Detection of Antioxidant Phytochemicals Isolated from Camellia japonica Seeds Using HPLC and EPR Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Camellia japonica Seeds Sample
2.3. Preparation of Extracts
2.4. Determination for In Vitro Antioxidant Activity
2.4.1. Scavenging Effects on Nitric Oxide (NO)
2.4.2. Scavenging Effects on the Superoxide Anion
2.4.3. Inhibitory Effect on Lipid Peroxidation
2.5. Determination of Antioxidant Activity in Cell-Based Study
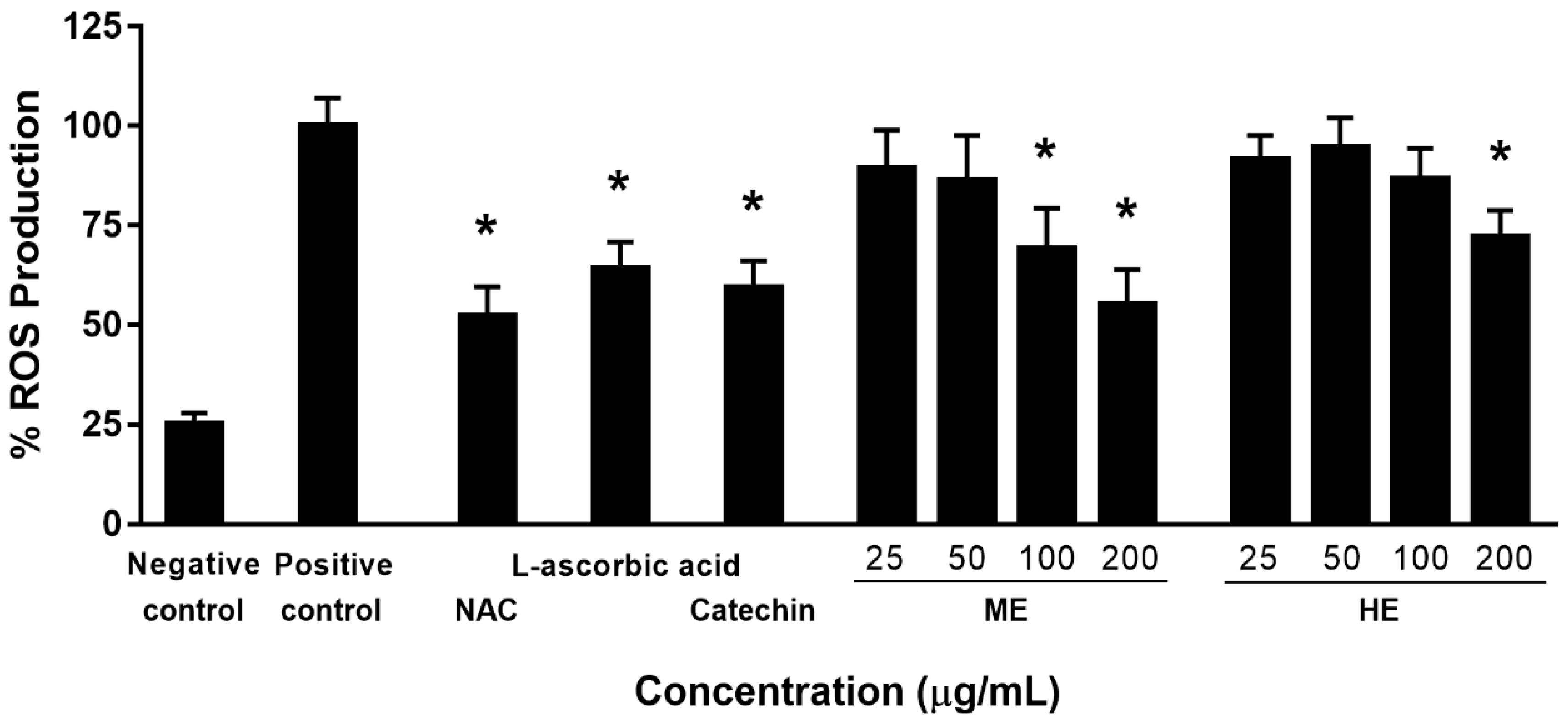
2.5.1. Determination of Inhibitory Effect on the Intracellular ROS Production
2.5.2. Determination of NO and Inducible Nitric Oxide Synthase (iNOS) Production
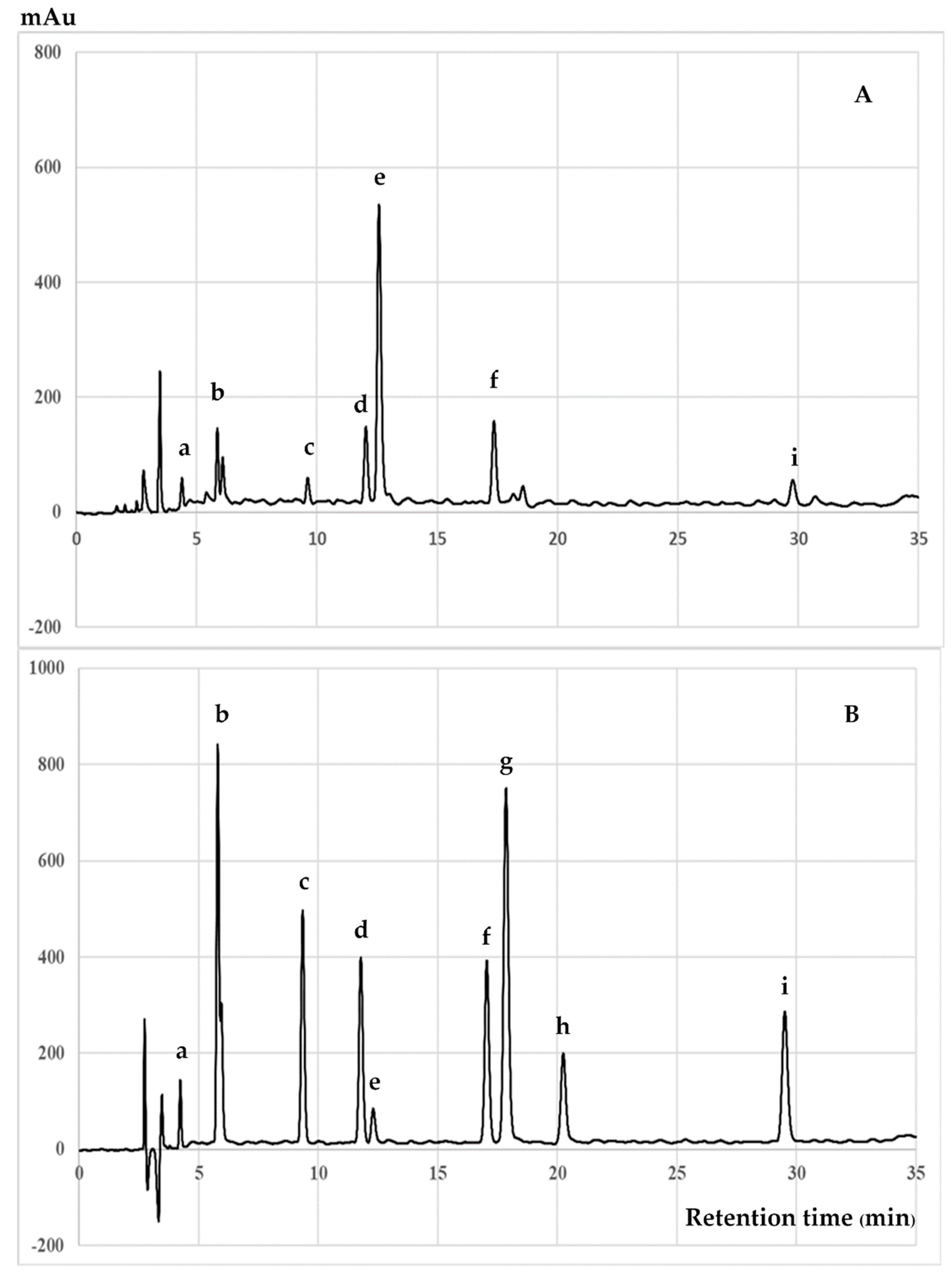
2.6. Chromatographic Analysis of Polar Phenolic Compounds
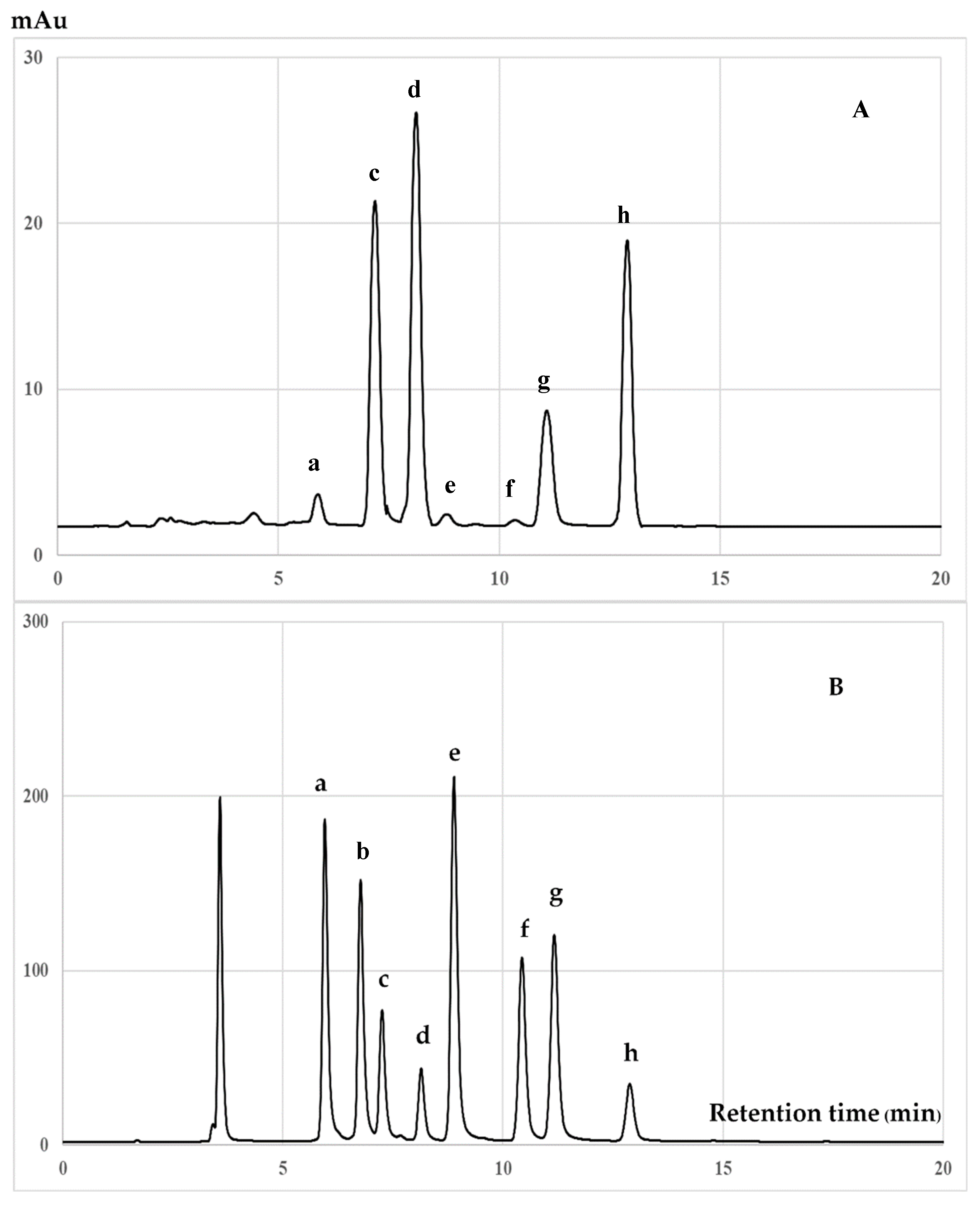
2.7. Chromatographic Analysis of Tocotrienols and Tocopherols
2.8. EPR
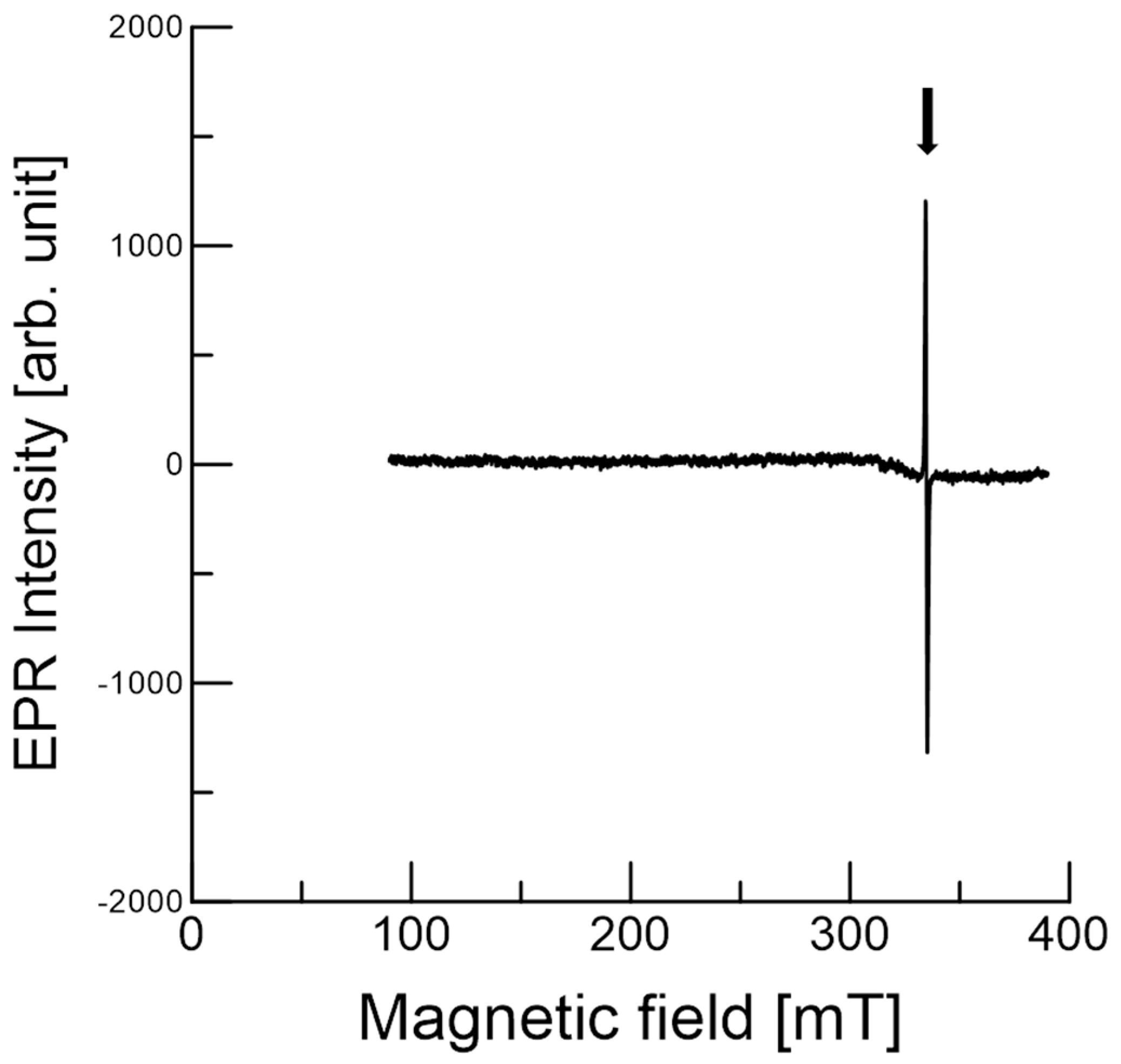
2.8.1. EPR Measurements
2.8.2. EPR Imaging Measurements
2.9. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity
3.2. Chromatographic Analysis of Biochemical Compounds
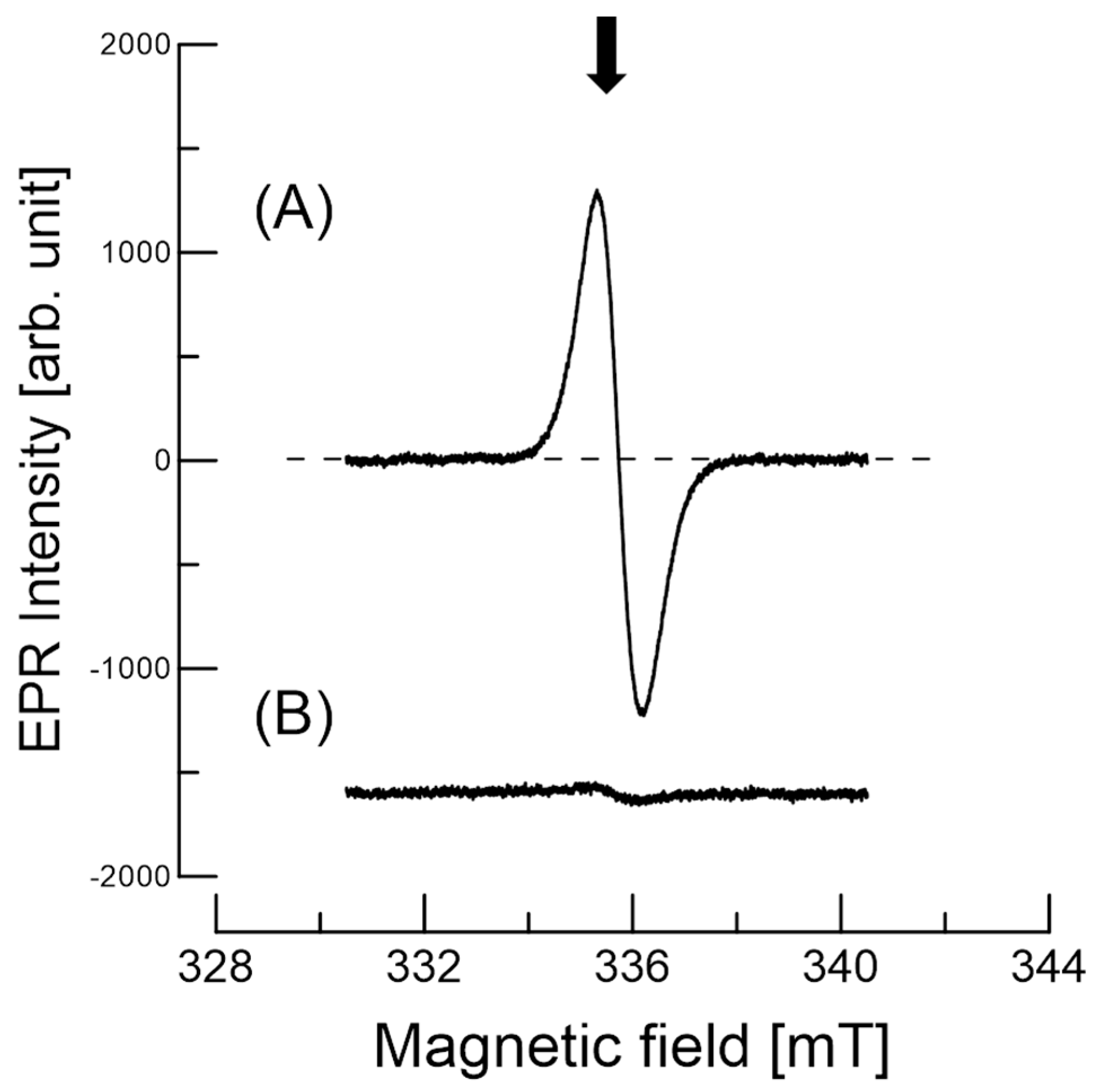
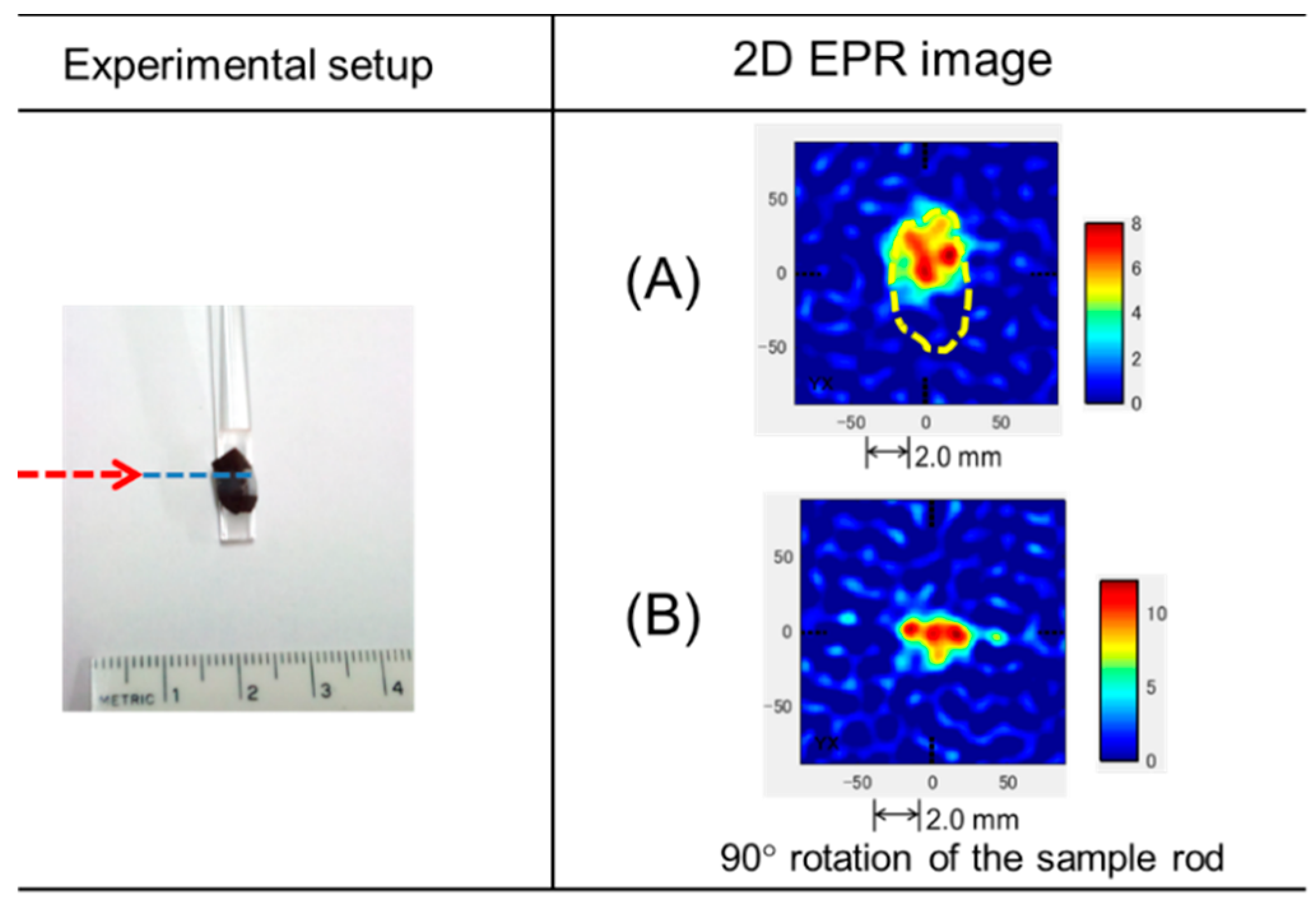
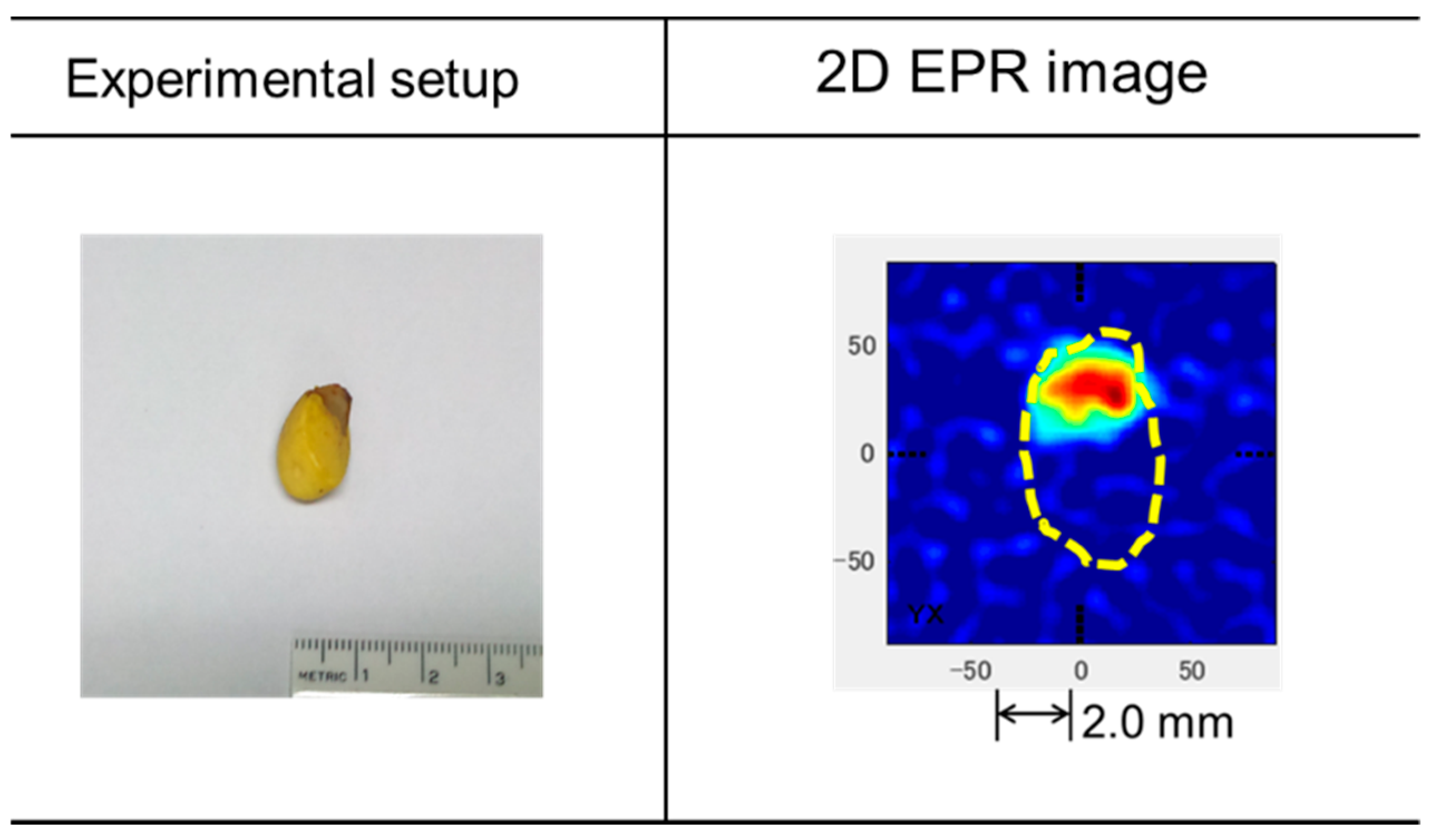
3.3. EPR of Stable Radicals in C. japonica Seed Coat
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zeb, A. Triacylglycerols composition, oxidation and oxidation compounds in camellia oil using liquid chromatography–mass spectrometry. Chem. Phys. Lipids 2012, 165, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Endo, Y. Lipid Characteristics of Camellia Seed Oil. J. Oleo Sci. 2019, 68, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Garcia, Q.S. Reactive oxygen species and seed germination. Boilogia 2013, 68, 351–357. [Google Scholar] [CrossRef]
- Nogala-Kalucka, M.; Rudzińska, M.; Zadernowski, R.; Siger, A.; Krzyzostaniak, I. Phytochemical Content and Antioxidant Properties of Seeds of Unconventional Oil Plants. J. Am. Oil Chem. Soc. 2010, 87, 1481–1487. [Google Scholar] [CrossRef]
