Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments and Experimental Design
2.2. Vine Yield Determination and Storage Experiment
2.3. Quality Parameters
2.4. Total Phenolics and Total and Individual Anthocyanin Quantifications
2.5. Statistical Analysis
3. Results
3.1. Vine Yield and Berry Ripening Process
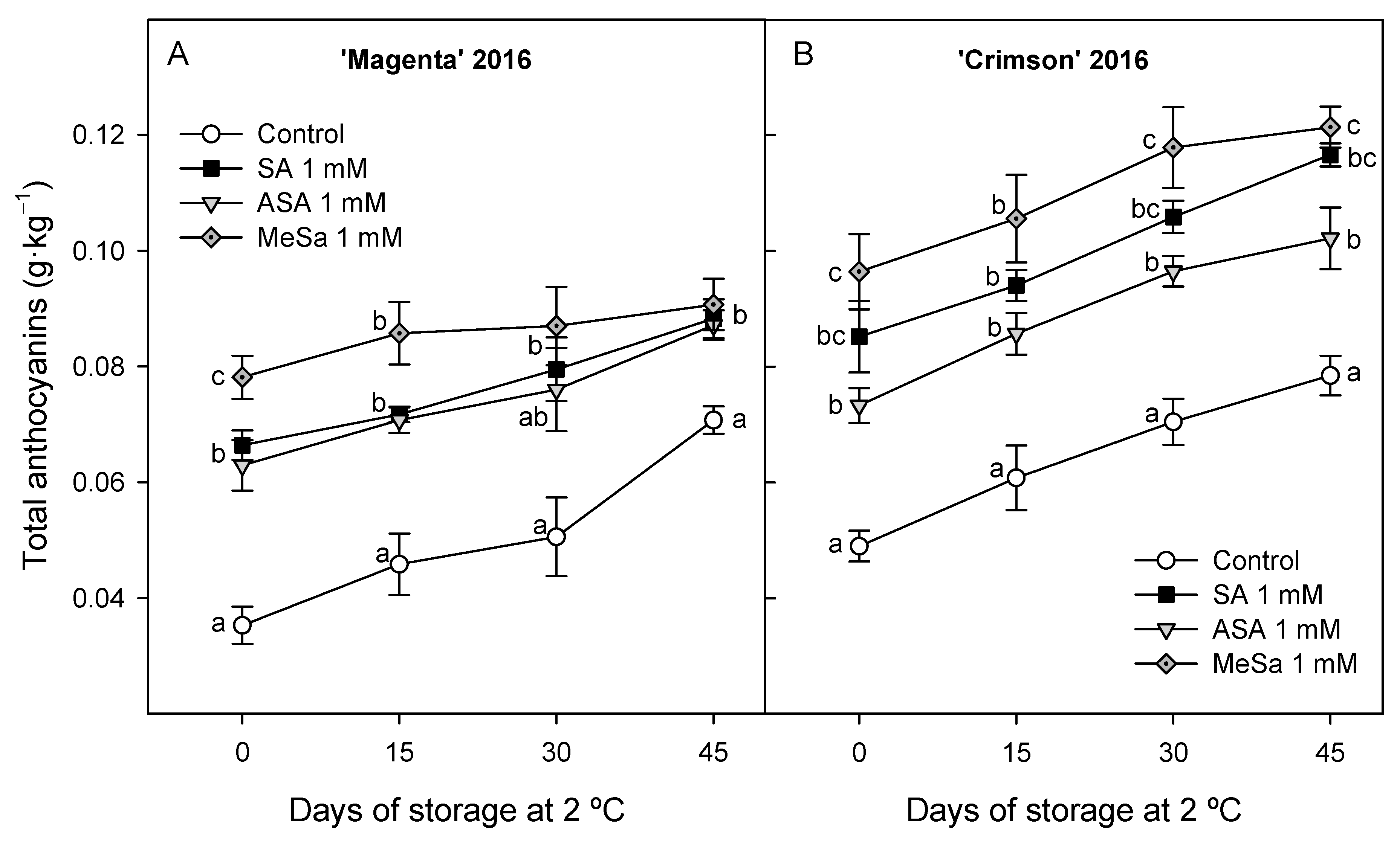
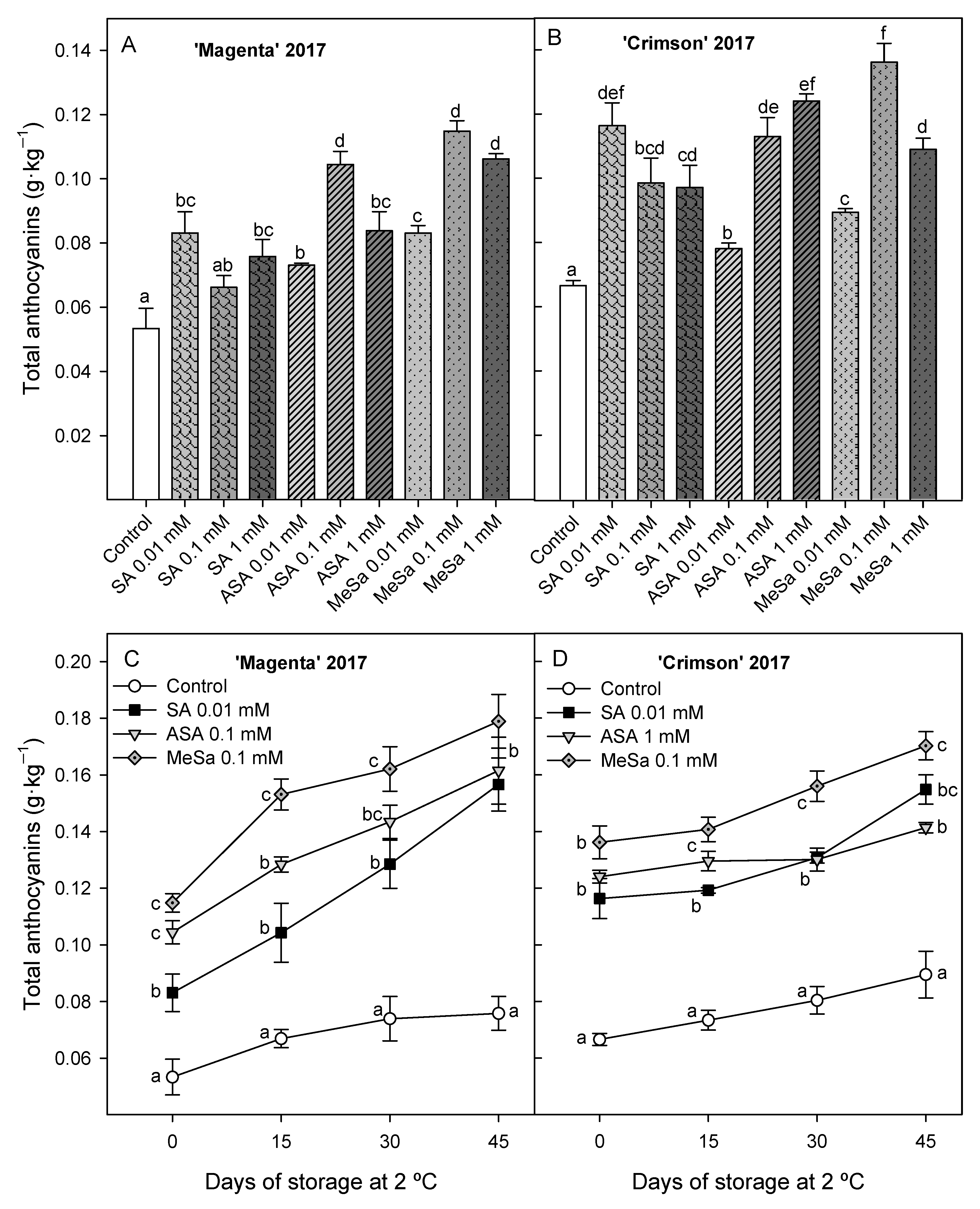
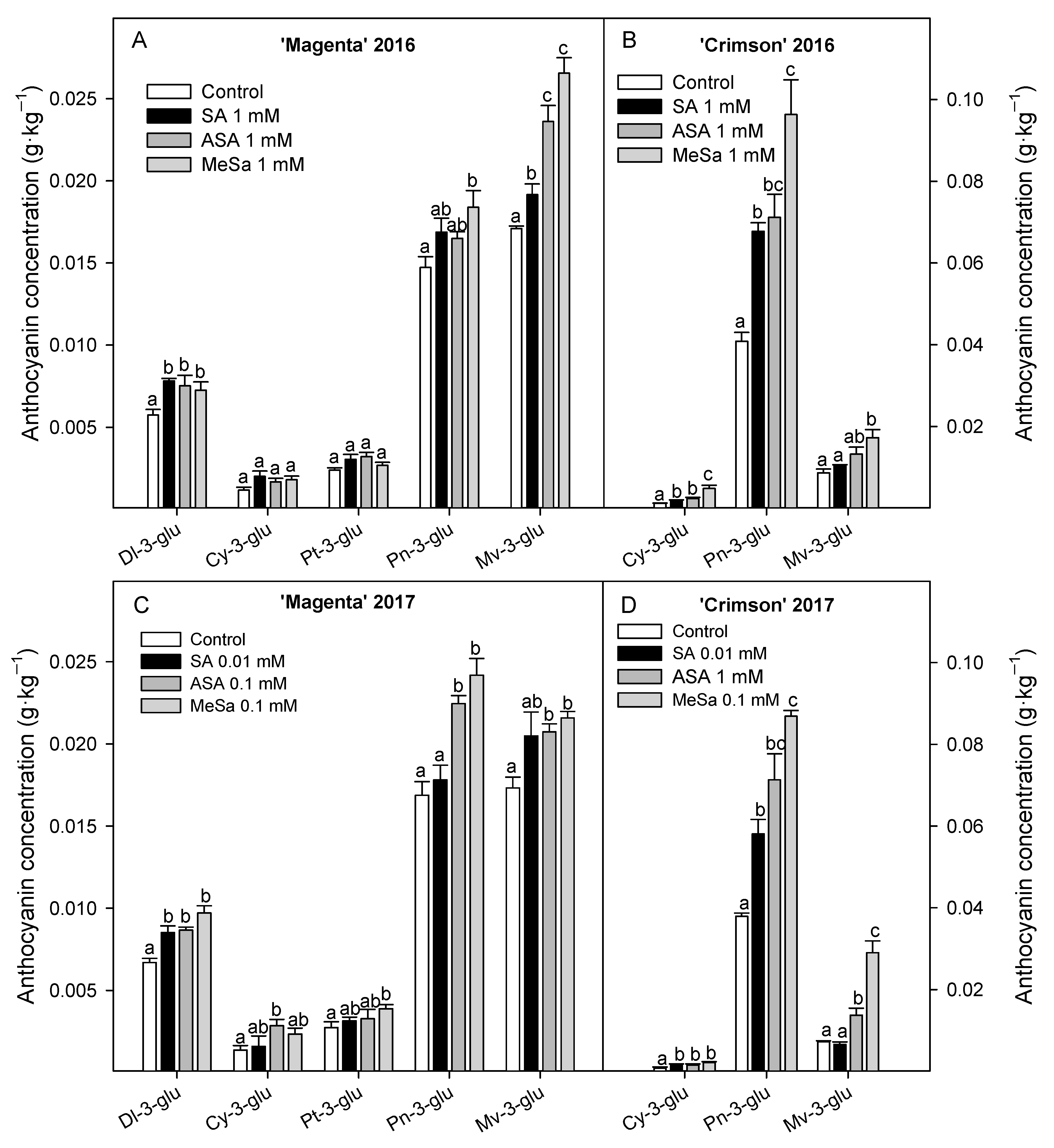
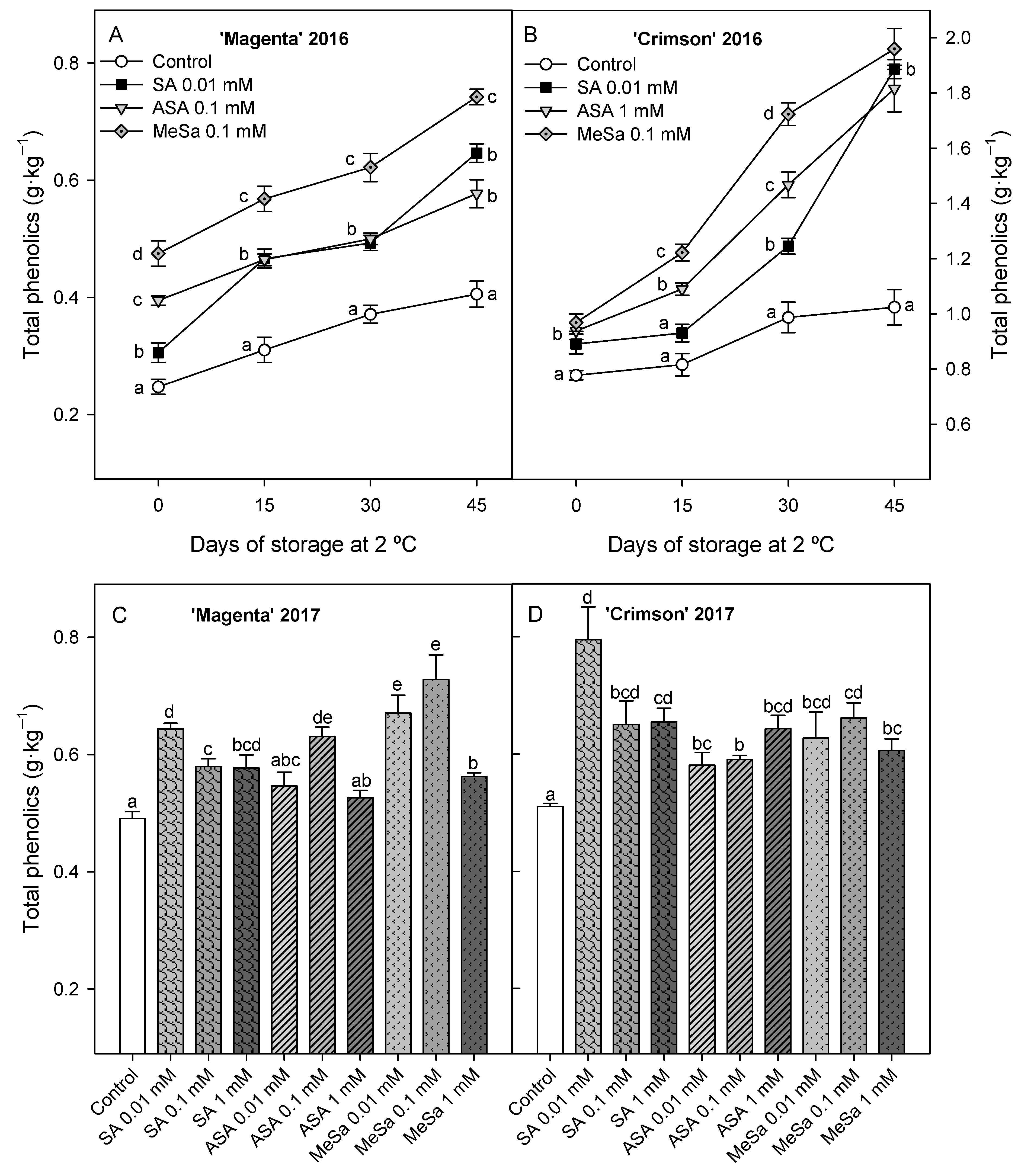
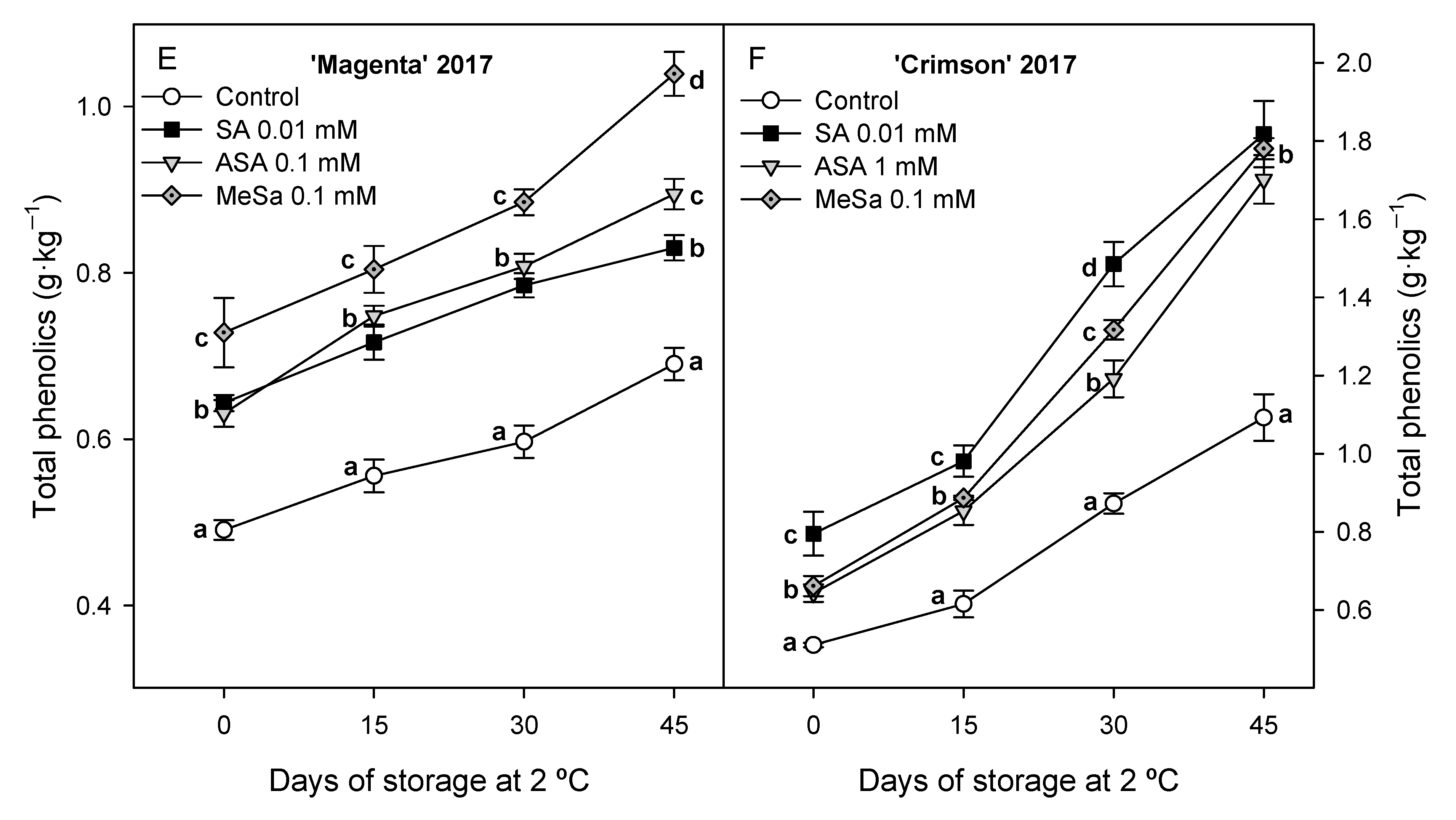
3.2. Bioactive Compounds: Anthocyanins and Phenolics
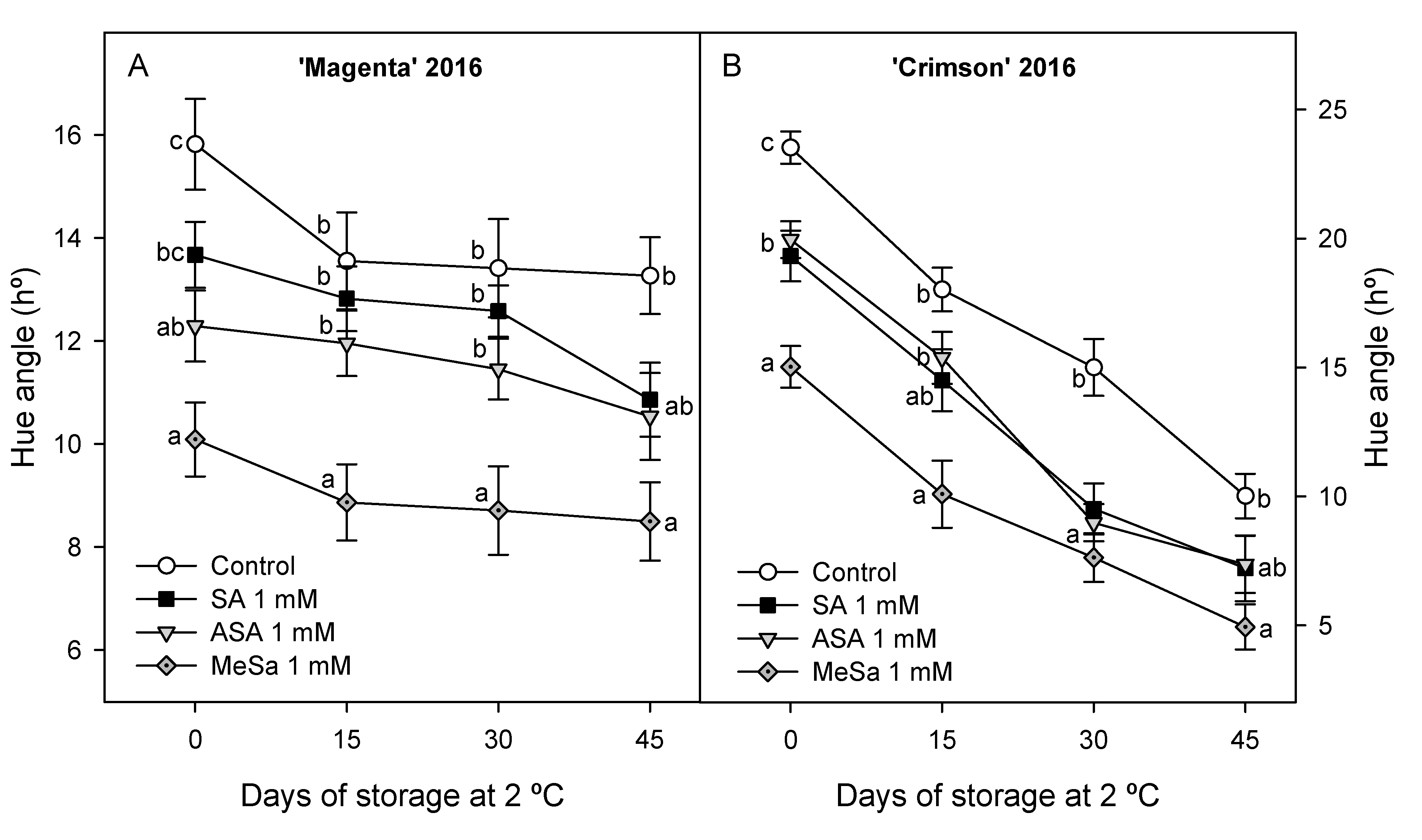
3.3. Quality Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.H.; Lauvergeat, V.; Gomès, E.; Li, S.H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening: recently heard through the grapevine. J. Exp. Bot. 2014, 65, 4543–4559. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Zhou, K.; Raffoul, J.J. Potential anticancer properties of grape antioxidants. J. Oncol. 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.M.S.; Pacucci, C.; Punzi, R.; Faccia, M.; Trani, A.; Gambacorta, G. Application of abscisic acid (S-ABA) and sucrose to improve color, anthocyanin content and antioxidant activity of cv. ‘Crimson Seedless’ grapes berries. Aust. J. Grape Wine Res. 2015, 21, 18–29. [Google Scholar] [CrossRef]
- Olivares, D.; Contreras, C.; Muñoz, V.; Rivera, S.; González-Agüero, M.; Retamales, J.; Defilippi, B.G. Relationship among color development, anthocyanin and pigment-related gene expression in ‘Crimson Seedless’ grapes treated with abscisic acid and sucrose. Plant Physiol. Biochem. 