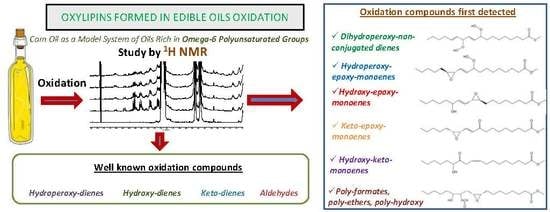
Oxylipins Associated to Current Diseases Detected for the First Time in the Oxidation of Corn Oil as a Model System of Oils Rich in Omega-6 Polyunsaturated Groups. A Global, Broad and in-Depth Study by 1H NMR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Subject of Study
2.1.1. Original Oil
2.1.2. Oxidized Oil Samples
2.2. Acquisition of the 1H Nuclear Magnetic Resonance Spectra (1H NMR)
2.2.1. Operating Conditions
2.2.2. Identification of Components
2.2.3. Quantification of the Components
2.3. Statistical Analysis
3. Results and Discussion
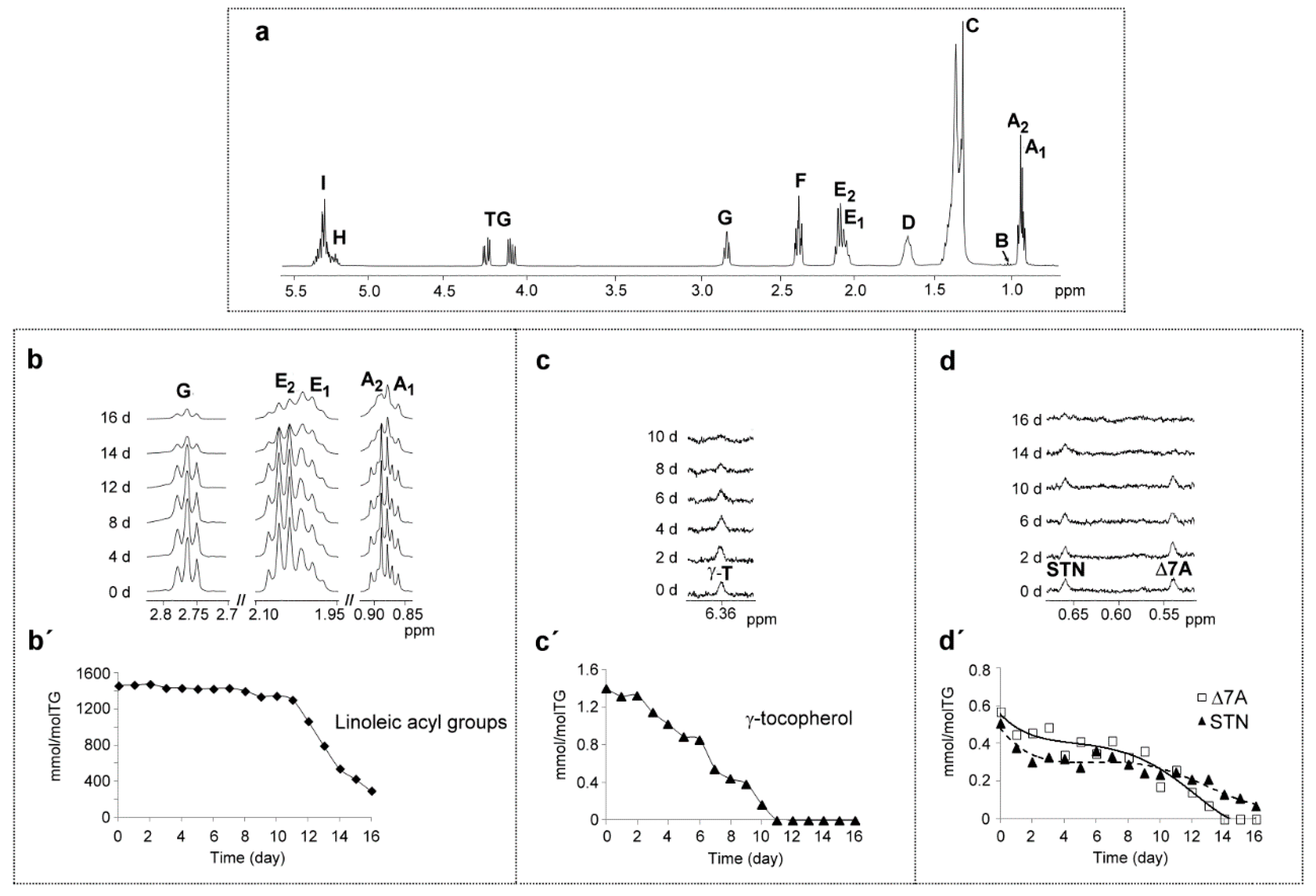
3.1. Evolution of Original Oil Components
3.1.1. Corn Oil Main Components
3.1.2. Corn Oil Minor Components
(a) Regarding Tocopherols
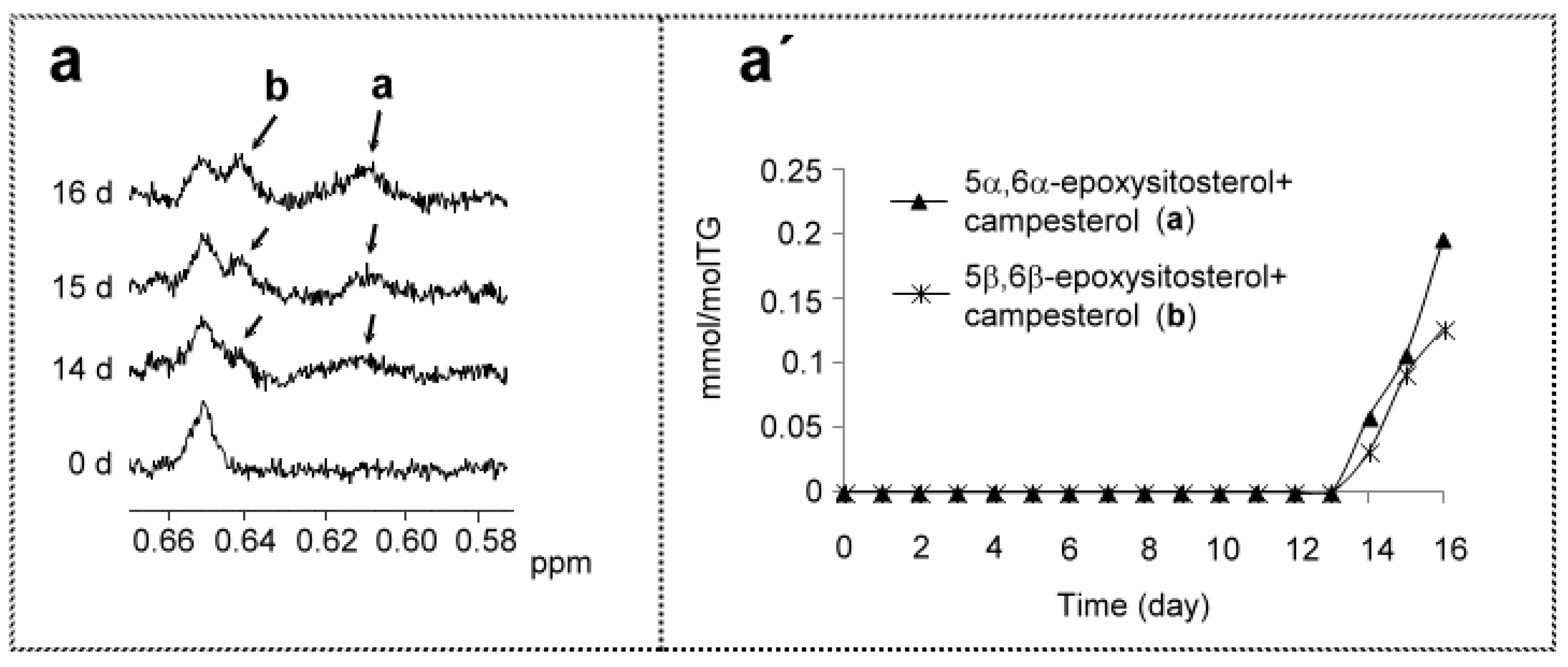
(b) Regarding Sterols-Stanols
3.2. Formation of New Compounds
3.2.1. Compounds Derived from Corn Oil Main Components
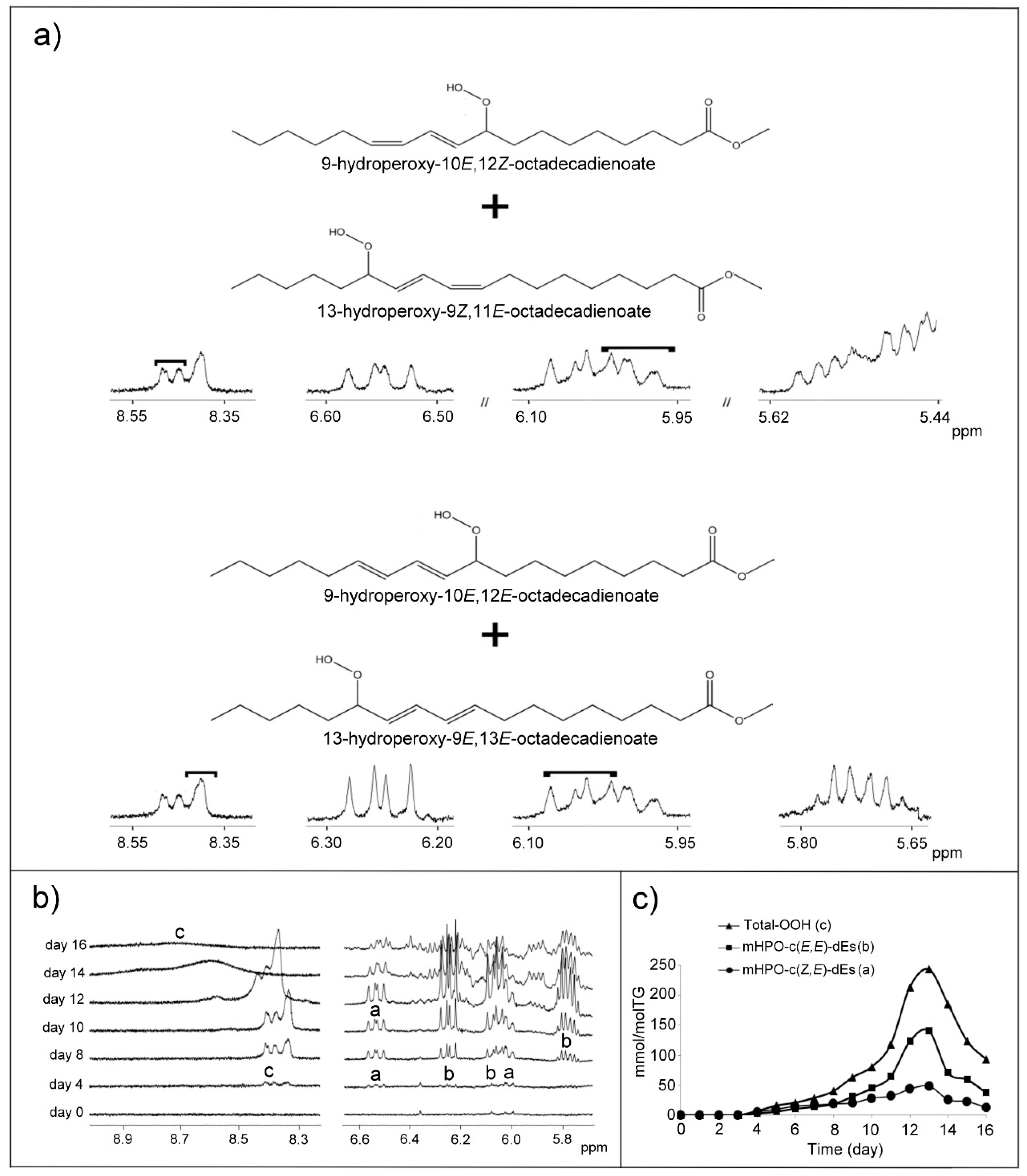
(a) Monohydroperoxides (mHPOs)
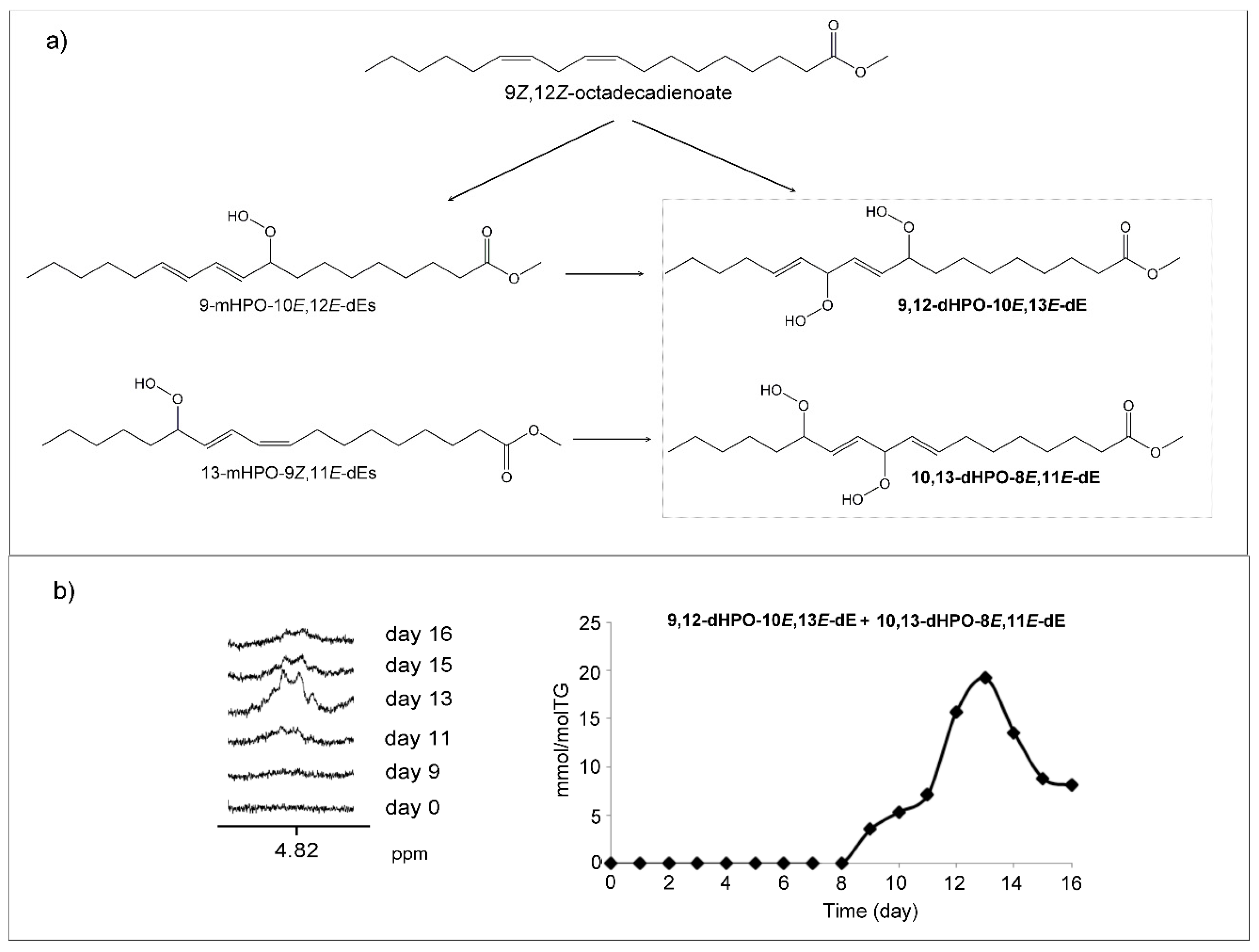
(b) Dihydroperoxides (dHPOs)
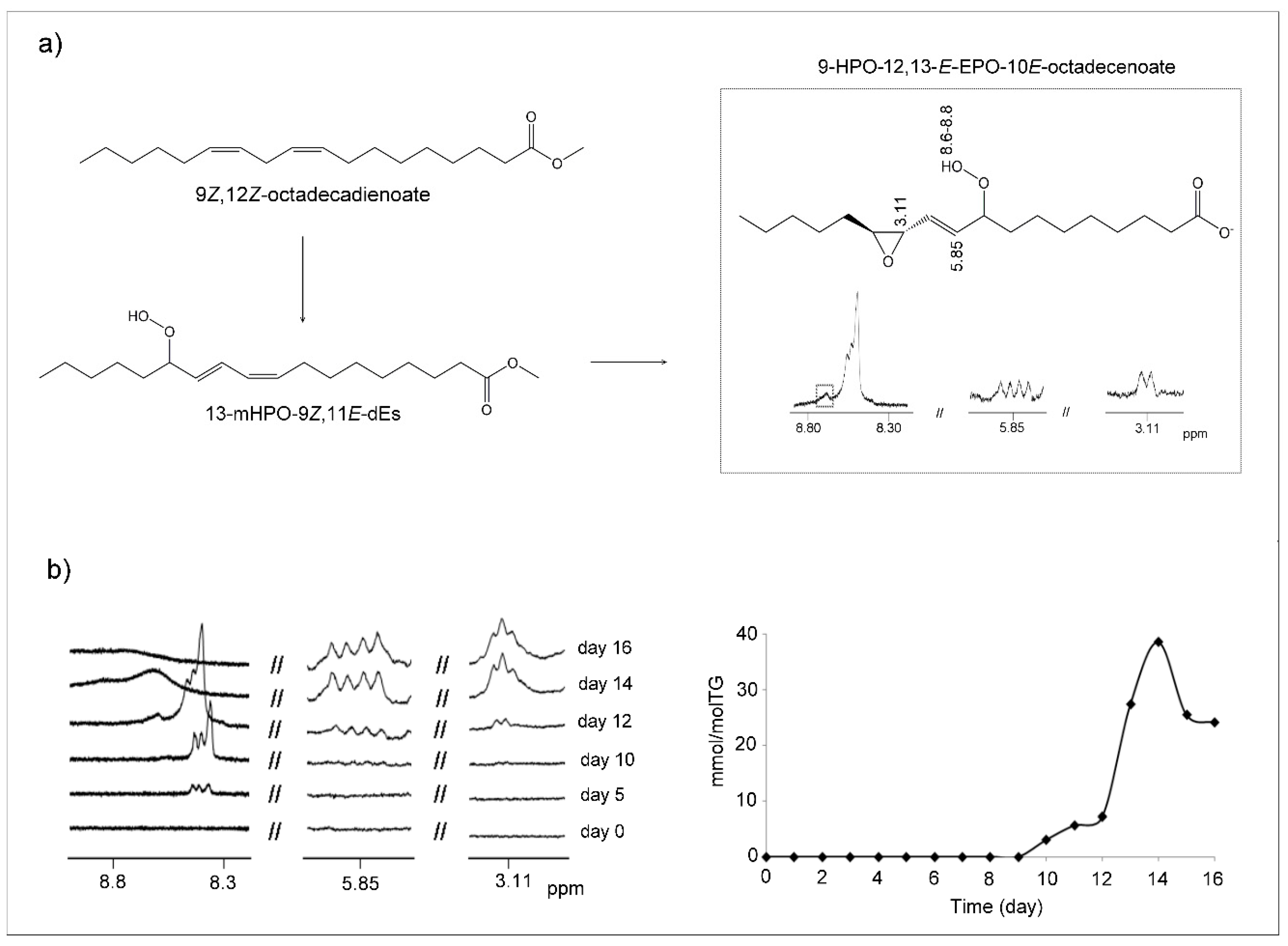
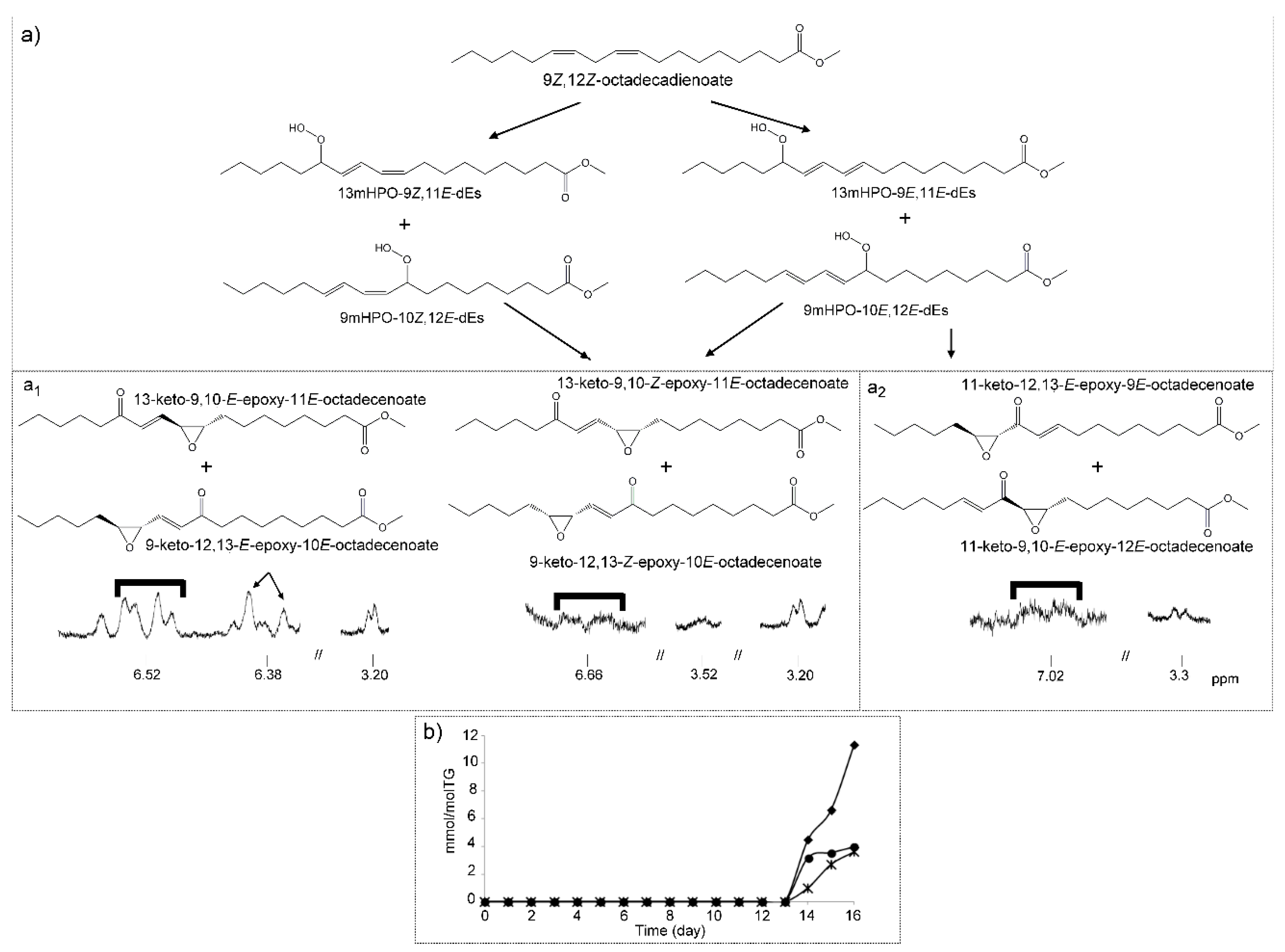
(c) Hydroperoxy-Epoxy-Monoenes (HPO-EPO-mEs)
(d) Monohydroxy-Conjugated Dienes (mHO-c-dEs)
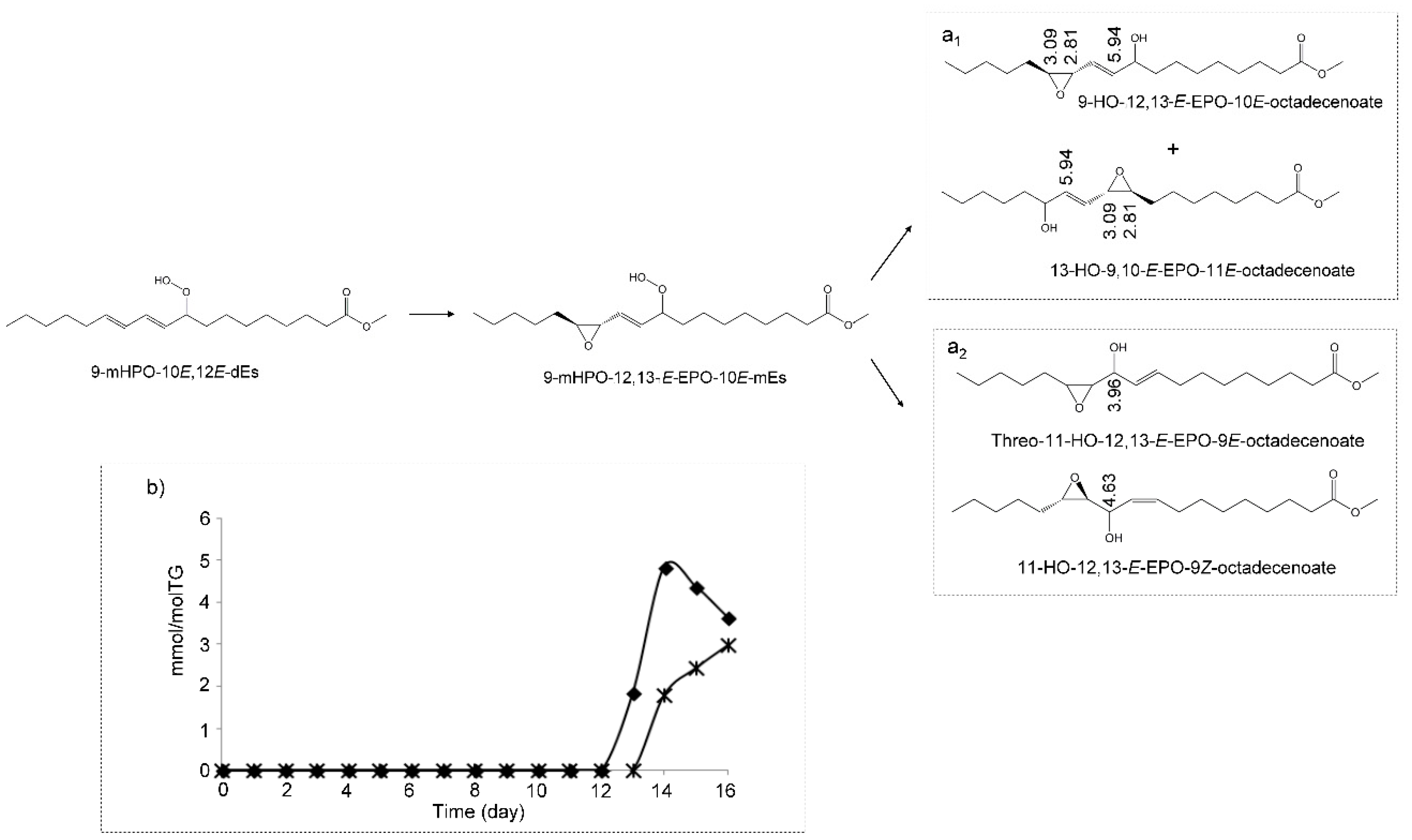
(e) Hydroxy-Epoxy-Monoenes (HO-EPO-mEs)
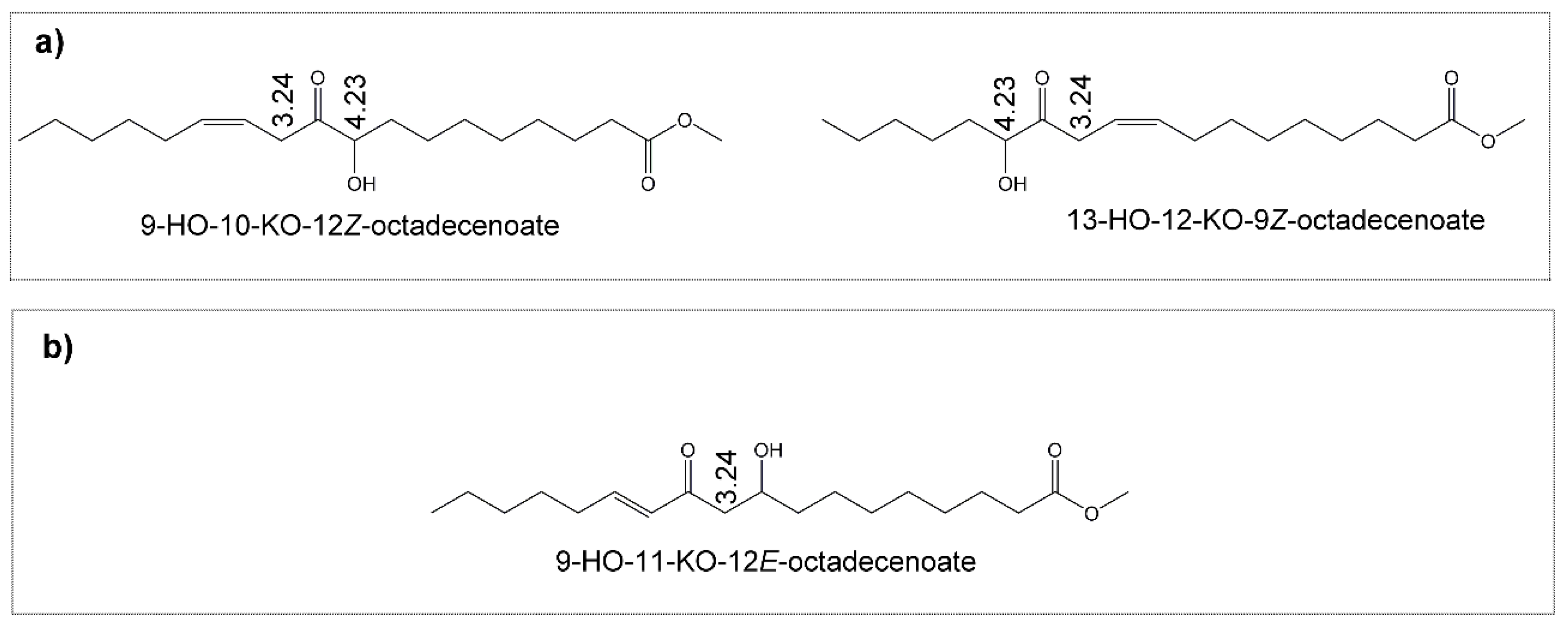
(f) Hydroxy-Keto-Monoenes (HO-KO-mEs)
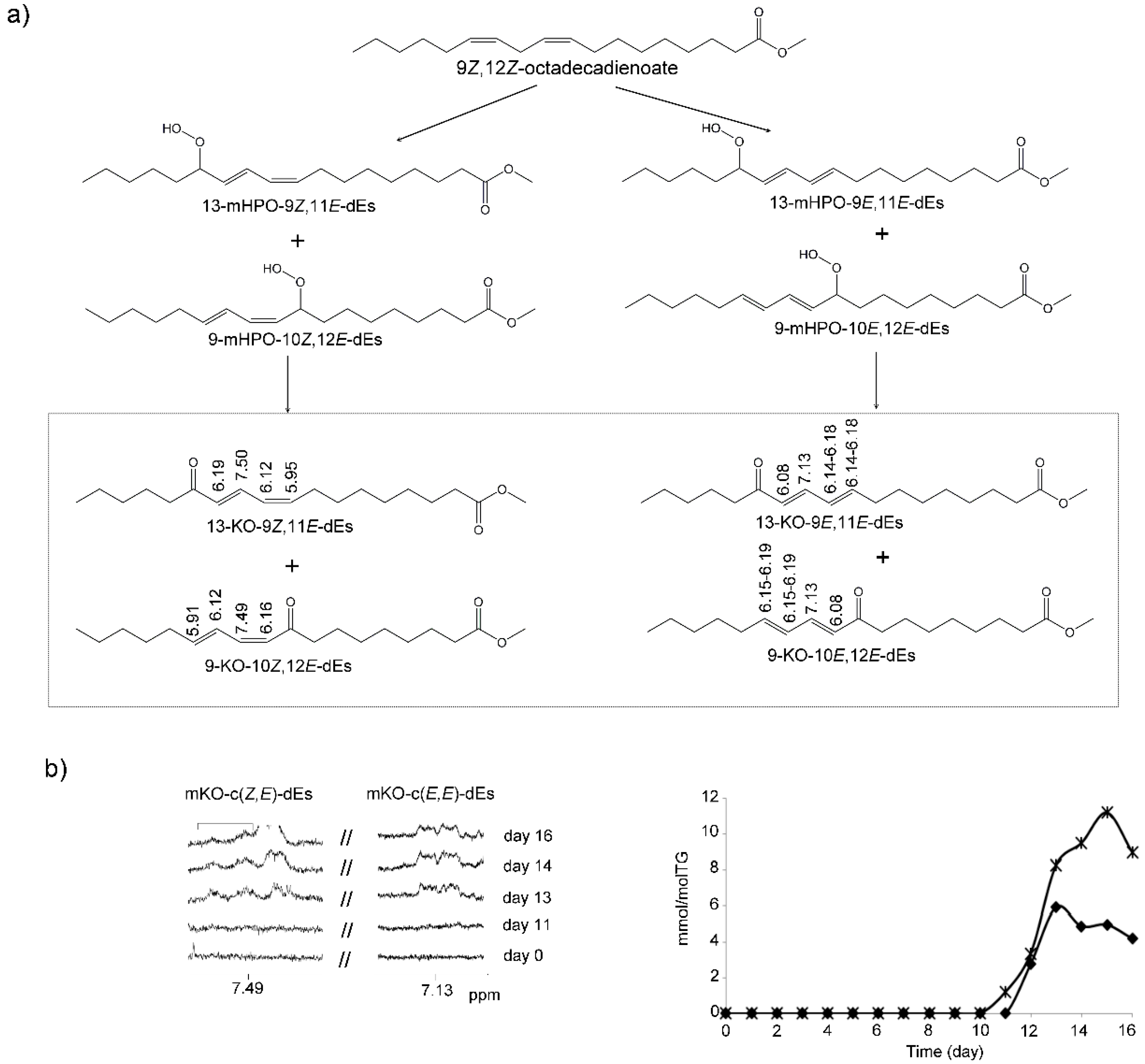
(g) Monoketo-Conjugated Dienes (mKO-c-dEs)
(h) Keto-Epoxy-Monoenes (KO-EPO-mEs)
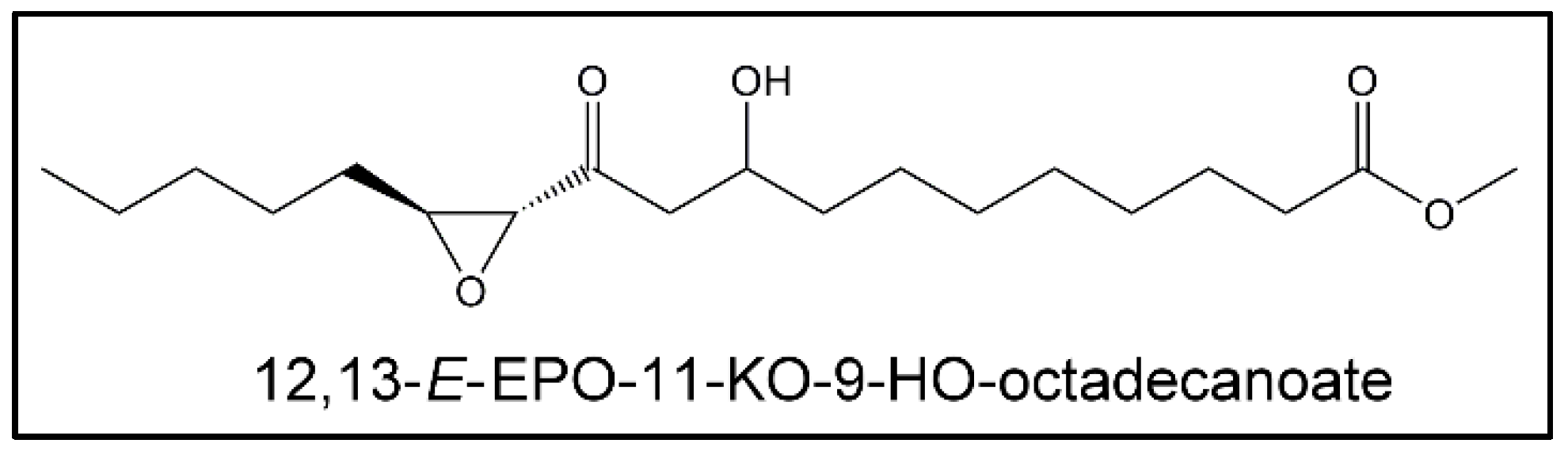
(i) Epoxy-Keto-Hydroxy Derivatives (EPO-KO-HOs)
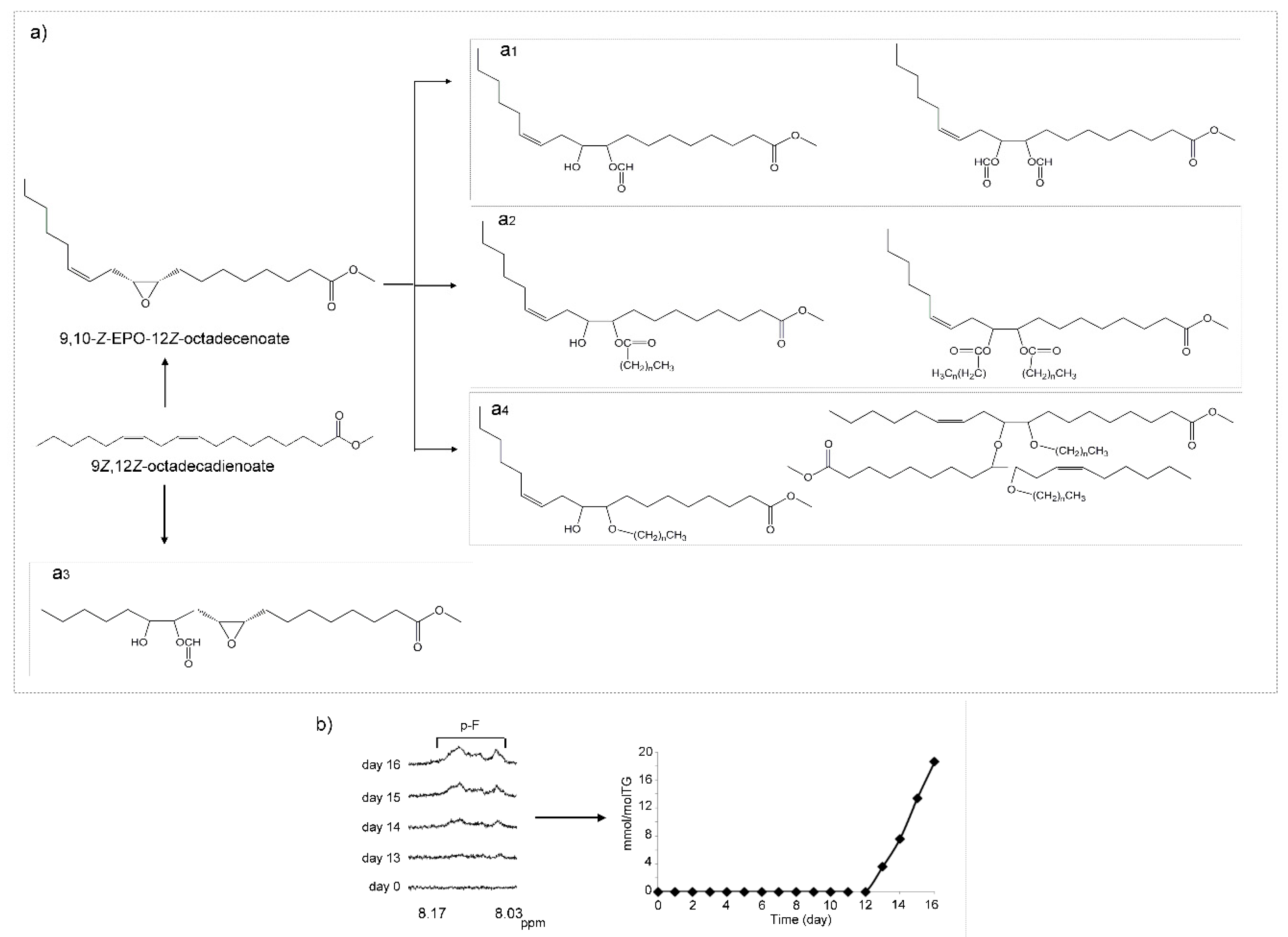
(j) Monoepoxy-Monoenes (mEPO-mEs) and Diepoxy (dEPO) Structures
(k) Dihydroxy (dHO) and/or Polyhydroxy (pHO) Structures
(l) Acids and Formic Acid
(m) Poly-Formate (pF), Poly-Ester (pEst) and Poly-Hydroxy (pHO) Structures
(n) Poly-Ether (pEt) and Poly-Hydroxy (pHO) Structures
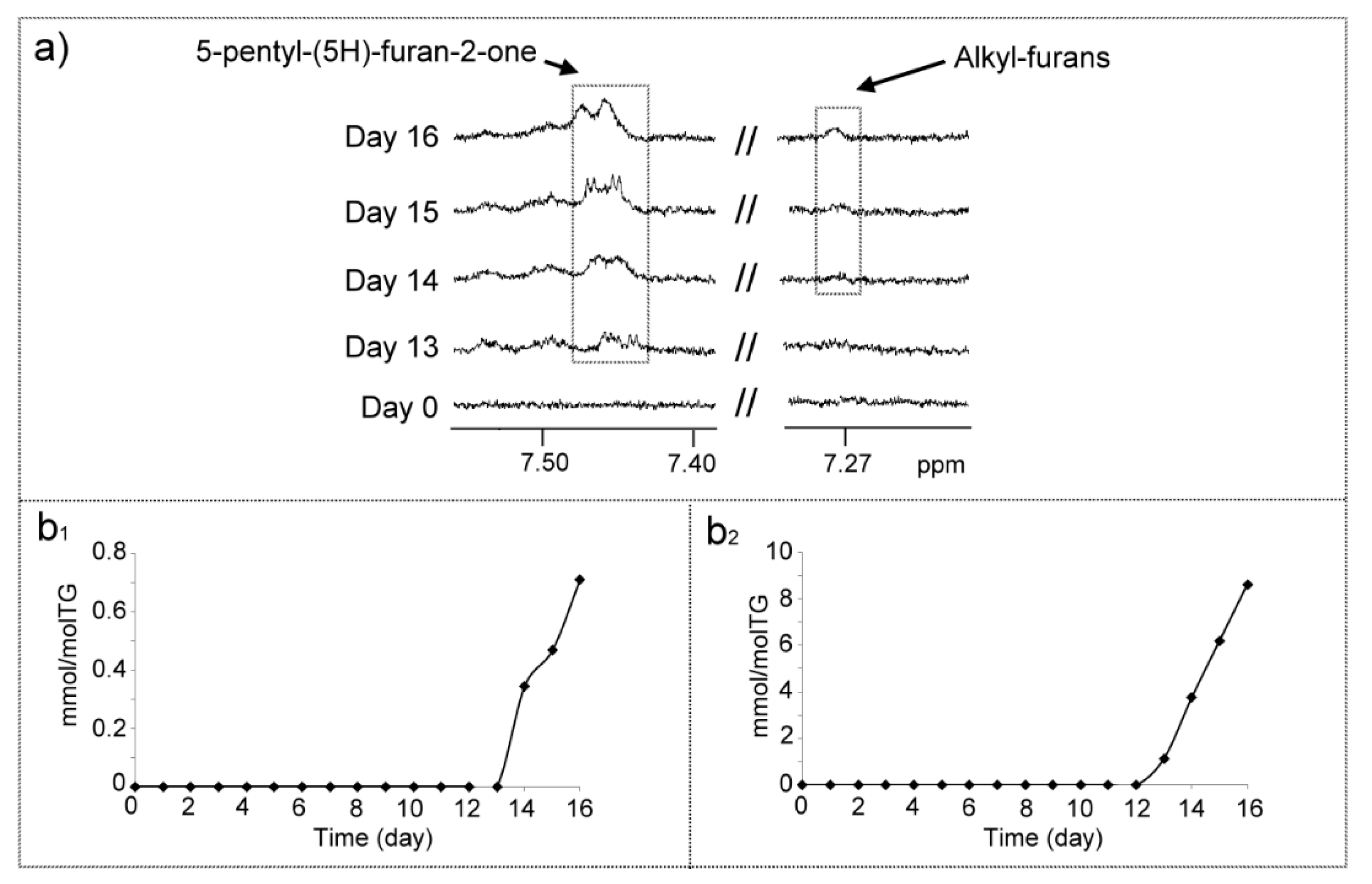
(o) Structures Supporting Furan Ring (Frs)
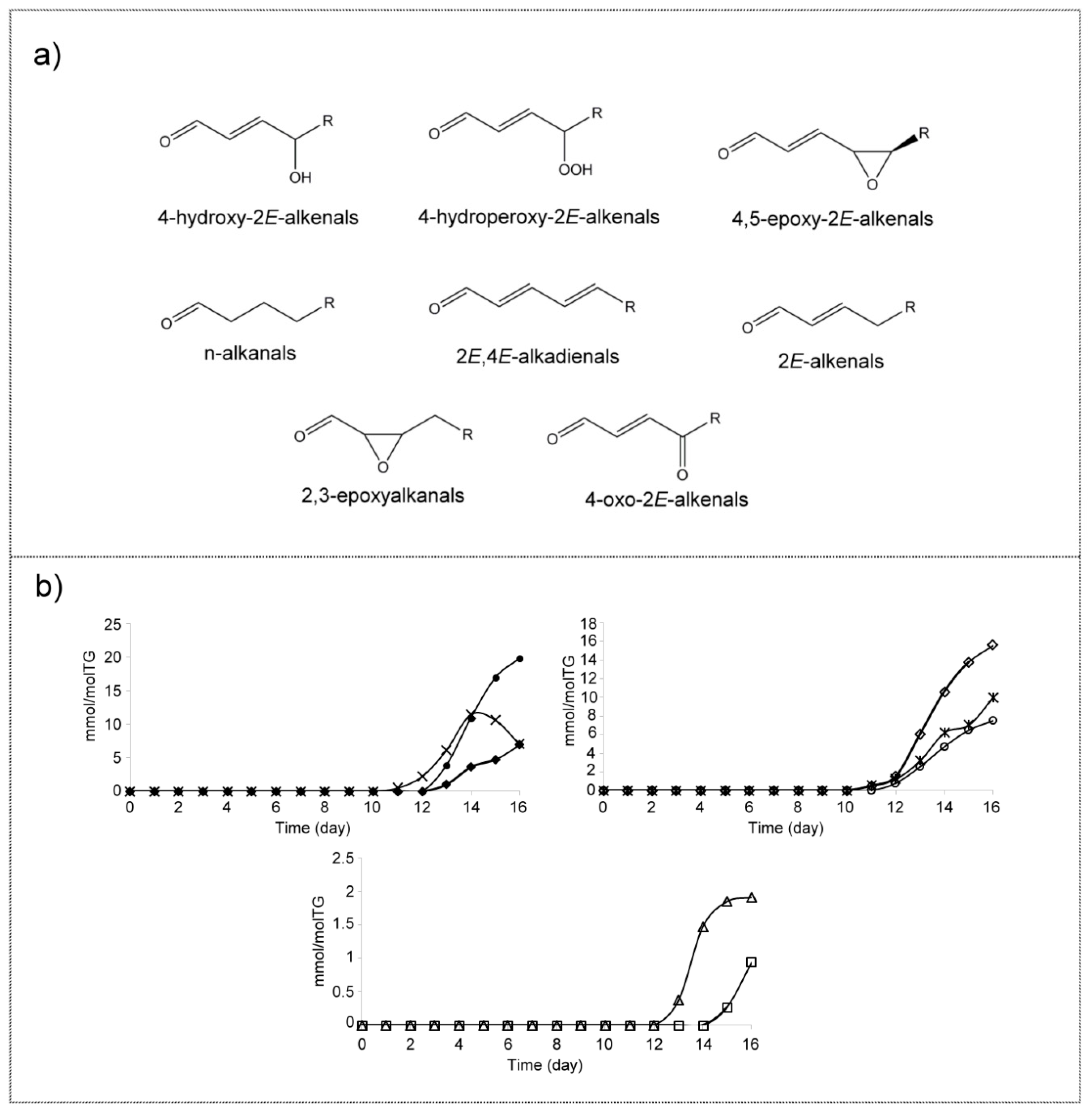
(p) Aldehydes (A)
3.2.2. Compounds Derived from Corn Oil Minor Components
3.3. View of the Evolution over Time of the Oxidation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guillén, M.D.; Ruiz, A. Formation of hydroperoxy-and hydroxyalkenals during thermal oxidative degradation of sesame oil monitored by proton NMR. Eur. J. Lipid Sci. Technol. 2004, 106, 680–687. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Monitoring the oxidation of unsaturated oils and formation of oxygenated aldehydes by proton NMR. Eur. J. Lipid Sci. Technol. 2005, 107, 36–47. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Oxidation process of oils with high content of linoleic acyl groups and formation of toxic hydroperoxy-and hydroxyalkenals. A study by 1H nuclear magnetic resonance. J. Sci. Food Agric. 2005, 85, 2413–2420. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Study by proton nuclear magnetic resonance of the thermal oxidation of oils rich in oleic acyl groups. J. Am. Oil Chem. Soc. 2005, 82, 349–355. [Google Scholar] [CrossRef]
