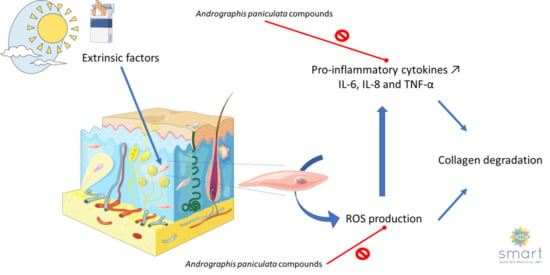
Andrographis paniculata and Its Bioactive Diterpenoids Protect Dermal Fibroblasts against Inflammation and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of A. paniculata Extract
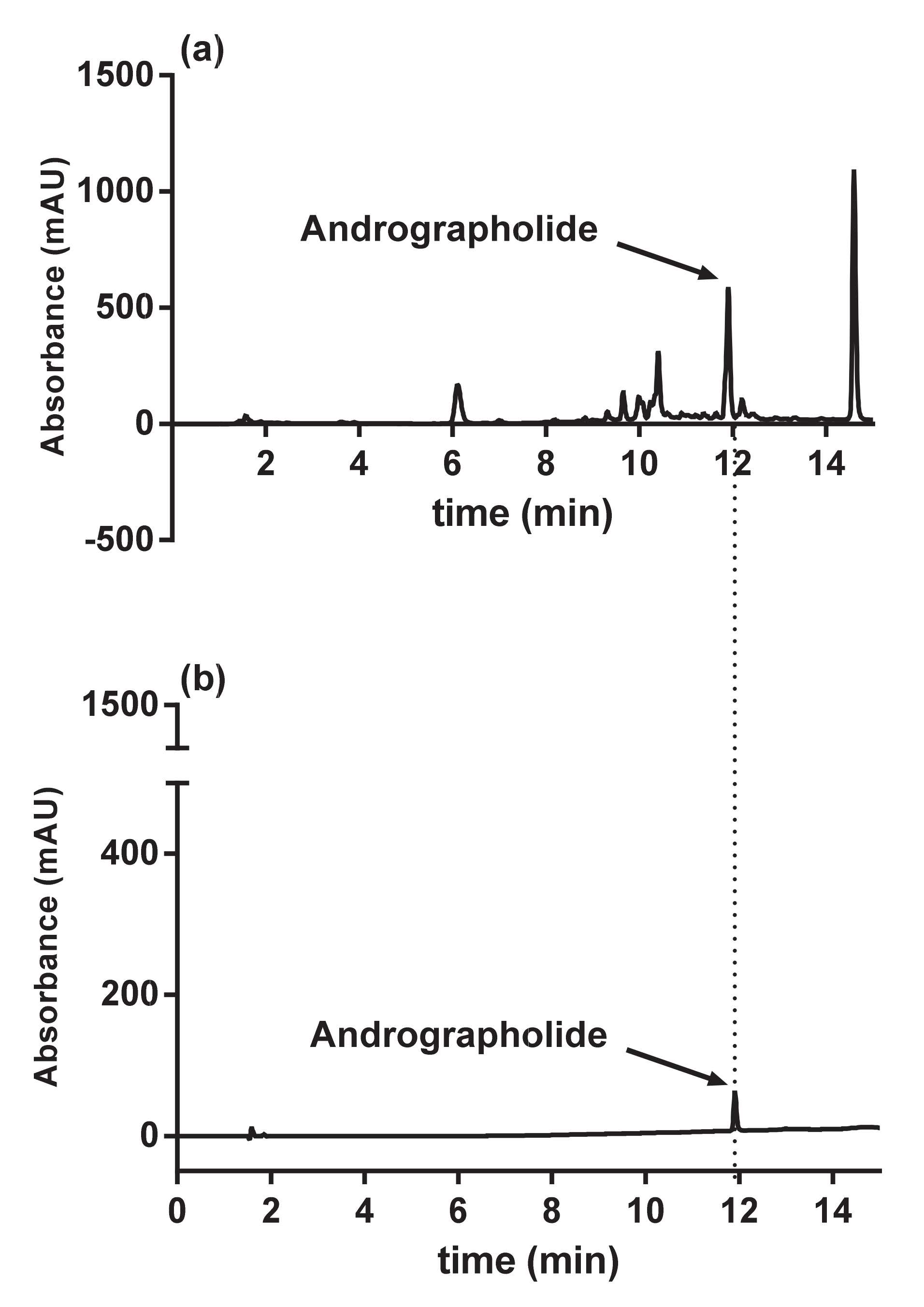
2.3. HPLC Analysis
2.4. Cell Culture
2.5. Cell Treatment
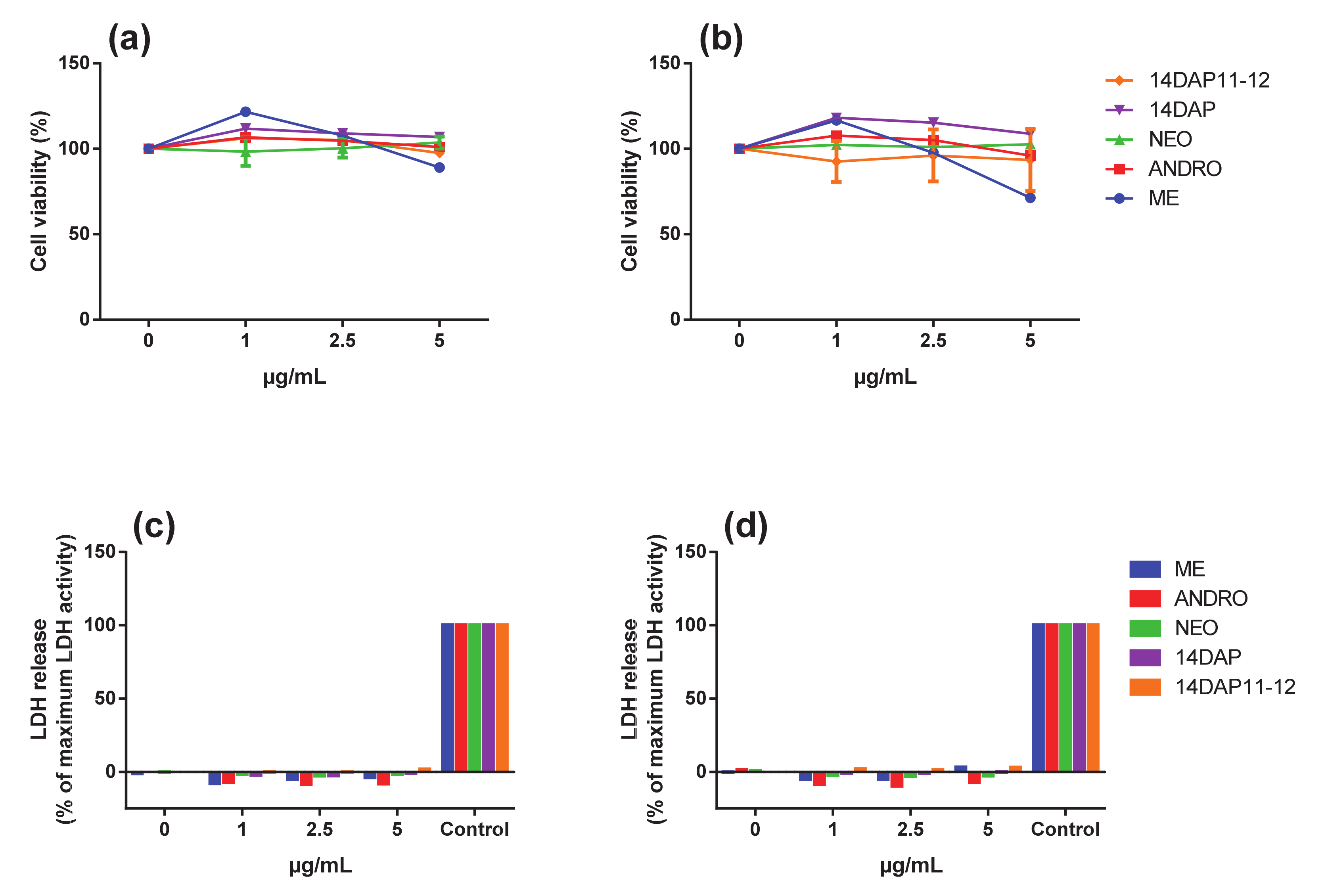
2.6. MTT Assay
2.7. Lactate Deshydrogénase Activity
2.8. Intracellular Reactive Oxygen Species (ROS)
2.9. Quantitative RT-PCR
2.10. Measurement of IL-6 and IL-8 Secretion
2.11. Measurement of Procollagen Type I Secretion
2.12. Statistic Test
3. Results
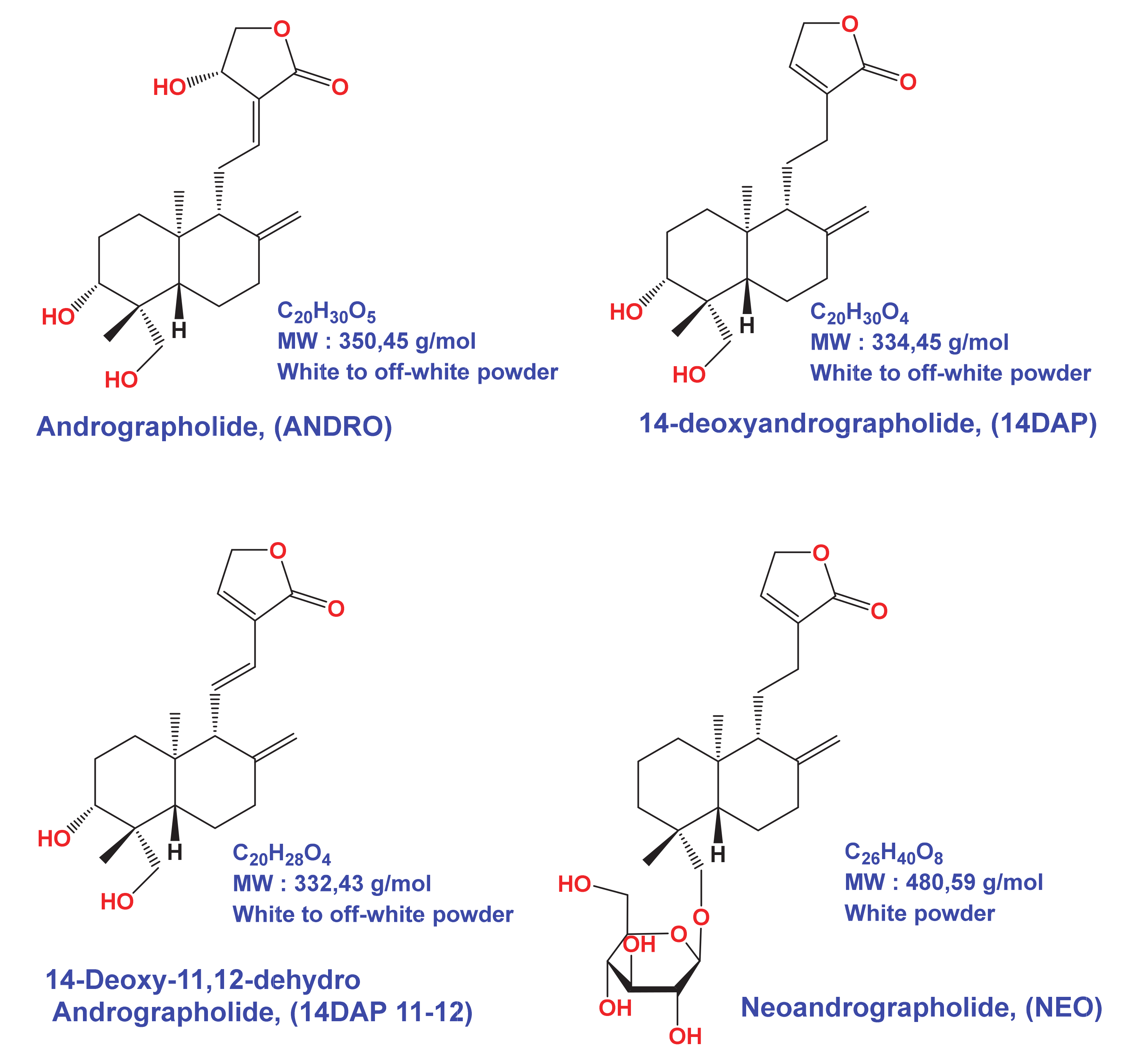
3.1. Analysis of Methanolic Extract from Andrographis paniculata
3.2. Cytotoxicity Assays
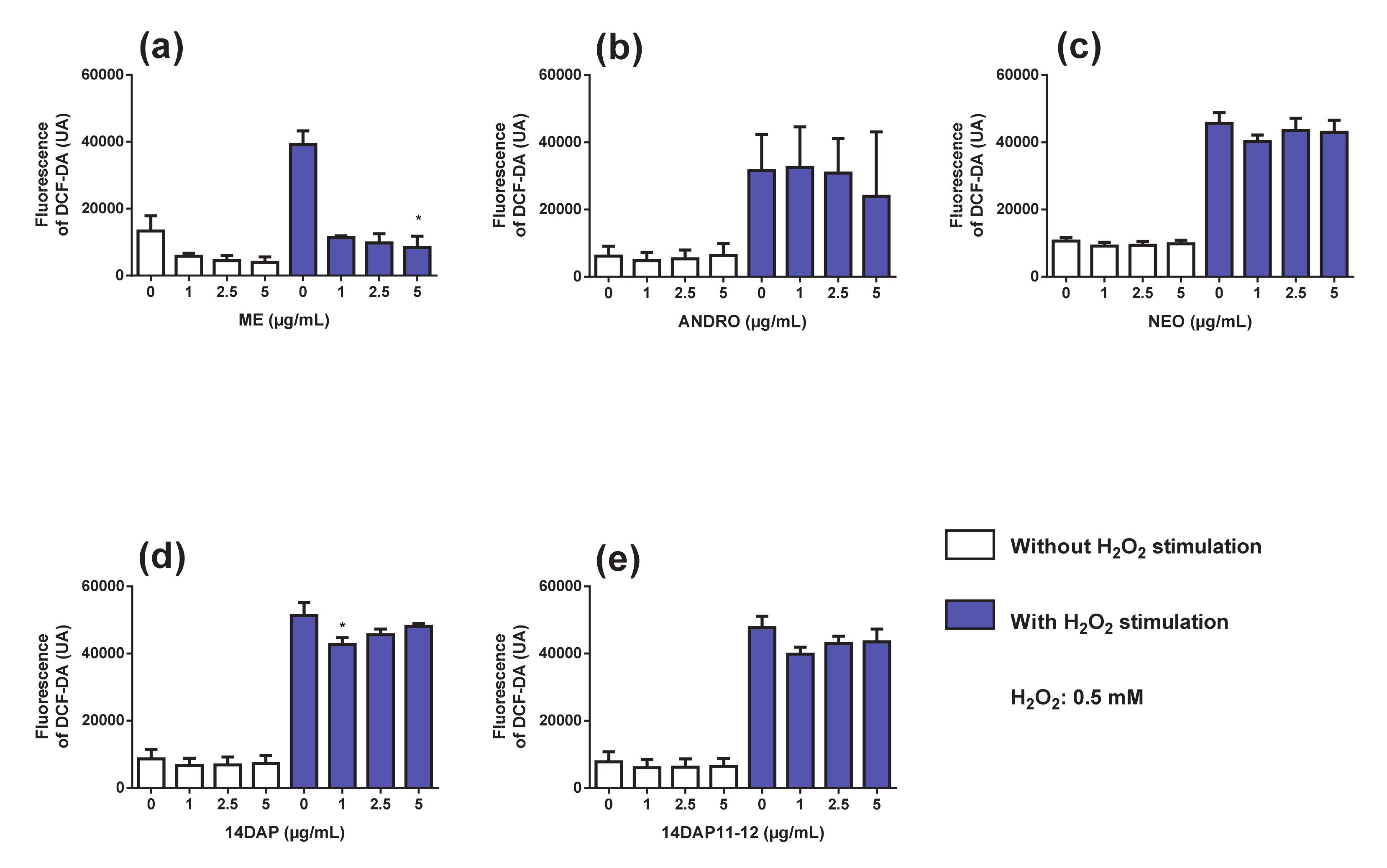
3.3. Antioxidant Activity
3.4. TNF-αExpression
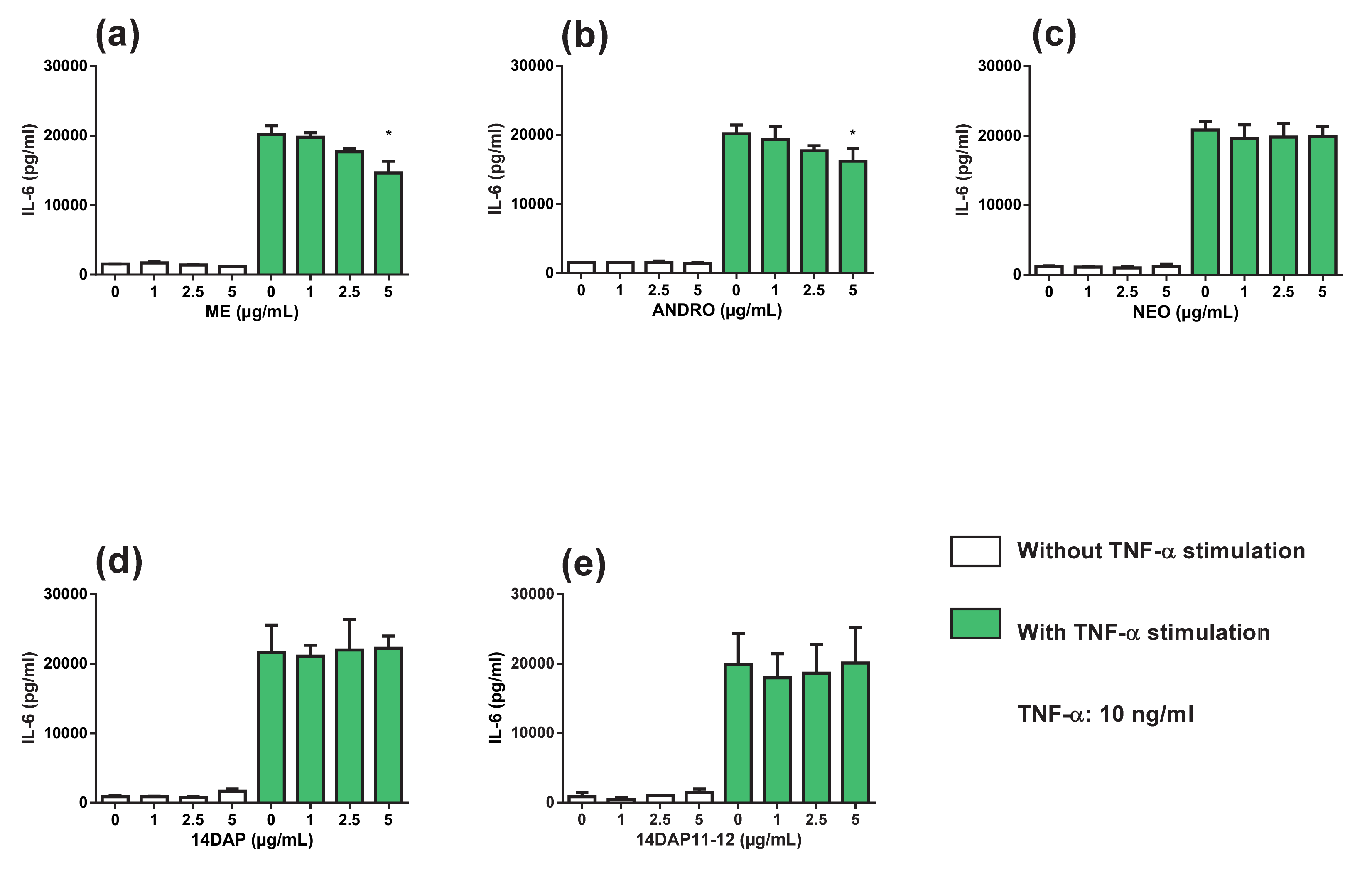
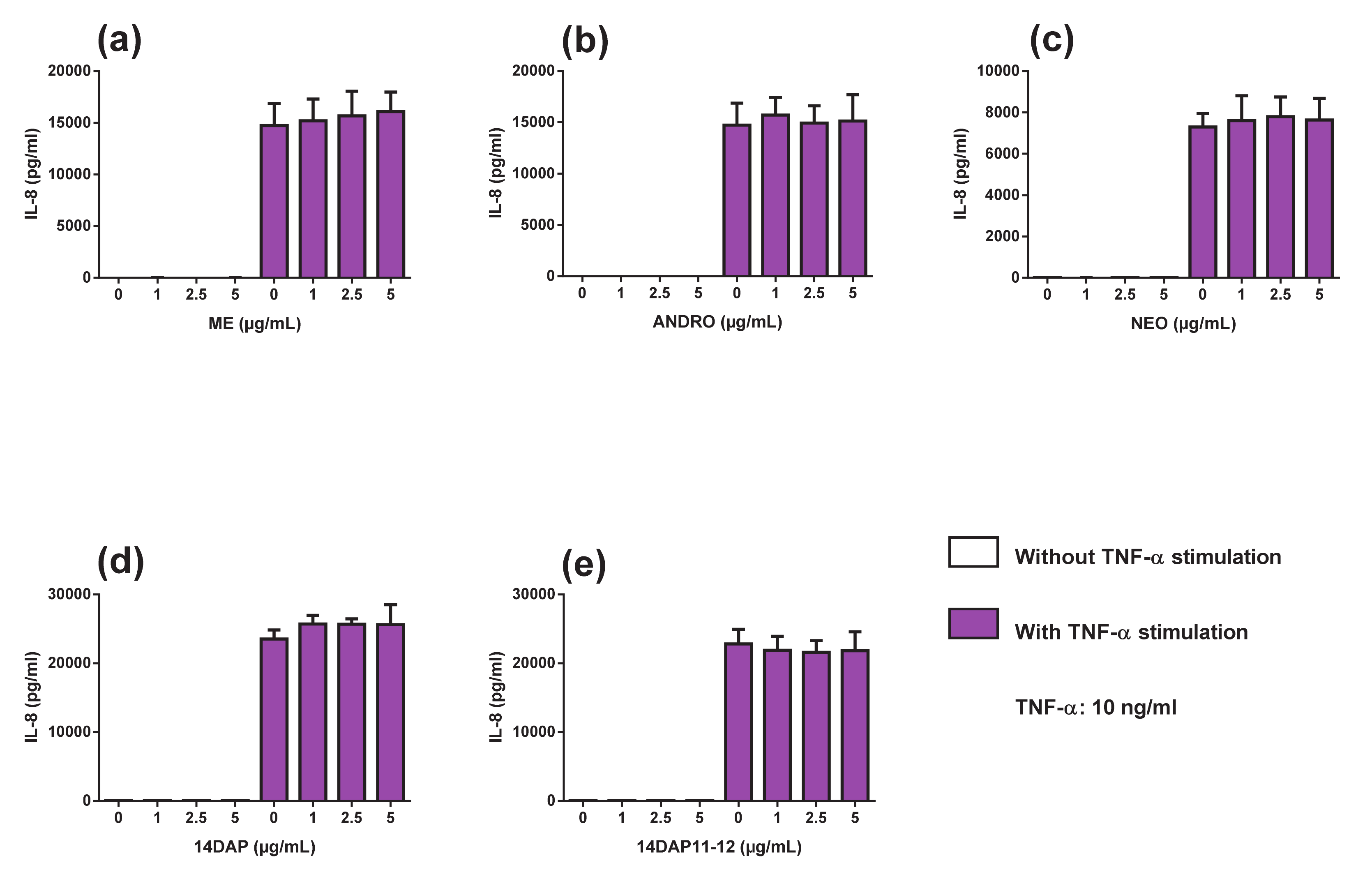
3.5. Cytokine Secretions
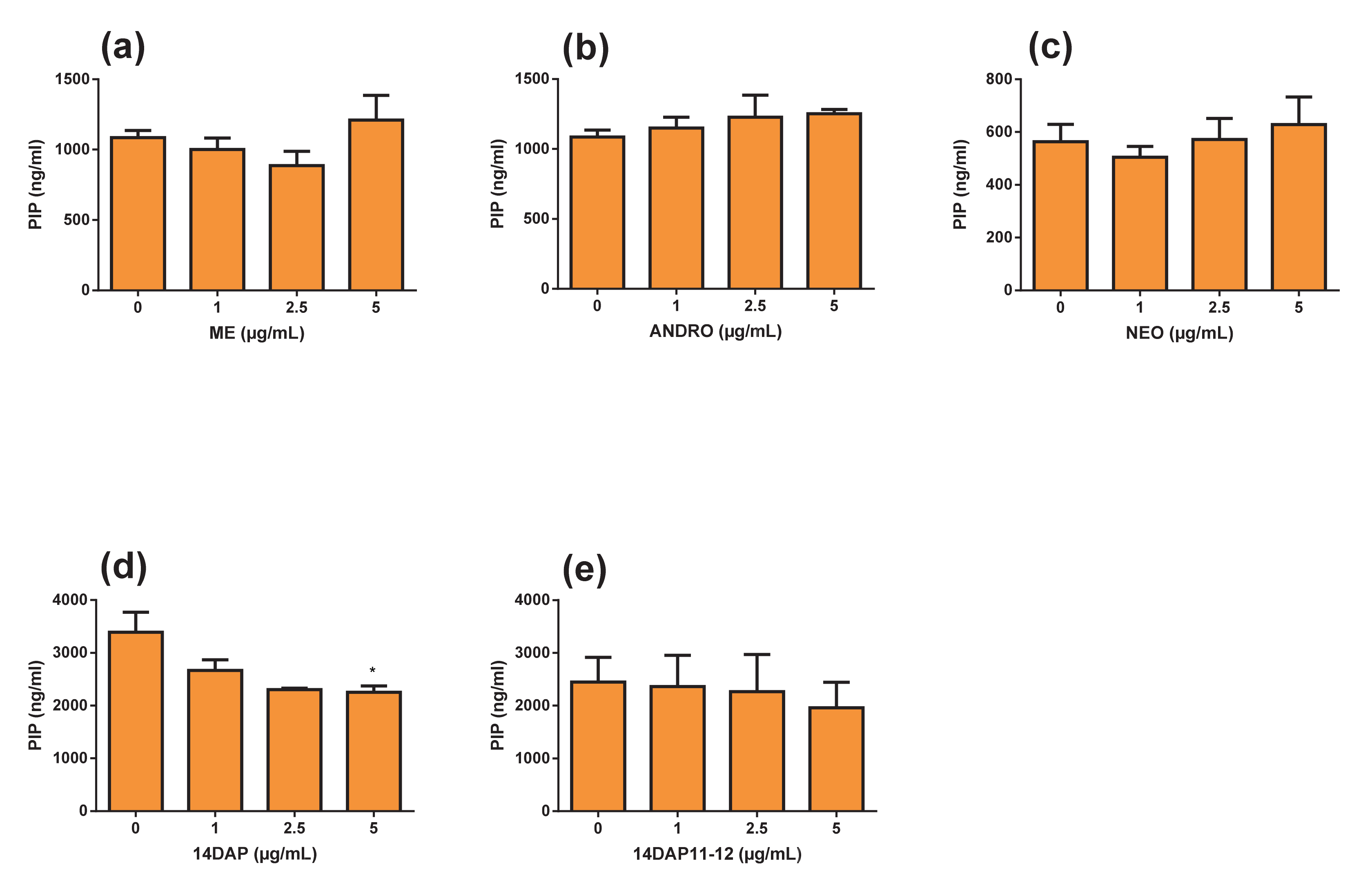
3.6. Pro-collagen Type I Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 14DAP | 14-deoxyandrographolide |
| 14DAP11-12 | 14-deoxy-11,12-didehydroandrographolide |
| ANDRO | andrographolide |
| HDFa | human dermal fibroblasts, adult |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| LDH | lactate dehydrogenase |