2017, 115, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Conesa, M.R.; Falagán, N.; de la Rosa, J.M.; Aguayo, E.; Domingo, R.; Pastor, A.P. Post-veraison deficit irrigation regimes enhance berry coloration and health-promoting bioactive compounds in ‘Crimson Seedless’ table grapes. Agric. Water Manage. 2016, 163, 9–18. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Methyl jasmonate effects on table grape ripening, vine yield, berry quality and bioactive compounds depend on applied concentration. Sci. Hortic. 2019, 247, 380–389. [Google Scholar] [CrossRef]
- Kumar, D. Salicylic acid signalling in disease resistance. Plant Sci. 2014, 228, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Glowacz, M.; Ree, D. Using jasmonates and salicylates to reduce losses within the fruit supply chain. Eur. Food Res. Technol. 2015, 242, 143–156. [Google Scholar] [CrossRef]
- Cao, J.K.; Yan, J.Q.; Zhao, Y.M.; Jiang, W.B. Effects of four pre-harvest foliar sprays with β-aminobutyric acid or salicylic acid on the incidence of post-harvest disease and induced defence responses in jujube (Zizyphus jujuba Mill.) fruit after storage. J. Hortic. Sci. Biotechnol. 2013, 88, 338–344. [Google Scholar] [CrossRef]
- Supapvanich, S.; Mitsang, P.; Youryon, P. Preharvest salicylic acid application maintains physicochemical quality of ‘Taaptimjaan’ wax apple fruit (Syzygium samarangenese) during short-term storage. Sci. Hortic. 2017, 215, 178–183. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Serrano, M.; Moral, J.; Castillo, S. Methyl salicylate treatments of sweet cherry trees improve fruit quality at harvest and during storage. Sci. Hortic. 2015, 197, 665–673. [Google Scholar] [CrossRef]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of antioxidant systems and storability of two plum cultivars by preharvest treatments with salicylates. Open Access. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; Martínez-Romero, D.; Díaz-Mula, H.M.; Serrano, M. Preharvest treatments with salicylates enhance nutrient and antioxidant compounds in plum at harvest and after storage. J. Sci. Food Agric. 2018, 98, 2742–2750. [Google Scholar] [CrossRef]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Technol. 2015, 52, 3607–3616. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; EL-Boray, M.S. Improving fruit cluster quality attributes of ‘Flame Seedless’ grapes using preharvest application of ascorbic and salicylic acid. Sci. Hortic. 2018, 233, 339–348. [Google Scholar] [CrossRef]
- Marzouk, H.A.; Kassem, H.A. Improving yield, quality, and shelf life of Thompson seedless grapevine by preharvest foliar applications. Sci. Hortic. 2011, 130, 425–430. [Google Scholar] [CrossRef]
- Alrashdi, A.M.A.; Al-Qurashi, A.D.; Awad, M.A.; Mohamed, S.A.; Al-rashdi, A.A. Quality, antioxidant compounds, antioxidant capacity and enzymes activity of ‘El-Bayadi’ table grapes at harvest as affected by preharvest salicylic acid and gibberellic acid spray. Sci. Hortic. 2017, 220, 243–249. [Google Scholar] [CrossRef]
