physiol. Res. 2008, 57, 1–13. [Google Scholar]
- Tribulova, N.; Knezl, V.; Szeiffova Bacova, B.; Egan Benova, T.; Viczenczova, C.; Gonçalvesova, E.; Slezak, J. Disordered myocardial Ca2+ homeostasis results in substructural alterations that may promote occurrence of malignant arrhythmias. Physiol. Res. 2016, 65, 139–148. [Google Scholar] [CrossRef]
- Tribulova, N.; Seki, S.; Radosinska, J.; Kaplan, P.; Babusikova, E.; Knezl, V.; Mochizuki, S. Myocardial Ca2+ handling and cell-to-cell coupling, key factors in prevention of sudden cardiac death1. Can. J. Physiol. Pharmacol. 2009, 87, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Veliotes, D.G.A.; Norton, G.R.; Correia, R.J.; Strijdom, H.; Badenhorst, D.; Brooksbank, R.; Woodiwiss, A.J. Impact of aldosterone receptor blockade on the deleterious cardiac effects of adrenergic activation in hypertensive rats. J. Cardiovasc. Pharmacol. 2010, 56, 203–211. [Google Scholar] [CrossRef]
- Soltysinska, E.; Olesen, S.P.; Osadchii, O.E. Myocardial structural, contractile and electrophysiological changes in the guinea-pig heart failure model induced by chronic sympathetic activation. Exp. Physiol. 2011, 96, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Lu, M.; Xie, Z.Z.; Hua, F.; Xie, L.; Gao, J.H.; Koh, Y.H.; Bian, J.S. Hydrogen sulfide prevents heart failure development via inhibition of renin release from mast cells in isoproterenol-treated rats. Antioxid. Redox Signal. 2013, 20, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Krenek, P.; Kmecova, J.; Kucerova, D.; Bajuszova, Z.; Musil, P.; Gazova, A.; Ochodnicky, P.; Klimas, J.; Kyselovic, J. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur. J. Heart Fail. 2009, 11, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Li, W. The study of ISO induced heart failure rat model. Exp. Mol. Pathol. 2010, 88, 299–304. [Google Scholar] [CrossRef]
- Mukherjee, D.; Roy, S.G.; Bandyopadhyay, A.; Chattopadhyay, A.; Basu, A.; Mitra, E.; Ghosh, A.K.; Reiter, R.J.; Bandyopadhyay, D. Melatonin protects against isoproterenol-induced myocardial injury in the rat: Antioxidative mechanisms. J. Pineal Res. 2010, 48, 251–262. [Google Scholar] [CrossRef]
- Challa, A.A.; Vukmirovic, M.; Blackmon, J.; Stefanovic, B. Withaferin-A reduces type I collagen expression in vitro and inhibits development of myocardial fibrosis in vivo. PLoS ONE 2012, 7, e42989. [Google Scholar] [CrossRef]
- Herrmann, J.E.; Heale, J.; Bieraugel, M.; Ramos, M.; Fisher, R.L.; Vickers, A.E.M. Isoproterenol effects evaluated in heart slices of human and rat in comparison to rat heart in vivo. Toxicol. Appl. Pharmacol. 2014, 274, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Ramachandran, J.; Xie, L.; Contreras, J.; Fraidenraich, D. Selective Connexin43 Inhibition Prevents Isoproterenol-Induced Arrhythmias and Lethality in Muscular Dystrophy Mice. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Meng, T.; Sun, C. Protective effect of diltiazem on myocardial ischemic rats induced by isoproterenol. Mol. Med. Rep. 2018, 17, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Kyle, J.W.; Makielski, J.C.; Dudley, S.C. Mechanisms of Sudden Cardiac Death: Oxidants and Metabolism. Circ. Res. 2015, 116, 1937–1955. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Smyth, J.W.; Hong, T.T.; Gao, D.; Vogan, J.M.; Jensen, B.C.; Fong, T.S.; Simpson, P.C.; Stainier, D.Y.R.; Chi, N.C.; Shaw, R.M. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J. Clin. Investig. 2010, 120, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Gopi, V.; Devi Km, S.; Balaji, N.; Vellaichamy, E. Aminoguanidine inhibits ventricular fibrosis and remodeling process in isoproterenol-induced hypertrophied rat hearts by suppressing ROS and MMPs. Life Sci. 2014, 118, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur. Heart J. 2020, 41, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Benova, T.; Viczenczova, C.; Radosinska, J.; Bacova, B.; Knezl, V.; Dosenko, V.; Weismann, P.; Zeman, M.; Navarova, J.; Tribulova, N. Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethalarrhythmias. Can. J. Physiol. Pharmacol. 2013, 91, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Benova, T.; Knezl, V.; Viczenczova, C.; Bacova, B.S.; Radosinska, J.; Tribulova, N. Acute anti-fibrillating and defibrillating potential of atorvastatin, melatonin, eicosapentaenoic acid and docosahexaenoic acid demonstrated in isolated heart model. J. Physiol. Pharmacol. 2015, 66, 83–89. [Google Scholar] [PubMed]
- Sedova, K.A.; Bernikova, O.G.; Cuprova, J.I.; Ivanova, A.D.; Kutaeva, G.A.; Pliss, M.G.; Lopatina, E.V.; Vaykshnorayte, M.A.; Diez, E.R.; Azarov, J.E. Association between antiarrhythmic, electrophysiological, and antioxidative effects of melatonin in ischemia/reperfusion. Int. J. Mol. Sci. 2019, 20, 6331. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Tribulova, N.; Szeiffova Bacova, B.; Benova, T.; Viczenczova, C. Can we protect from malignant arrhythmias by modulation of cardiac cell-to-cell coupling? J. Electrocardiol. 2015, 48, 434–440. [Google Scholar] [CrossRef]
- Bacova, B.S.; Radosinska, J.; Wallukat, G.; Barancik, M.; Wallukat, A.; Knezl, V.; Sykora, M.; Paulis, L.; Tribulova, N. Suppression of β1-adrenoceptor autoantibodies is involved in the antiarrhythmic effects of omega-3 fatty acids in male and female hypertensive rats. Int. J. Mol. Sci. 2020, 21, 526. [Google Scholar] [CrossRef]
- Egan Beňová, T.; Knezl, V.; Viczenczová, C.; Szeiffová Bačová, B.; Radošinská, J.; Tribulová, N. Anti-fibrillating and defibrillating capability of atorvastatin, melatonin and omega-3 fatty acids demonstrated in acute conditions on isolated heart model. Cardiol. Lett. 2016, 25, 376–383. [Google Scholar]
- Prado, N.J.; Egan Beňová, T.; Diez, E.R.; Knezl, V.; Lipták, B.; Ponce Zumino, A.Z.; Llamedo-Soria, M.; Szeiffová Bačová, B.; Miatello, R.M.; Tribulová, N. Melatonin receptor activation protects against low potassium—Induced ventricular fibrillation by preserving action potentials and connexin-43 topology in isolated rat hearts. J. Pineal Res. 2019, 67, e12605. [Google Scholar] [CrossRef]
- Sykora, M.; Szeiffova Bacova, B.; Egan Benova, T.; Barancik, M.; Zurmanova, J.; Rauchova, H.; Weismann, P.; Pavelka, S.; Kurahara, L.H.; Slezak, J.; et al. Cardiac Cx43 and ECM Responses to Altered Thyroid Status Are Blunted in Spontaneously Hypertensive versus Normotensive Rats. Int. J. Mol. Sci. 2019, 20, 3758. [Google Scholar] [CrossRef]
- Radosinska, J.; Bacova, B.; Knezl, V.; Benova, T.; Zurmanova, J.; Soukup, T.; Arnostova, P.; Slezak, J.; Goncalvesova, E.; Tribulova, N. Dietary omega-3 fatty acids attenuate myocardial arrhythmogenic factors and propensity of the heart to lethal arrhythmias in a rodent model of human essential hypertension. J. Hypertens. 2013, 31, 1876–1885. [Google Scholar] [CrossRef]