| ME | methanolic extract |
| NEO | neoandrographolide |
| PIP | procollagen type I carboxy-terminal |
| ROS | reactive oxygen species |
| TNF-α | tumor necrosis factor-α |
References
- Cavinato, M.; Jansen-Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. Skin aging and photoaging: An overview. J. Am. Acad. Dermatol. 1989, 21, 610–613. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Li, N.; Karin, M. Is NF-κB the sensor of oxidative stress? FASEB J. 1999, 13, 1137–1143. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, S.-S.; Bang, M.-H.; Jo, S.K.; Jhee, K.-H.; Yang, S.-A. Protection against UVB-induced damages in human dermal fibroblasts: Efficacy of tricin isolated from enzyme-treated Zizania latifolia extract. Biosci. Biotechnol. Biochem. 2019, 83, 551–560. [Google Scholar] [CrossRef]
- Tanaka, K.; Hasegawa, J.; Asamitsu, K.; Okamoto, T. Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor kappaB inhibitor, parthenolide. J. Pharmacol. Exp. Ther. 2005, 315, 624–630. [Google Scholar] [CrossRef]
- Lee, K.-J.; Park, K.H.; Hahn, J.-H. Alleviation of Ultraviolet-B Radiation-Induced Photoaging by a TNFR Antagonistic Peptide, TNFR2-SKE. Mol. Cells 2019, 42, 151–160. [Google Scholar] [CrossRef]
- Wang, C.; Eskiw, C.H. Cytoprotective effects of Avenathramide C against oxidative and inflammatory stress in normal human dermal fibroblasts. Sci. Rep. 2019, 9, 2932. [Google Scholar] [CrossRef] [PubMed]