- Goicoechea, E.; Guillén, M.D. Analysis of hydroperoxides, aldehydes and epoxides by 1H nuclear magnetic resonance in sunflower oil oxidized at 70 and 100 C. J. Agric. Food Chem. 2010, 58, 6234–6245. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α, β unsaturated aldehydes. Food Chem. 2012, 131, 915–926. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A review of thermo-oxidative degradation of food lipids studied by 1H NMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Vidal, N.P.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. Influence of smoking with smoke flavorings on the oxidative stability of farmed sea bass fillets monitored by 1H NMR and FTIR. Eur. J. Lipid Sci. Technol. 2017, 119, 1600023. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. Prooxidant effect of α-tocopherol on soybean oil. Global monitoring of its oxidation process under accelerated storage conditions by 1H nuclear magnetic resonance. Food Chem. 2018, 245, 312–323. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Monitoring of minor compounds in corn oil oxidation by direct immersion-solid phase microextraction-gas chromatography/mass spectrometry. New oil oxidation markers. Food Chem. 2019, 290, 286–294. [Google Scholar] [CrossRef]
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000, 33, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Lapidot, T.; Shaham, I.; Granit, R.; Ligumsky, M.; Kohen, R.; Kanner, J. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: Health implications. J. Agric. Food Chem. 2005, 53, 3397–3402. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Food lipid oxidation under gastrointestinal digestion conditions: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Effect of the Enrichment of Corn Oil With alpha- or gamma-Tocopherol on Its In Vitro Digestion Studied by 1H NMR and SPME-GC/MS.; Formation of Hydroperoxy-, Hydroxy-, Keto-Dienes and Keto-E-epoxy-E-Monoenes in the More alpha-Tocopherol Enriched Samples. Antioxidants 2020, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Leonarduzzi, G.; Chiarpotto, E.; Biasi, F.; Poli, G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol. Nutr. Food Res. 2005, 49, 1044–1049. [Google Scholar] [CrossRef]
- Catalá, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Schaich, K.M. Lipid oxidation: Theoretical aspects. Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 1, pp. 269–356. [Google Scholar]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4–hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Seppanen, C.M.; Csallany, A.S. Incorporation of the toxic aldehyde 4-hydroxy-2-trans-nonenal into food fried in thermally oxidized soybean oil. J. Am. Oil Chem. Soc. 2004, 81, 1137–1141. [Google Scholar] [CrossRef]
- Guillén, M.D.; Goicoechea, E. Formation of oxygenated α, β-unsaturated aldehydes and other toxic compounds in sunflower oil oxidation at room temperature in closed receptacles. Food Chem. 2008, 111, 157–164. [Google Scholar] [CrossRef]
- Guillén, M.D.; Goicoechea, E. Toxic oxygenated α, β-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Han, I.H.; Csallany, A.S. Formation of toxic α,β-unsaturated 4-hydroxy-aldehydes in thermally oxidized fatty acid methyl esters. J. Am. Oil Chem. Soc. 2009, 86, 253–260. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Domenichiello, A.F.; Yuan, Z.X.; Sapio, M.R.; Keyes, G.S.; Mishra, S.K.; Gross, J.R.; Majchrzak-Hong, S.; Zamora, D.; Horowitz, M.S.; et al. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci. Signal. 2017, 10, eaal5241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillén, M.D.; Cabo, N. Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils. Food Chem. 2002, 77, 503–510. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Boloor, K.K.; Ramasarma, T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J. Biochem. Biol. 2003, 40, 300–308. [Google Scholar]