- Lee, K.J.; Baek, D.-Y.; Lee, G.-A.; Cho, G.-T.; So, Y.-S.; Lee, J.-R.; Ma, K.-H.; Chung, J.-W.; Hyun, D.Y. Phytochemicals and Antioxidant Activity of Korean Black Soybean (Glycine max L.) Landraces. Antioxidants 2020, 9, 213. [Google Scholar] [CrossRef]
- Elkhamlichi, A.; Hajaji, H.E.; Faraj, H.; Alami, A.; Bali, B.E.; Lachkar, M. Phytochemical screening and evoluation of antioxidant and antibacterial activities of seeds and pods extracts of Calycotome villosa subsp. Intermedia. J. Appl. Pharm. Sci. 2017, 7, 192–198. [Google Scholar]
- Nakagawa, K.; Epel, B. Locations of radical species in black pepper seeds investigated by CW EPR and 9 GHz EPR imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 342–346. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hara, H. Investigation of radical locations in various sesame seeds by CW EPR and 9-GHz EPR imaging. Free. Radic. Res. 2014, 49, 1–6. [Google Scholar] [CrossRef]
- Nakagawa, K.; Epel, B. Investigating the Distribution of Stable Paramagnetic Species in an Apple Seed Using X-Band EPR and EPR Imaging. J. Oleo Sci. 2017, 66, 315–319. [Google Scholar] [CrossRef]