- Andrulewicz-Botulińska, E.; Kuźmicz, I.; Nazaruk, J.; Wosek, J.; Galicka, A. The concentration-dependent effect of anethole on collagen, MMP-2 and GAG in human skin fibroblast cultures. Adv. Med. Sci. 2019, 64, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Shin, J.S.; Myung, D.B.; Ahn, H.S.; Lee, S.H.; Kim, H.J.; Lee, K.T. Hydrangea serrata (Thunb.) Ser. Extract Attenuate UVB-Induced Photoaging through MAPK/AP-1 Inactivation in Human Skin Fibroblasts and Hairless Mice. Nutrients 2019, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Son, D.; Shin, S.; Park, D.; Byun, S.; Jung, E. Protective effects of Camellia japonica flower extract against urban air pollutants. BMC Complement. Altern. Med. 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Park, N.H.; Kang, Y.G.; Kim, S.H.; Bae, I.H.; Lee, S.H.; Kim, D.Y.; Lee, E.S. Dehydroabietic Acid Induces Regeneration of Collagen Fibers in Ultraviolet B-Irradiated Human Dermal Fibroblasts and Skin Equivalents. Skin Pharmacol. Physiol. 2019, 32, 109–116. [Google Scholar] [CrossRef]
- Kielty, C.M.; Shuttleworth, C.A. Microfibrillar elements of the dermal matrix. Microsc. Res. Tech. 1997, 38, 413–427. [Google Scholar] [CrossRef]