- Frankel, E.N. Recent advances in lipid oxidation. J. Sci. Food Agric. 1991, 54, 495–511. [Google Scholar] [CrossRef]
- Steenhorst-Slikkerveer, L.; Louter, A.; Janssen, H.G.; Bauer-Plank, C. Analysis of nonvolatile lipid oxidation products in vegetable oils by normal-phase high-performance liquid chromatography with mass spectrometric detection. J. Am. Oil Chem. Soc. 2000, 77, 837. [Google Scholar] [CrossRef]
- Byrdwell, W.C.; Neff, W.E. Electrospray ionization MS of high MW TAG oligomers. J. Am. Oil Chem. Soc. 2004, 81, 13–26. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N.; Ibargoitia, M.L.; Ruiz, A. Study of both sunflower oil and its headspace throughout the oxidation process. Occurrence in the headspace of toxic oxygenated aldehydes. J. Agric. Food Chem. 2005, 53, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Dobarganes, M.C.; Márquez Ruiz, G. Formation and analysis of oxidized monomeric, dimeric, and higher oligomeric triglycerides. In Deep Frying; Erickson, M.D., Ed.; AOCS Press: Champaign, IL, USA, 2007; pp. 87–110. [Google Scholar]
- Velasco, J.; Morales-Barroso, A.; Ruiz-Méndez, M.V.; Márquez-Ruiz, G. Quantitative determination of major oxidation products in edible oils by direct NP-HPLC-DAD analysis. J. Chromatogr. A 2018, 1547, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Ruiz, A. High resolution 1H nuclear magnetic resonance in the study of edible oils and fats. Trends Food Sci. Technol. 2001, 12, 328–338. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Uriarte, P.S.; Guillén, M.D. Formation of toxic alkylbenzenes in edible oils submitted to frying temperature: Influence of oil composition in main components and heating time. Food Res. Int. 2010, 43, 2161–2170. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. A thorough insight into the complex effect of gamma-tocopherol on the oxidation process of soybean oil by means of 1H Nuclear Magnetic Resonance. Comparison with alpha-tocopherol. Food Res. Int. 2018, 114, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Ruiz, A. 1H nuclear magnetic resonance as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. Eur. J. Lipid Sci. Technol. 2003, 105, 502–507. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Contribution to further understanding of the evolution of sunflower oil submitted to frying temperature in a domestic fryer: Study by 1H nuclear magnetic resonance. J. Agric. Food Chem. 2009, 57, 7790–7799. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Cristillo, G.; Sopelana, P.; Guillén, M.D. A new methodology capable of characterizing most volatile and less volatile minor edible oils components in a single chromatographic run without solvents or reagents. Detection of new components. Food Chem. 2017, 221, 1135–1144. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Bioactive compounds detected for the first time in corn oil: Cyclic dipeptides and other nitrogenated compounds. J. Food Compos. Anal. 2017, 62, 197–204. [Google Scholar] [CrossRef]