- Bačová, B.S.; Vinczenzová, C.; Žurmanová, J.; Kašparová, D.; Knezl, V.; Beňová, T.E.; Pavelka, S.; Soukup, T.; Tribulová, N. Altered thyroid status affects myocardial expression of connexin-43 and susceptibility of rat heart to malignant arrhythmias that can be partially normalized by red palm oil intake. Histochem. Cell Biol. 2017, 147, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Szeiffová Bačova, B.; Egan Beňová, T.; Viczenczová, C.; Soukup, T.; Rauchová, H.; Pavelka, S.; Knezl, V.; Barancík, M.; Tribulová, N. Cardiac connexin-43 and PKC signaling in rats with altered thyroid status without and with omega-3 fatty acids intake. Physiol. Res. 2016, 65, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Barancik, M.; Bohacova, V.; Gibalova, L.; Sedlak, J.; Sulova, Z.; Breier, A. Potentiation of anticancer drugs: Effects of pentoxifylline on neoplastic cells. Int. J. Mol. Sci. 2012, 13, 369–382. [Google Scholar] [CrossRef]
- Sykora, M.; Kamocsaiova, L.; Benova, T.E.; Frimmel, K.; Ujhazy, E.; Mach, M.; Barancik, M.; Tribulova, N.; Bacova, B.S. Alterations in myocardial connexin-43 and matrix metalloproteinase-2 signaling in response to pregnancy and oxygen deprivation of Wistar rats: A pilot study. Can. J. Physiol. Pharmacol. 2019, 97, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Pelouch, V.; Dixon, I.M.C.; Sethi, R.; Dhalla, N.S. Alteration of collagenous protein profile in congestive heart failure secondary to myocardial infarction. Mol. Cell. Biochem. 1993, 129, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.; Enwemeka, C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef]
- Lojda, Z.; Gutmann, E. Histochemistry of some acid hydrolases in striated muscles of the rat. Histochemistry 1976, 49, 337–342. [Google Scholar] [CrossRef]
- Shlafer, M.; Shepard, B.M. A method to reduce interference by sucrose in the detection of thiobarbituric acid-reactive substances. Anal. Biochem. 1984, 137, 269–276. [Google Scholar] [CrossRef]
- Szobi, A.; Farkašová-Ledvényiová, V.; Lichý, M.; Muráriková, M.; Čarnická, S.; Ravingerová, T.; Adameová, A. Cardioprotection of ischaemic preconditioning is associated with inhibition of translocation of MLKL within the plasma membrane. J. Cell. Mol. Med. 2018, 22, 4183–4196. [Google Scholar] [CrossRef]
- Aarvik, M.D.; Sandven, I.; Dondo, T.B.; Gale, C.P.; Ruddox, V.; Munkhaugen, J.; Atar, D.; Otterstad, J.E. Effect of oral β-blocker treatment on mortality in contemporary post-myocardial infarction patients: A systematic review and meta-analysis. Eur. Hear. J.-Cardiovasc. Pharmacother. 2019, 5, 12–20. [Google Scholar] [CrossRef]
- Simko, F.; Bednarova, K.R.; Krajcirovicova, K.; Hrenak, J.; Celec, P.; Kamodyova, N.; Gajdosechova, L.; Zorad, S.; Adamcova, M. Melatonin reduces cardiac remodeling and improves survival in rats with isoproterenol-induced heart failure. J. Pineal Res. 2014, 57, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Duran, J.M.; Mohsin, S.; Troupes, C.D.; Vanhoutte, D.; Borghetti, G.; Vagnozzi, R.J.; Gross, P.; Yu, D.; Trappanese, D.M.; et al. Acute Catecholamine Exposure Causes Reversible Myocyte Injury without Cardiac Regeneration. Circ. Res. 2016, 119, 865–879. [Google Scholar] [CrossRef]