- Oraei, M.; Panahirad, S.; Zaare-Nahandi, F.; Gohari, G. Pre-véraison treatment of salicylic acid to enhance anthocyanin content of grape (Vitis vinifera L.) berries. J. Sci. Food Agric. 2019, 99, 5946–5952. [Google Scholar] [CrossRef]
- Blanch, G.P.; Gómez-Jiménez, M.C.; del Castillo, M.L.R. Exogenous salicylic acid improves phenolic content and antioxidant activity in table grapes. Plant Foods Hum. Nutr. 2020, 75, 177–183. [Google Scholar] [CrossRef]
- Kraeva, E.; Tesnière, C.; Terrier, N.; Romieu, C.; Sauvage, F.X.; Bierne, J.; Deloire, A. Transcription of a β-1,3-glucanase gene in grape berries in a late developmental period, or earlier after wounding treatments. Vitis 1998, 37, 107–111. [Google Scholar]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Preciado-Rangel, P.; Reyes-Pérez, J.J.; Ramírez-Rodríguez, S.C.; Salas-Pérez, L.; Fortis-Hernández, M.; Murillo-Amador, B.; Troyo-Diéguez, E. Foliar aspersion of salicylic acid improves phenolic and flavonoid compounds, and also the fruit yield in cucumber (Cucumis sativus L). Plants 2019, 8, 44. [Google Scholar] [CrossRef]
- Elwan, M.W.M.; El-Hamahmy, M.A.M. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 2009, 112, 521–526. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wen, P.F.; Kong, W.F.; Pan, Q.H.; Zhan, J.C.; Li, J.M.; Wan, S.B.; Huang, W.D. Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biol. Technol. 2006, 40, 64–72. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.H.; Chung, I.M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Harindra Champa, W.A.; Gill, M.I.S.; Mahajan, B.V.C.; Bedi, S. Exogenous treatment of spermine to maintain quality and extend postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless under low temperature storage. LWT Food Sci. Technol. 2015, 60, 412–419. [Google Scholar] [CrossRef]