- Baker, J.K.; Myers, C.W. One-dimensional and two-dimensional 1H-and 13C-nuclear magnetic resonance (NMR) analysis of vitamin E raw materials or analytical reference standards. Pharm. Res. 1991, 8, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Ibargoitia, M.L.; Sopelana, P.; Guillén, M.D. 1H Nuclear Magnetic Resonance monitoring of the degradation of margarines of varied compositions when heated to high temperature. Food Chem. 2014, 165, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Sopelana, P.; Arizabaleta, I.; Ibargoitia, M.L.; Guillén, M.D. Characterisation of the lipidic components of margarines by 1H Nuclear Magnetic Resonance. Food Chem. 2013, 141, 3357–3364. [Google Scholar] [CrossRef]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct study of minor extra-virgin olive oil components without any sample modification. 1H NMR multisupression experiment: A powerful tool. Food Chem. 2017, 228, 301–314. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Detection of monohydroperoxides with unconjugated diene systems as minor products of the autoxidation of methyl linoleate Nachweis von Monohydroperoxiden mit unkonjugierten Dien-Systemen als Nebenprodukte der Autoxidation von Linolsauremethylester. Zeitschrift für Lebensmittel-Untersuchung Und Forschung 1981, 173, 199–203. [Google Scholar] [CrossRef]
- Haslbeck, F.; Grosch, W.; Firl, J. Formation of hydroperoxides with unconjugated diene systems during autoxidation and enzymic oxygenation of linoleic acid. BBA Lipid Lipid Met. 1983, 750, 185–193. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, M.; Salomon, R.G. Preparative singlet oxygenation of linoleate provides doubly allylic dihydroperoxides: Putative intermediates in the generation of biologically active aldehydes in vivo. J. Org. Chem. 2006, 71, 5607–5615. [Google Scholar] [CrossRef]
- Brash, A.R. Autoxidation of methyl linoleate: Identification of the bis-allylic 11-hydroperoxide. Lipids 2000, 35, 947–952. [Google Scholar] [CrossRef]
- Schneider, C.; Tallman, K.A.; Porter, N.A.; Brash, A.R. Two distinct pathways of formation of 4-hydroxynonenal mechanisms of nonenzymatic transformation of the 9-and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001, 276, 20831–20838. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Boeglin, W.E.; Yin, H.; Stec, D.F.; Hachey, D.L.; Porter, N.A.; Brash, A.R. Synthesis of dihydroperoxides of linoleic and linolenic acids and studies on their transformation to 4-hydroperoxynonenal. Lipids 2005, 40, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Synthesis and Fragmentation Reactions of Linoleic Acid-Derived Hydroperoxides. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, January 2008. [Google Scholar]
- Neff, W.E.; Frankel, E.N.; Selke, E.; Weisleder, D. Photosensitized oxidation of methyl linoleate monohydroperoxides: Hydroperoxy cyclic peroxides, dihydroperoxides, keto esters and volatile thermal decomposition products. Lipids 1983, 18, 868–876. [Google Scholar] [CrossRef]