- Zhang, G.X.; Kimura, S.; Nishiyama, A.; Shokoji, T.; Rahman, M.; Yao, L.; Nagai, Y.; Fujisawa, Y.; Miyatake, A.; Abe, Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc. Res. 2005, 65, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Mikaelian, I.; Coluccio, D.; Morgan, K.T.; Johnson, T.; Ryan, A.L.; Rasmussen, E.; Nicklaus, R.; Kanwal, C.; Hilton, H.; Frank, K.; et al. Temporal gene expression profiling indicates early up-regulation of interleukin-6 in isoproterenol-induced myocardial necrosis in rat. Toxicol. Pathol. 2008, 36, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Ghosh, A.K.; Bandyopadhyay, A.; Basu, A.; Datta, S.; Pattari, S.K.; Reiter, R.J.; Bandyopadhyay, D. Melatonin protects against isoproterenol-induced alterations in cardiac mitochondrial energy-metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 2012, 53, 166–179. [Google Scholar] [CrossRef]
- Sagor, M.A.T.; Tabassum, N.; Potol, M.A.; Alam, M.A. Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats. Oxid. Med. Cell. Longev. 2015, 2015, 478039. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Q.; Leng, J.; Zheng, Y.; Li, J. The role of Pyruvate Dehydrogenase Complex in cardiovascular diseases. Life Sci. 2015, 121, 97–103. [Google Scholar] [CrossRef]
- Jelinek, M.; Wallach, C.; Ehmke, H.; Schwoerer, A.P. Genetic background dominates the susceptibility to ventricular arrhythmias in a murine model of β-adrenergic stimulation. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Mikušová, A.; Kráľová, E.; Tylková, L.; Novotová, M.; Stankovičová, T. Myocardial remodelling induced by repeated low doses of isoproterenol. Can. J. Physiol. Pharmacol. 2009, 87, 641–651. [Google Scholar] [CrossRef]
- Seidel, T.; Salameh, A.; Dhein, S. A simulation study of cellular hypertrophy and connexin lateralization in cardiac tissue. Biophys. J. 2010, 99, 2821–2830. [Google Scholar] [CrossRef]
- Dhein, S.; Gaertner, C.; Georgieff, C.; Salameh, A.; Schlegel, F.; Mohr, F.W. Effects of isoprenaline on endothelial connexins and angiogenesis in a human endothelial cell culture system. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 101–108. [Google Scholar] [CrossRef]
- Salameh, A.; Dhein, S. Pharmacology of Gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim. Biophys. Acta (BBA)-Biomembr. 2005, 1719, 36–58. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, J.; Wei, B.; Wang, Y. CaMKII inhibition reduces isoproterenol-induced ischemia and arrhythmias in hypertrophic mice. Oncotarget 2017, 8, 17504. [Google Scholar] [CrossRef]
- Peters, N.S. New insights into myocardial arrhythmogenesis: Distribution of gap-junctional coupling in normal, ischaemic and hypertrophied human hearts. Clin. Sci. 1996, 90, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Tribulova, N.; Novakova, S.; Macsaliova, A.; Sass, S.; Thomas, S.; Goetzfried, S.; Podzuweit, T.; Manoach, M. Histochemical and ultrastructural characterisation of an arrhythmogenic substrate in ischemic pig heart. Acta Histochem. 2002, 104, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Escobar, G.P.; Mukherjee, R.; Goshorn, D.K.; Sheats, N.J.; Bruce, J.A.; Mains, I.M.; Hendrick, J.K.; Hewett, K.W.; Gourdie, R.G.; et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 2006, 113, 2919–2928. [Google Scholar] [CrossRef]