- Nakagawa, K.; Matsumoto, K.; Chaiserm, N.; Priprem, A. X-band Electron Paramagnetic Resonance Investigation of Stable Organic Radicals Present under Cold Stratification in ‘Fuji’ Apple Seeds. J. Oleo Sci. 2017, 66, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Szöcs, F. Free Radicals in Wheat Seeds Studied by Electron Spin Resonance. J. Food Sci. 2002, 67, 2079–2082. [Google Scholar] [CrossRef]
- Watanabe, J.; Oki, T.; Takebayashi, J.; Takano-Ishikawa, Y. Extraction Efficiency of Hydrophilic and Lipophilic Antioxidants from Lyophilized Foods Using Pressurized Liquid Extraction and Manual Extraction. J. Food Sci. 2014, 79, 1665. [Google Scholar] [CrossRef]
- Rao, M.N.A. Nitric Oxide Scavenging by Curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef]
- Kidarn, S.; Saenjum, C.; Hongwiset, D.; Phrutivorapongkul, A. Furanocoumarins from Kaffir lime and their inhibitory effects on inflammatory mediator production. Cogent Chem. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant Activity of a Flavonoid-Rich Extract ofHypericum perforatumL.in Vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Saenjum, C. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J. Med. Plants Res. 2012, 6, 1070–1077. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Wudtiwai, B.; Khaw-On, P.; Rachakhom, W.; Duangnil, N.; Kongtawelert, P. Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumor Boil. 2015, 37, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Hur, S.K.; Oh, O.-J.; Kim, S.S.; Nam, K.A.; Lee, S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J. Ethnopharmacol. 2002, 83, 153–159. [Google Scholar] [CrossRef]
- Hu, C.; Zawistowski, J.; Ling, W.; Kitts, D.D. Black Rice (Oryza sativaL.indica) Pigmented Fraction Suppresses both Reactive Oxygen Species and Nitric Oxide in Chemical and Biological Model Systems. J. Agric. Food Chem. 2003, 51, 5271–5277. [Google Scholar] [CrossRef]