- Meigel, W.N.; Gay, S.; Weber, L. Dermal architecture and collagen type distribution. Arch. Dermatol. Res. 1977, 259, 1–10. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lovell, C.R.; Smolenski, K.A.; Duance, V.C.; Light, N.D.; Young, S.; Dyson, M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C.; Sandby-Møller, J.; Kobayasi, T.; Gniadecki, R. Skin aging and natural photoprotection. Micron. Oxf. Engl. 1993 2004, 35, 185–191. [Google Scholar] [CrossRef]
- Aust, M.C.; Fernandes, D.; Kolokythas, P.; Kaplan, H.M.; Vogt, P.M. Percutaneous Collagen Induction Therapy: An Alternative Treatment for Scars, Wrinkles, and Skin Laxity. Plast Reconstr. Surg. 2008, 121, 1421. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Saraf, S.; Kaur, C.D. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn. Rev. 2010, 4, 1–11. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential role of natural compounds against skin aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. PTR 2003, 17, 987–1000. [Google Scholar] [CrossRef]
- Okhuarobo, A.; Ehizogie Falodun, J.; Erharuyi, O.; Imieje, V.; Falodun, A.; Langer, P. Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: A review of its phytochemistry and pharmacology. Asian Pac. J. Trop. Dis. 2014, 4, 213–222. [Google Scholar] [CrossRef]