- Fialová, M.; Dlugošová, K.; Okruhlicová, L.; Kristek, F.; Manoach, M.; Tribulová, N. Adaptation of the heart to hypertension is associated with maladaptive gap junction connexin-43 remodeling. Physiol. Res. 2008, 57, 7–11. [Google Scholar]
- Salameh, A.; Dhein, S. Adrenergic control of cardiac gap junction function and expression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 331–346. [Google Scholar] [CrossRef]
- Saffitz, J.E.; Kléber, A.G. Gap junctions, slow conduction, and ventricular tachycardia after myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.L.; Clymer, B.D.; Billman, G.E. Myocardial electrotonic response to submaximal exercise in dogs with healed myocardial infarctions: Evidence for β-adrenoceptor mediated enhanced coupling during exercise testing. Front. Physiol. 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Karl, S.; Djilali, H.; Dhein, S.; Janousek, J.; Daehnert, I. Opposing and synergistic effects of cyclic mechanical stretch and α- or β-adrenergic stimulation on the cardiac gap junction protein Cx43. Pharmacol. Res. 2010, 62, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G.; Service, M.; Einstein, A. The Biological Basis for Cardiac Repair after Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2017, 119, 91–112. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Díaz-Araya, G.; Chiong, M.; Muñoz, D.; Riveros, J.P.; Ebensperger, R.; Sabat, S.; Irarrázaval, P.; Jalil, J.E.; Lavandero, S. Isoproterenol and angiotensin I-converting enzyme in lung, left ventricle, and plasma during myocardial hypertrophy and fibrosis. J. Cardiovasc. Pharmacol. 2002, 40, 246–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, J.; Ma, S.Y.; Ding, C.H. hua Curcumin reduces cardiac fibrosis by inhibiting myofibroblast differentiation and decreasing transforming growth factor β1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinase 1. Chin. J. Integr. Med. 2017, 23, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Angert, D.; Berretta, R.M.; Kubo, H.; Zhang, H.; Chen, X.; Wang, W.; Ogorek, B.; Barbe, M.; Houser, S.R. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ. Res. 2011, 108, 1226–1237. [Google Scholar] [CrossRef]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef]
- Tribulova, N.; Szeiffova Bacova, B.; Egan Benova, T.; Knezl, V.; Barancik, M.; Slezak, J. Omega-3 index and anti-arrhythmic potential of omega-3 PUFAs. Nutrients 2017, 9, 1191. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Oliveira, T.L.; Castro, M.C.M.; Almeida, A.P.; Castro, C.H.; Caliari, M.V.; Gava, E.; Kitten, G.T.; Santos, R.A.S. Isoproterenol-induced impairment of heart function and remodeling are attenuated by the nonpeptide angiotensin-(1-7) analogue AVE 0991. Life Sci. 2007, 81, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, B.; de las Heras, N.; Miana, M.; Ballesteros, S.; Valero-Muñoz, M.; Vassallo, D.; Davel, A.P.; Rossoni, L.V.; Cachofeiro, V.; Lahera, V. Spironolactone prevents alterations associated with cardiac hypertrophy produced by isoproterenol in rats: Involvement of serum- and glucocorticoid-regulated kinase type 1. Exp. Physiol. 2012, 97, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Folino, A.; Sprio, A.E.; Di Scipio, F.; Berta, G.N.; Rastaldo, R. Alpha-linolenic acid protects against cardiac injury and remodelling induced by beta-adrenergic overstimulation. Food Funct. 2015, 6, 2231–2239. [Google Scholar] [CrossRef]