- Ustun, D.; Candir, E.; Ozdemir, A.E.; Kamiloglu, O.; Soylu, E.M.; Dilbaz, R. Effects of modified atmosphere packaging and ethanol vapor treatment on the chemical composition of ‘Red Globe’ table grapes during storage. Postharvest Biol. Technol. 2012, 68, 8–15. [Google Scholar] [CrossRef]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- de Rosas, I.; Ponce, M.T.; Malovini, E.; Deis, L.; Cavagnaro, B.; Cavagnaro, P. Loss of anthocyanins and modification of the anthocyanin profiles in grape berries of Malbec and Bonarda grown under high temperature conditions. Plant Sci. 2017, 258, 137–145. [Google Scholar] [CrossRef]
- Costa, C.; Graça, A.; Fontes, N.; Teixeira, M.; Gerós, H.; Santos, J.A. The interplay between atmospheric conditions and grape berry quality parameters in Portugal. Appl. Sci. 2020, 10, 4943. [Google Scholar] [CrossRef]
- Valero, D.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Castillo, S.; Serrano, M. The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol. 2006, 41, 317–327. [Google Scholar] [CrossRef]
- Guillén, F.; Zapata, P.J.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Improvement of the overall quality of table grapes stored under modified atmosphere packaging in combination with natural antimicrobial compounds. J. Food Sci. 2007, 72, S185–S190. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2010; ISBN 9781439802663. [Google Scholar]