- Tan, W.S.D.; Liao, W.; Zhou, S.; Wong, W.S.F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem. Pharmacol. 2017, 139, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sheeja, K.; Shihab, P.K.; Kuttan, G. Antioxidant and anti-inflammatory activities of the plant Andrographis paniculata Nees. Immunopharmacol. Immunotoxicol. 2006, 28, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Razak, Z.N.R.A.; Saad, W.M.M.; Mustakim, M. Mechanism of antagonistic effects of Andrographis paniculata methanolic extract against Staphylococcus aureus. Asian Pac. J. Trop. Med. 2017, 10, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Radhika, P.; Annapurna, A.; Rao, S.N. Immunostimulant, cerebroprotective & nootropic activities of Andrographis paniculata leaves extract in normal & type 2 diabetic rats. Indian J. Med. Res. 2012, 135, 636–641. [Google Scholar]
- Suzuki, R.; Matsushima, Y.; Okudaira, N.; Sakagami, H.; Shirataki, Y. Cytotoxic Components Against Human Oral Squamous Cell Carcinoma Isolated from Andrographis paniculata. Anticancer Res. 2016, 36, 5931–5935. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S.; Sharma, A. Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J. Med. Res. 1990, 92, 276–283. [Google Scholar]
- Tang, L.I.C.; Ling, A.P.K.; Koh, R.Y.; Chye, S.M.; Voon, K.G.L. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern. Med. 2012, 12, 3. [Google Scholar] [CrossRef]