- Gourdie, R.G.; Dimmeler, S.; Kohl, P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat. Rev. Drug Discov. 2016, 15, 620–638. [Google Scholar] [CrossRef] [PubMed]
- Nardo, L.; Rezzani, R.; Facchetti, L.; Favero, G.; Franco, C.; Abdelhafez, Y.G.; Badawi, R.D.; Guindani, M.; Seo, Y.; Pampaloni, M. Beneficial Effects of Melatonin on Apolipoprotein-E Knockout Mice by Morphological and 18F-FDG PET/CT Assessments. Int. J. Mol. Sci. 2020, 21, 2920. [Google Scholar] [CrossRef] [PubMed]
- Panasiuk, O.S.; Shysh, A.M.; Moĭbenko, O.O. The influence of dietary omega-3 polyunsaturated fatty acids on functional parameters of myocardial mitochondria during isoproterenol-induced heart injury. Fiziolohichnyi Zhurnal (Kiev Ukraine 1994) 2014, 60, 18–24. [Google Scholar] [CrossRef]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Huang, C.-C.; Liu, S.-C.; Tang, C.-H. Reconsidering the Role of Melatonin in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 8, 2877. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.R.; Dolmatova, E.; Tan, A.; Duffy, H.S. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Front. Physiol. 2012, 3, 272. [Google Scholar] [CrossRef] [PubMed]
- Bačová, B.; Viczenczová, C.; Žurmanová, J.; Kašparová, D.; Knezl, V.; Radošinská, J.; Beňová, T.; Pavelka, S.; Soukup, T.; Tribulová, N. Susceptibility of rats with altered thyroid status to malignant arrhythmias is primarily related to myocardial levels of connexin-43 and can be partially ameliorated by supplementation with red palm oil. Exp. Cardiol. 2013, 18, 41–46. [Google Scholar]
- Prado, N.J.; Muñoz, E.M.; Farias Altamirano, L.E.; Aguiar, F.; Ponce Zumino, A.Z.; Sánchez, F.J.; Miatello, R.M.; Pueyo, E.; Diez, E.R. Reperfusion Arrhythmias Increase after Superior Cervical Ganglionectomy Due to Conduction Disorders and Changes in Repolarization. Int. J. Mol. Sci. 2020, 21, 1804. [Google Scholar] [CrossRef]

| Antibody | Dilution | Host | Type | Supplier/# Catalogue |
|---|---|---|---|---|
| anti-Cx43 | 1:5000 | Rabbit | Polyclonal | Sigma-Aldrich, St.Louis, MO, USA, #C6219 |
| anti-phospho-serine 368-Cx43 | 1:1000 | Rabbit | Polyclonal | Santa Cruz Biotechnology, Dallas, TX, USA, #sc-101660 |
| anti-PKC-epsilon | 1:2000 | Rabbit | Polyclonal | Santa Cruz Biotechnology, Dallas, TX, USA, #sc-214 |
| anti-TGF-β | 1:1000 | Rabbit | Polyclonal | Sigma-Aldrich, St.Louis, MO, USA, #SAB4502954 |
| anti-SMAD2/3 | 1:1000 | Rabbit | Polyclonal | Cell Signaling Technology, Danvers, MA, USA, #3102 |
| anti-Collagen I | 1:1000 | Rabbit | Polyclonal | Abcam Inc.,Toronto, ON, Canada, #ab34710 |
| anti-Keap1 | 1:500 | Goat | Polyclonal | Abcam Inc.,Toronto, ON, Canada, #ab118285 |
| anti-SOD-1 | 1:1000 | Rabbit | Polyclonal | Santa Cruz Biotechnology, Dallas, TX, USA, #sc-11407 |
| anti-SOD-2 | 1:1000 | Rabbit | Polyclonal | Santa Cruz Biotechnology, Dallas, TX, USA, #sc-30080 |
| anti-GAPDH | 1:1000 | Rabbit | polyclonal | Santa Cruz Biotechnology, Dallas, TX, USA#sc-25778 |
| Variables | W | Wi | Wim | Wio | S | Si | Sim | Sio |
|---|---|---|---|---|---|---|---|---|
| BP (mmHg) | 114 ± 17 | 118 ± 11 | 120 ± 25 | 140 ± 19 | 203 ± 24 * | 190 ± 16 | 219 ± 41 | 185 ± 25 |