- Ranjbaran, E.; Sarikhani, H.; Bakhshi, D.; Pouya, M. Investigation of salicylic acid application to reduce postharvest losses in stored ‘Bidaneh Ghermez’ table grapes. Int. J. Fruit Sci. 2011, 11, 430–439. [Google Scholar] [CrossRef]
- Khalil, H.A. Effects of pre- and postharvest salicylic acid application on quality and shelf life of ‘Flame Seedless’ grapes. Eur. J. Hortic. Sci. 2014, 79, 8–15. [Google Scholar]
- Zhang, Y.; Chen, K.; Zhang, S.; Ferguson, I. The role of salicylic acid in postharvest ripening of kiwifruit. Postharvest Biol. Technol. 2003, 28, 67–74. [Google Scholar] [CrossRef]
- Valverde, J.M.; Giménez, M.J.; Guillén, F.; Valero, D.; Martínez-Romero, D.; Serrano, M. Methyl salicylate treatments of sweet cherry trees increase antioxidant systems in fruit at harvest and during storage. Postharvest Biol. Technol. 2015, 109, 106–113. [Google Scholar] [CrossRef]
| Cultivar | ‘Magenta’ | ‘Crimson’ | ||
|---|---|---|---|---|
| Treatment | 2016 | 2017 | 2016 | 2017 |
| T1 | 23rd June | 27th June | 24th June | 28th June |
| T2 | 8th July | 12th July | 9th July | 15th July |
| T3 | 18th July | 21st July | 25th July | 28th July |
| Cultivar | ‘Magenta’ 2016 | ‘Crimson’ 2016 | ||
|---|---|---|---|---|
| Day 0 | Day 45 | Day 0 | Day 45 | |
| Control | 195.5 ± 1.0 aA | 204.5 ± 3.2 aB | 186.3 ± 1.5 aA | 204.3 ± 2.1 aB |
| SA 1 mM | 206.1 ± 3.1 bA | 214.2 ± 1.5 bA | 195.5 ± 2.0 bA | 218.2 ± 3.7 bB |
| ASA 1 mM | 207.8 ± 2.9 bA | 218.9 ± 3.7 bA | 198.2 ± 1.6 bA | 223.0 ± 2.3 bB |
| MeSa 1 mM | 207.2 ± 2.7 bA | 216.8 ± 2.9 bA | 196.4 ± 1.3 bA | 217.3 ± 1.7 bB |