- Gardner, H.W.; Weisleder, D.; Kleiman, R. Formation of trans-12,13-epoxy-9-hydroperoxy-trans-10-octadecenoic acid from 13-L-hydroperoxy-cis-9, trans-11-octadecadienoic acid catalyzed by either a soybean extract or cysteine-FeC13. Lipids 1978, 13, 246–252. [Google Scholar] [CrossRef]
- Schneider, C.; Boeglin, W.E.; Yin, H.; Porter, N.A.; Brash, A.R. Intermolecular peroxyl radical reactions during autoxidation of hydroxy and hydroperoxy arachidonic acids generate a novel series of epoxidized products. Chem. Res. Toxicol. 2008, 21, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, T.; Kasai, S.; Matsui, K.; Nakamura, T. Methyl hydroperoxy-epoxy-octadecenoate as an autoxidation product of methyl linoleate: A new inhibitor-uncoupler of mitochondrial respiration. J. Biochem. 1982, 92, 1109–1121. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nishikawa, Y.; Mori, H.; Tamura, H.; Matsushita, Y.I.; Matsui, T. Antitumor activity of (10E, 12Z)-9-hydroxy-10, 12-octadecadienoic acid from rice bran. J. Ferment. Bioeng. 1998, 86, 149–153. [Google Scholar] [CrossRef]
- Jira, W.; Spiteller, G.; Richter, A. Increased levels of lipid oxidation products in rheumatically destructed bones of patients suffering from rheumatoid arthritis. Zeitschrift für Naturforschung C 1998, 53, 1061–1071. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Christie, W.W.; Durand, T.; Rossi, J.C.; Vidal, J.P.; Kasyanov, S.P.; Akulin, V.N.; Bezuglov, V.V. Synthesis of keto-and hydroxydienoic compounds from linoleic acid. Chem. Phys. Lipids 1997, 85, 125–134. [Google Scholar] [CrossRef]
- Schieberle, P.; Trebert, Y.; Firl, J.; Grosch, W. Photolysis of unsaturated fatty acid hydroperoxides 4. Fatty acid products from the aerobic decomposition of methyl 13(S)-hydroperoxy-9(Z),11(E)-octadecadienoate dissolved in cyclohexane. Chem. Phys. Lipids 1988, 48, 281–288. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R.; Vioque, E. Syntheses and reactions of methyl (Z)-9,10-epoxy-13-oxo-(E)-11-octadecenoate and methyl (E)-9,10-epoxy-13-oxo-(E)-11-octadecenoate. Chem. Phys. Lipids 1992, 60, 225–233. [Google Scholar] [CrossRef]
- Gardner, H.W.; Kleiman, R.; Weisleder, D. Homolytic decomposition of linoleic acid hydroperoxide: Identification of fatty acid products. Lipids 1974, 9, 696–706. [Google Scholar] [CrossRef]
- Gardner, H.W.; Crawford, C.G. Degradation of linoleic acid hydroperoxides by a cysteine FeCl3 catalyst as a model for similar biochemical reactions: III. A novel product, trans-12,13-epoxy-11-oxo-trans-9-octadecenoic acid, from 13-L(S)-hydroperoxy-cis-9,trans-11-octadecadienoic acid. BBA Lipid Lipid Met. 1981, 665, 126–133. [Google Scholar] [CrossRef]
- Gardner, H.W.; Kleiman, R. Degradation of linoleic acid hydroperoxides by a cysteine FeCl3 catalyst as a model for similar biochemical reactions: II. Specificity in formation of fatty acid epoxides. BBA Lipid Lipid Met. 1981, 665, 113–125. [Google Scholar] [CrossRef]
- Doolen, S.; Keyes, G.S.; Ramsden, C.E. Hydroxy-epoxide and keto-epoxide derivatives of linoleic acid activate trigeminal neurons. Neurobiol. Pain 2020, 7, 100046. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W.; Kleiman, R.; Christianson, D.D.; Weisleder, D. Positional specificity of γ-ketol formation from linoleic acid hydroperoxides by a corn germ enzyme. Lipids 1975, 10, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhang, J.; Sayre, L.M. Synthesis of six epoxyketooctadecenoic acid (EKODE) isomers, their generation from nonenzymatic oxidation of linoleic acid, and their reactivity with imidazole nucleophiles. J. Org. Chem. 2007, 72, 9471–9480. [Google Scholar] [CrossRef]