- Chao, W.-W.; Lin, B.-F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- Sareer, O.; Ahmad, S.; Umar, S. Andrographis paniculata: A critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Nat. Prod. Res. 2014, 28, 2081–2101. [Google Scholar] [CrossRef]
- Paemanee, A.; Hitakarun, A.; Wintachai, P.; Roytrakul, S.; Smith, D.R. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 322–332. [Google Scholar] [CrossRef]
- Forestier-Román, I.S.; López-Rivas, A.; Sánchez-Vázquez, M.M.; Rohena-Rivera, K.; Nieves-Burgos, G.; Ortiz-Zuazaga, H.; Martínez-Ferrer, M. Andrographolide induces DNA damage in prostate cancer cells. Oncotarget 2019, 10, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, F.; Zhu, L.L.; Liu, P.; Shang, Y.R.; Liu, W.H.; Wang, Z.Y. Andrographolide impairs alpha-naphthylisothiocyanate-induced cholestatic liver injury in vivo. J. Nat. Med. 2019, 73, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.D.; Liao, W.; Peh, H.Y.; Vila, M.; Dong, J.; Shen, H.M.; Wong, W.F. Andrographolide simultaneously augments Nrf2 antioxidant defense and facilitates autophagic flux blockade in cigarette smoke-exposed human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2018, 360, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Yu, Q.-L.; Huang, Y.