| ‘Magenta’ 2017 | ‘Crimson’ 2017 | |||
| Day 0 | Day 45 | Day 0 | Day 45 | |
| Control | 180.2 ± 2.2 aA | 191.9 ± 2.5 aB | 175.8 ± 1.2 aA | 199.0 ± 2.3 aB |
| SA 0.01 mM | 185.5 ± 6.4 abA | 198.0 ± 0.5 aA | 178.7 ± 0.5 abA | 200.3 ± 1.4 aB |
| SA 0.1 mM | 186.5 ± 5.2 abA | 201.2 ± 3.2 aA | 179.9 ± 2.6 abA | 204.0 ± 1.3 aB |
| SA 1 mM | 199.0 ± 2.6 bA | 206.8 ± 2.1 bA | 181.3 ± 0.9 bA | 210.3 ± 1.2 bB |
| ASA 0.01 mM | 186.5 ± 5.6 abA | 198.8 ± 1.6 aA | 179.5 ± 1.0 abA | 193.7 ± 2.4 aB |
| ASA 0.1 | 189.3 ± 3.2 abA | 191.8 ± 0.9 aA | 178.7 ± 3.8 abA | 199.7 ± 1.8 aB |
| ASA 1 mM | 205.7 ± 6.9 bA | 214.7 ± 3.1 bA | 190.3 ± 2.1 cA | 214.0 ± 1.6 bB |
| MeSa 0.01 mM | 190.7 ± 4.8 abA | 196.7 ± 1.4 aA | 178.7 ± 2.0 abA | 192.2 ± 1.4 aB |
| MeSa 0.1 mM | 187.2 ± 3.4 abA | 195.2 ± 1.4 aA | 174.2 ± 5.5 abA | 191.5 ± 1.5 aB |
| MeSa 1 mM | 197.8 ± 5.4 bA | 207.7 ± 4.2 bA | 195.2 ± 2.6 cA | 212.5 ± 2.5 bB |
| ‘Magenta’ 2016 | ‘Crimson’ 2016 | |||
|---|---|---|---|---|
| Day 0 | Day 45 | Day 0 | Day 45 | |
| Control | 7.5 ± 0.2 aA | 6.1 ± 0.2 aB | 9.0 ± 0.1 aA | 6.6 ± 0.2 aB |
| SA 1 mM | 8.6 ± 0.3 bA | 7.0 ± 0.2 bB | 11.8 ± 0.4 bA | 7.7 ± 0.2 bB |
| ASA 1 mM | 8.5 ± 0.2 bA | 6.9 ± 0.1 bB | 10.8 ± 0.4 bA | 7.6 ± 0.3 bB |
| MeSa 1 mM | 8.7 ± 0.1 bA | 7.2 ± 0.3 bB | 12.4 ± 0.1 bA | 7.8 ± 0.3 bB |
| ‘Magenta’ 2017 | ‘Crimson’ 2017 | |||
| Day 0 | Day 45 | Day 0 | Day 45 | |
| Control | 8.6 ± 0.2 aA | 7.3 ± 0.3 aB | 10.2 ± 0.1 aA | 8.1 ± 0.2 aB |
| SA 0.01 mM | 11.3 ± 0.1 cA | 9.6 ± 0.2 cdB | 14.1 ± 0.2 cA | 12.3 ± 0.3 cB |