- Dufour, C.; Loonis, M. Regio-and stereoselective oxidation of linoleic acid bound to serum albumin: Identification by ESI–mass spectrometry and NMR of the oxidation products. Chem. Phys. Lipids 2005, 138, 60–68. [Google Scholar] [CrossRef]
- Zhao, B.; Sakurai, Y.; Shibata, K.; Kikkawa, F.; Tomoda, Y.; Mizukami, H. Cytotoxic fatty acid ketodienes from eggplants. Jpn. J. Food Chem. Saf. 2014, 21, 42–47. [Google Scholar]
- Lundström, S.L.; Balgoma, D.; Wheelock, A.M.; Haeggstrom, J.Z.; Dahlen, S.E.; Wheelock, C.E. Lipid mediator profiling in pulmonary disease. Curr. Pharm. Biotechnol. 2011, 12, 1026–1052. [Google Scholar] [CrossRef]
- Goodfriend, T.L.; Ball, D.L.; Gardner, H.W. An oxidized derivative of linoleic acid affects aldosterone secretion by adrenal cells in vitro. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Burstyn, P.; Horrobin, D. Possible mechanism of action for aldosterone-induced hypertension. Lancet 1970, 295, 973–976. [Google Scholar] [CrossRef]
- Nilewski, C.; Chapelain, C.L.; Wolfrum, S.; Carreira, E.M. Synthesis and Biological Evaluation of Chlorinated Analogs of Leukotoxin Diol. Org. Lett. 2015, 17, 5602–5605. [Google Scholar] [CrossRef] [PubMed]
- Anuar, S.T.; Zhao, Y.Y.; Mugo, S.M.; Curtis, J.M. Monitoring the epoxidation of canola oil by non-aqueous reversed phase liquid chromatography/mass spectrometry for process optimization and control. J. Am. Oil Chem. Soc. 2012, 89, 1951–1960. [Google Scholar] [CrossRef]
- Aerts, H.A.; Jacobs, P.A. Epoxide yield determination of oils and fatty acid methyl esters using 1H NMR. J. Am. Oil Chem. Soc. 2004, 81, 841–846. [Google Scholar] [CrossRef]
- Shah, D.U. Towards Sustainable Polymers and Plastics: NMR Spectroscopic Analysis and Characterisation of Vernonia Seed (Vernonia Galamensis) Oil and Epoxidised Soya Bean Seed (Glycine Max) Oil. Sci. Technol. 2004, 1, 13. [Google Scholar]
- Xia, W.; Budge, S.M.; Lumsden, M.D. 1H-NMR Characterization of Epoxides Derived from Polyunsaturated Fatty Acids. J. Am. Oil Chem. Soc. 2016, 93, 467–478. [Google Scholar] [CrossRef]
- Moghaddam, M.F.; Grant, D.F.; Cheek, J.M.; Greene, J.F.; Williamson, K.C.; Hammock, B.D. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 1997, 3, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Hayakawa, M.; Kosaka, K.; Sugiyama, S.; Ogawa, T.; Yokoo, K.; Aoyama, H.; Izawa, Y. Leukotoxin, 9,10-epoxy-12-octadecenoate, as a burn toxin causing adult respiratory distress syndrome. Adv. Prostaglandin Thromboxane Leukotriene Res. 1991, 21, 569–572. [Google Scholar]
- Greene, J.F.; Newman, J.W.; Williamson, K.C.; Hammock, B.D. Toxicity of epoxy fatty acids and related compounds to cells expressing human soluble epoxide hydrolase. Chem. Res. Toxicol. 2000, 13, 217–226. [Google Scholar] [CrossRef]
- Kuksis, A.; Pruzanski, W. Epoxy Fatty Acids: Chemistry and Biological Effects. In Fatty Acids; Ahmad, M., Ed.; AOCS Press: North York, ON, Canada, 2017; pp. 83–119. [Google Scholar]