| BW (g) | 360 ± 18 | 359 ± 17 | 331 ± 36 * | 369 ± 31 | 307 ± 29 * | 322 ± 20 | 313 ± 17 | 313 ± 20 |
| HW (g) | 0.95 ± 0.26 | 1.26 ± 0.23 * | 0.96 ± 0.15 • | 1.02± 0.15 • | 1.33± 0.15 * | 1.40 ± 0.20 | 1.34 ± 0.21 | 1.24 ± 0.14 |
| LVW (g) | 0.72 ± 0.09 | 0,95 ± 0.22 * | 0.72 ± 0.07 • | 0.73 ± 0.13 • | 1.00 ± 0.14 * | 1.08 ± 0.14 | 0.96 ± 0.18 | 0.84 ± 0.26 • |
| EPI (g) | 5.15 ± 0.40 | 5.17 ± 0.82 | 4.64 ± 1.32 | 5.27 ± 0.77 | 3.38 ± 0.34 * | 3.19 ± 0.63 | 2.99 ± 0.53 | 2.98 ± 0.26 |
| RET (g) | 3.18 ± 0.61 | 3.43 ± 0.67 | 3.02 ± 0.98 | 3.99 ± 1.10 | 4.01 ± 0.76 | 3.39 ± 1.04 | 2.91 ± 0.49 # | 3.15 ± 0.42 |
| EPI/BW (%) | 100 ± 7.5 | 101 ± 16 | 97 ± 25 | 100 ± 12 | 78 ± 10 * | 70 ± 14 | 67 ± 13 | 67 ± 6.0 |
| RET/BW (%) | 100 ± 20 | 108 ± 22 | 102 ± 27 | 123 ± 34 | 149 ± 32 * | 118 ± 31 # | 106 ± 18 # | 114 ± 16 # |
| Variables | W | Wi | Wim | Wio | Sc | Si | Sim | Sio |
|---|---|---|---|---|---|---|---|---|
| HR (beats/min) | 261 ± 33 | 254 ± 37 | 259 ± 5 | 222 ± 33 | 252 ± 18 | 256 ± 50 | 247 ± 22 | 234 ± 35 |
| CF (mL/min) | 9.90 ± 0.79 | 15.77 ± 3.21 * | 14.20 ± 4.80 | 15.47 ± 4.43 * | 12.63 ± 0.89 | 19.19 ± 1.08 # | 16.65 ± 0.99 | 13.9 ± 0.14 • |
| LVP (mmHg) | 14.07 ± 3.27 | 15.77 ± 3.21 | 16.77 ± 3.55 | 16.14 ± 2.49 | 13.98 ± 2.25 | 21.07 ± 3.46 # | 17.84 ± 3.08 | 18.23 ± 1.92 |
| +(dP/dt)max | 327 ± 15 | 470 ± 127 * | 458 ± 88 | 497 ± 8 * | 291 ± 13 | 604 ± 75 # | 564 ± 85 # | 630 ± 81 # |
| −(dP/dt)max | 222 ± 6 | 246 ± 74 | 253 ± 2 | 260 ± 35 | 199 ± 59 | 328 ± 39 # | 314 ± 40 # | 364 ± 35 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeiffova Bacova, B.; Viczenczova, C.; Andelova, K.; Sykora, M.; Chaudagar, K.; Barancik, M.; Adamcova, M.; Knezl, V.; Egan Benova, T.; Weismann, P.; et al. Antiarrhythmic Effects of Melatonin and Omega-3 Are Linked with Protection of Myocardial Cx43 Topology and Suppression of Fibrosis in Catecholamine Stressed Normotensive and Hypertensive Rats. Antioxidants 2020, 9, 546. https://doi.org/10.3390/antiox9060546
Szeiffova Bacova B, Viczenczova C, Andelova K, Sykora M, Chaudagar K, Barancik M, Adamcova M, Knezl V, Egan Benova T, Weismann P, et al. Antiarrhythmic Effects of Melatonin and Omega-3 Are Linked with Protection of Myocardial Cx43 Topology and Suppression of Fibrosis in Catecholamine Stressed Normotensive and Hypertensive Rats. Antioxidants. 2020; 9(6):546. https://doi.org/10.3390/antiox9060546
Chicago/Turabian StyleSzeiffova Bacova, Barbara, Csilla Viczenczova, Katarina Andelova, Matus Sykora, Kiranj Chaudagar, Miroslav Barancik, Michaela Adamcova, Vladimir Knezl, Tamara Egan Benova, Peter Weismann, and et al. 2020. "Antiarrhythmic Effects of Melatonin and Omega-3 Are Linked with Protection of Myocardial Cx43 Topology and Suppression of Fibrosis in Catecholamine Stressed Normotensive and Hypertensive Rats" Antioxidants 9, no. 6: 546. https://doi.org/10.3390/antiox9060546
APA StyleSzeiffova Bacova, B., Viczenczova, C., Andelova, K., Sykora, M., Chaudagar, K., Barancik, M., Adamcova, M., Knezl, V., Egan Benova, T., Weismann, P., Slezak, J., & Tribulova, N. (2020). Antiarrhythmic Effects of Melatonin and Omega-3 Are Linked with Protection of Myocardial Cx43 Topology and Suppression of Fibrosis in Catecholamine Stressed Normotensive and Hypertensive Rats. Antioxidants, 9(6), 546. https://doi.org/10.3390/antiox9060546