-S.; Yang, G. Neuroprotective effects of andrographolide derivative CX-10 in transient focal ischemia in rat: Involvement of Nrf2/AE and TLR/NF-κB signaling. Pharmacol. Res. 2019, 144, 227–234. [Google Scholar] [CrossRef]
- Batkhuu, J.; Hattori, K.; Takano, F.; Fushiya, S.; Oshiman, K.; Fujimiya, Y. Suppression of NO production in activated macrophages in vitro and ex vivo by neoandrographolide isolated from Andrographis paniculata. Biol. Pharm. Bull. 2002, 25, 1169–1174. [Google Scholar] [CrossRef]
- Kamdem, R.E.; Sang, S.; Ho, C.-T. Mechanism of the superoxide scavenging activity of neoandrographolide—A natural product from Andrographis paniculata Nees. J. Agric. Food Chem. 2002, 50, 4662–4665. [Google Scholar] [CrossRef]
- Lee, M.-J.; Rao, Y.K.; Chen, K.; Lee, Y.-C.; Chung, Y.-S.; Tzeng, Y.-M. Andrographolide and 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata attenuate high glucose-induced fibrosis and apoptosis in murine renal mesangeal cell lines. J. Ethnopharmacol. 2010, 132, 497–505. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.-T.; Ji, L.-L.; Ge, B.-X. Inhibitory effects of neoandrographolide on nitric oxide and prostaglandin E2 production in LPS-stimulated murine macrophage. Mol. Cell Biochem. 2007, 298, 49–57. [Google Scholar] [CrossRef]