- Hanaki, Y.; Kamiya, H.; Ohno, M.; Hayakawa, M.; Sugiyama, S.; Ozawa, T. Leukotoxin, 9,10-epoxy-12-octadecenoate: A possible responsible factor in circulatory shock and disseminated intravascular coagulation. Jpn. J. Med. 1991, 30, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Morton, M.D.; Hill, D.W.; Grant, D.F. NMR and HPLC-MS/MS analysis of synthetically prepared linoleic acid diol glucuronides. Chem. Phys. Lipids 2006, 140, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Zheng, J.; Plopper, C.G.; Lakritz, J.; Storms, D.H.; Hammock, B.D. Leukotoxin-diol: A putative toxic mediator involved in acute respiratory distress syndrome. Am. J. Resp. Cell Mol. 2001, 25, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Markaverich, B.M.; Crowley, J.R.; Alejandro, M.A.; Shoulars, K.; Casajuna, N.; Mani, S.; Reyna, A.; Sharp, J. Leukotoxin diols from ground corncob bedding disrupt estrous cyclicity in rats and stimulate MCF-7 breast cancer cell proliferation. Environ. Health Perspect. 2005, 113, 1698. [Google Scholar] [CrossRef]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef] [Green Version]
- Loury, M. Possible mechanisms of autoxidative rancidity. Lipids 1972, 7, 671–675. [Google Scholar] [CrossRef]
- DeMan, J.M.; Tie, F.; DeMan, L. Formation of short chain volatile organic acids in the automated AOM method. J. Am. Oil Chem. Soc. 1987, 64, 993–996. [Google Scholar] [CrossRef]
- Harry-O’kuru, R.E.; Biresaw, G.; Tisserat, B.; Evangelista, R. Synthesis of polyformate esters of vegetable oils: Milkweed, pennycress, and soy. J. Lipids 2016, 2016, 3128604. [Google Scholar] [CrossRef] [Green Version]
- Caillol, S.; Desroches, M.; Boutevin, G.; Loubat, C.; Auvergne, R.; Boutevin, B. Synthesis of new polyester polyols from epoxidized vegetable oils and biobased acids. Eur. J. Lipid Sci. Technol. 2012, 114, 1447–1459. [Google Scholar] [CrossRef]
- Brasil, M.C.; Gerbase, A.E.; de Luca, M.A.; Gregório, J.R. Organic-inorganic hybrid films based on hydroxylated soybean oils. J. Am. Oil Chem. Soc. 2007, 84, 289–295. [Google Scholar] [CrossRef]
- Ionescu, M.; Petrović, Z.S.; Wan, X. Primary hydroxyl content of soybean polyols. J. Am. Oil Chem. Soc. 2008, 85, 465–473. [Google Scholar] [CrossRef]
- Abdullah, B.M.; Zubairi, S.I.; Huri, H.Z.; Hairunisa, N.; Yousif, E.; Basu, R.C. Polyesters based on linoleic acid for biolubricant basestocks: Low-temperature, Tribological and Rheological properties. PLoS ONE 2016, 11, e0151603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lligadas, G.; Ronda, J.C.; Galia, M.; Biermann, U.; Metzger, J.O. Synthesis and characterization of polyurethanes from epoxidized methyl oleate based polyether polyols as renewable resources. J. Polym. Sci. Pol. Chem. 2006, 44, 634–645. [Google Scholar] [CrossRef]
- De Souza, V.H.R.; Silva, S.A.; Ramos, L.P.; Zawadzki, S.F. Synthesis and characterization of polyols derived from corn oil by epoxidation and ozonolysis. J. Am. Oil Chem. Soc. 2012, 89, 1723–1731. [Google Scholar] [CrossRef]
- Bonete, P.; Najera, C. Lithium 3-Lithio-3-tosylalkanoates: Beta-Acylvinyl Anion Equivalents of beta-Lithiated alpha,beta-Unsaturated Carboxylic Acids. J. Org. Chem. 1994, 59, 3202–3209. [Google Scholar] [CrossRef]