| SA 0.1 mM | 10.2 ± 0.4 bA | 8.5 ± 0.2 bB | 12.9 ± 0.3 bA | 10.8 ± 0.1 bB |
| SA 1 mM | 10.3 ± 0.3 bA | 8.6 ± 0.3 bB | 13.0 ± 0.2 bA | 10.9 ± 0.3 bB |
| ASA 0.01 mM | 10.6 ± 0.3 bcA | 9.3 ± 0.4 bcB | 14.3 ± 0.2 cdA | 11.0 ± 0.4 bB |
| ASA 0.1 | 14.5 ± 0.7 dA | 11.1 ± 0.5 dB | 14.2 ± 0.5 bcdA | 11.1 ± 0.2 bB |
| ASA 1 mM | 11.0 ± 0.1 bcA | 9.1 ± 0.2 bcB | 15.0 ± 0.2 dA | 12.7 ± 0.4 cB |
| MeSa 0.01 mM | 11.1 ± 0.3 bcA | 9.0 ± 0.3 bcB | 14.3 ± 0.3 cdA | 10.7 ± 0.3 bB |
| MeSa 0.1 mM | 11.4 ± 0.2 cA | 9.9 ± 0.2 cdB | 14.9 ± 0.2 dA | 12.5 ± 0.2 cB |
| MeSa 1 mM | 11.0 ± 0.2 bcA | 8.9 ± 0.2 bcB | 13.7 ± 0.4 bcA | 10.6 ± 0.1 bB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M.; Guillén, F. Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage. Antioxidants 2020, 9, 832. https://doi.org/10.3390/antiox9090832
García-Pastor ME, Zapata PJ, Castillo S, Martínez-Romero D, Valero D, Serrano M, Guillén F. Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage. Antioxidants. 2020; 9(9):832. https://doi.org/10.3390/antiox9090832
Chicago/Turabian StyleGarcía-Pastor, María E., Pedro J. Zapata, Salvador Castillo, Domingo Martínez-Romero, Daniel Valero, María Serrano, and Fabián Guillén. 2020. "Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage" Antioxidants 9, no. 9: 832. https://doi.org/10.3390/antiox9090832
APA StyleGarcía-Pastor, M. E., Zapata, P. J., Castillo, S., Martínez-Romero, D., Valero, D., Serrano, M., & Guillén, F. (2020). Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage. Antioxidants, 9(9), 832. https://doi.org/10.3390/antiox9090832