- Jomha, N.M.; Elliott, J.A.W.; Law, G.; McGann, L.E. Evaluation of chondrocyte survival in situ using WST-1 and membrane integrity stains. Cell Tissue Bank. 2006, 8, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sirithunyalug, B.; Saenjum, C.; Charumanee, S.; Sivamaruthi, B.S.; Chaiyasut, C.; Sirithunyalug, J.; Tipduangta, P. Development of Colorectal-Targeted Dietary Supplement Tablets Containing Natural Purple Rice Bran Oil as a Colorectal Chemopreventive. Nutrients 2018, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Maeda, H. EPR imaging and HPLC characterization of the pigment-based organic free radical in black soybean seeds. Free. Radic. Res. 2017, 51, 187–192. [Google Scholar] [CrossRef]
- Lee, C.-P.; Yen, G.-C. Antioxidant Activity and Bioactive Compounds of Tea Seed (Camellia oleifera Abel.) Oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Leelapornpisid, P.; Jakmunee, J.; Korsamphan, C. Antioxidant and Moisturizing Effect of Camellia assamica Seed Oil and Its Development into Microemulsion. Cosmetics 2018, 5, 40. [Google Scholar] [CrossRef]
- Hu, J.B.; Yang, G. Physiochemical characteristics, fatty acid profile and tocopherol composition of the oil from Camellia oleifera Abel cultivated in Henan, China. Grasas Aceites 2018, 69, 255. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and their therapeutic benefits to inflammatory bower disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular Aspects of α-Tocotrienol Antioxidant Action and Cell Signalling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Krasuska, U.; Czajkowska, K.; Bogatek, R. Nitric oxide, hydrogen cyanide and ethylene are required in the control of germination and undisturbed development of young apple seedlings. Plant Growth Regul. 2010, 61, 75–84. [Google Scholar] [CrossRef]
- Dębska, K.; Krasuska, U.; Budnicka, K.; Bogatek, R.; Gniazdowska, A. Dormancy removal of apple seeds by cold stratification is associated with fluctuation in H2O2, NO production and protein carbonylation level. J. Plant Physiol. 2013, 170, 480–488. [Google Scholar] [CrossRef] [PubMed]
- EPR Imaging Toolbox Collection User Manual. 2015. Available online: http://epr-it.specman4epr.com/ (accessed on 10 February 2020).