- Braukmüller, S.; Brückner, R. Enantioselective butenolide preparation for straightforward asymmetric syntheses of γ-lactones-paraconic acids, avenaciolide, and hydroxylated eleutherol. Eur. J. Org. Chem. 2006, 2006, 2110–2118. [Google Scholar] [CrossRef]
- Gu, X. Oxidative lipid fragmentation; New mechanisms, synthesis and reactions of putative intermediates. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, August 2010. [Google Scholar]
- Martínez-Yusta, A.; Guillén, M.D. Deep-frying food in extra virgin olive oil: A study by 1H nuclear magnetic resonance of the influence of food nature on the evolving composition of the frying medium. Food Chem. 2014, 150, 429–437. [Google Scholar] [CrossRef]
- Goicoechea, E.; Guillén, M.D. Volatile compounds generated in corn oil stored at room temperature. Presence of toxic compounds. Eur. J. Lipid Sci. Technol. 2014, 116, 395–406. [Google Scholar] [CrossRef]
- Chung, F.L.; Chen, H.J.; Guttenplan, J.B.; Nishikawa, A.; Hard, G.C. 2,3-Epoxy-4-hydroxynonanal as a potential tumor-initiating agent of lipid peroxidation. Carcinogenesis 1993, 14, 2073–2077. [Google Scholar] [CrossRef]
- Dianzani, M.U. 4-hydroxynonenal from pathology to physiology. Mol. Aspects Med. 2003, 24, 263–272. [Google Scholar] [CrossRef]
- Eckl, P.M.; Ortner, A.; Esterbauer, H. Genotoxic properties of 4-hydroxyalkenals and analogous aldehydes. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1993, 290, 183–192. [Google Scholar] [CrossRef]
- McCarthy, F.O.; Chopra, J.; Ford, A.; Hogan, S.A.; Kerry, J.P.; O’Brien, N.M.; Ryan, E.; Maguire, A.R. Synthesis, isolation and characterisation of β-sitosterol and β-sitosterol oxide derivatives. Org. Biomol. Chem. 2005, 3, 3059–3065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Geoffroy, P.; Miesch, M.; Julien-David, D.; Raul, F.; Aoudé-Werner, D.; Marchioni, E. Gram-scale chromatographic purification of β-sitosterol: Synthesis and characterization of β-sitosterol oxides. Steroids 2005, 70, 886–895. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Oxylipins Associated to Current Diseases Detected for the First Time in the Oxidation of Corn Oil as a Model System of Oils Rich in Omega-6 Polyunsaturated Groups. A Global, Broad and in-Depth Study by 1H NMR Spectroscopy. Antioxidants 2020, 9, 544. https://doi.org/10.3390/antiox9060544
Alberdi-Cedeño J, Ibargoitia ML, Guillén MD. Oxylipins Associated to Current Diseases Detected for the First Time in the Oxidation of Corn Oil as a Model System of Oils Rich in Omega-6 Polyunsaturated Groups. A Global, Broad and in-Depth Study by 1H NMR Spectroscopy. Antioxidants. 2020; 9(6):544. https://doi.org/10.3390/antiox9060544
Chicago/Turabian StyleAlberdi-Cedeño, Jon, María L. Ibargoitia, and María D. Guillén. 2020. "Oxylipins Associated to Current Diseases Detected for the First Time in the Oxidation of Corn Oil as a Model System of Oils Rich in Omega-6 Polyunsaturated Groups. A Global, Broad and in-Depth Study by 1H NMR Spectroscopy" Antioxidants 9, no. 6: 544. https://doi.org/10.3390/antiox9060544
APA StyleAlberdi-Cedeño, J., Ibargoitia, M. L., & Guillén, M. D. (2020). Oxylipins Associated to Current Diseases Detected for the First Time in the Oxidation of Corn Oil as a Model System of Oils Rich in Omega-6 Polyunsaturated Groups. A Global, Broad and in-Depth Study by 1H NMR Spectroscopy. Antioxidants, 9(6), 544. https://doi.org/10.3390/antiox9060544