- Lu, Y.; Foo, L.Y. Constitution of some chemical components of apple seed. Food Chem. 1998, 61, 29–33. [Google Scholar] [CrossRef]
| Samples/Positive Control | IC50 (µg/mL) | ||
|---|---|---|---|
| Nitric Oxide | Superoxide Anion | Lipid Peroxidation | |
| ME | 22.64 ± 1.07 c | 14.5 ± 0.82 c | 59.58 ± 2.81 d |
| HE | 88.84 ± 2.23 d | 74.5 ± 2.26 d | 20.98 ± 2.17 b |
| Catechin | 14.86 ± 0.84 b | 9.61 ± 0.31 b | 30.64 ± 1.32 c |
| Curcumin | 9.14 ± 0.68 a | ND | ND |
| L-ascorbic acid | ND | 6.91 ± 0.26 a | ND |
| α-Tocopherol | ND | ND | 14.32 ± 0.93 a |
| Samples/Positive Control | IC50 (µg/mL) | |
|---|---|---|
| Nitric Oxide | iNOS | |
| ME | 22.78 ± 1.79 c | 32.68 ± 1.16 C |
| HE | 33.95 ± 2.08 d | 39.63 ± 1.78 D |
| Catechin | 12.55 ± 0.77 b | 17.45 ± 1.29 B |
| Curcumin | 7.51 ± 0.69 a | 9.52 ± 0.63 A |
| Compounds | Amount (mg/g Extract) | Compounds | Amount (mg/g Extract) | ||
|---|---|---|---|---|---|
| ME | HE | ME | HE | ||
| Gallic acid | 0.64 ± 0.10 | nd. | δ-Tocotrienol | nd. | 1.44 ± 0.35 |
| Gallocatechin | 1.98 ± 0.22 | nd. | β-Tocotrienol | nd. | nd. |
| Epigallocatechin | 1.24 ± 0.11 | nd. | γ-Tocotrienol | 1.16 ± 0.28 | 15.7 ± 0.52 |
| Caffeine | 3.69 ± 0.27 | 0.86 ± 0.12 | α-Tocotrienol | 1.49 ± 0.23 | 18.2 ± 0.76 |
| Catechin | 9.46 ± 0.45 | nd. | δ-Tocopherol | nd. | 0.82 ± 0.29 |
| Epicatechin | 3.55 ± 0.20 | nd. | β-Tocopherol | nd. | 0.37 ± 0.14 |
| Epigallocatechin gallate | 0.52 ± 0.08 | nd. | γ-Tocopherol | 0.47 ± 0.15 | 6.27 ± 0.39 |
| Epicatechin gallate | 0.86 ± 0.13 | nd. | α-Tocopherol | 0.65 ± 0.19 | 13.6 ± 0.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saenjum, C.; Pattananandecha, T.; Nakagawa, K. Detection of Antioxidant Phytochemicals Isolated from Camellia japonica Seeds Using HPLC and EPR Imaging. Antioxidants 2020, 9, 493. https://doi.org/10.3390/antiox9060493
Saenjum C, Pattananandecha T, Nakagawa K. Detection of Antioxidant Phytochemicals Isolated from Camellia japonica Seeds Using HPLC and EPR Imaging. Antioxidants. 2020; 9(6):493. https://doi.org/10.3390/antiox9060493
Chicago/Turabian StyleSaenjum, Chalermpong, Thanawat Pattananandecha, and Kouichi Nakagawa. 2020. "Detection of Antioxidant Phytochemicals Isolated from Camellia japonica Seeds Using HPLC and EPR Imaging" Antioxidants 9, no. 6: 493. https://doi.org/10.3390/antiox9060493
APA StyleSaenjum, C., Pattananandecha, T., & Nakagawa, K. (2020). Detection of Antioxidant Phytochemicals Isolated from Camellia japonica Seeds Using HPLC and EPR Imaging. Antioxidants, 9(6), 493. https://doi.org/10.3390/antiox9060493