- Mandal, S.; Nelson, V.K.; Mukhopadhyay, S.; Bandhopadhyay, S.; Maganti, L.; Ghoshal, N.; Biswas, T. 14-Deoxyandrographolide targets adenylate cyclase and prevents ethanol-induced liver injury through constitutive NOS dependent reduced redox signaling in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 59, 236–248. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Suthisisang, C.; Dhepakson, P.; Herunsalee, A. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression. Int. Immunopharmacol. 2010, 10, 1361–1373. [Google Scholar] [CrossRef]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, A natural Antioxydant: An Update. Antioxydants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Zhari, I.; Norhayati, I.; Mariam, A. HPLC and HPTLC densitometric determination of andrographolides and antioxidant potential of Andrographis paniculata. J. Food Compos. Anal. 2006, 19, 118–126. [Google Scholar] [CrossRef]
- Gu, L.; Yu, Q.; Li, Q.; Zhang, L.; Lu, H.; Zhang, X. Andrographolide Protects PC12 Cells Against β-Amyloid-Induced Autophagy-Associated Cell Death Through Activation of the Nrf2-Mediated p62 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2844. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.; Liu, X.; Du, Z.; Yang, R.; Zhao, Y. Andrographolide Ameliorates Diabetic Cardiomyopathy in Mice by Blockage of Oxidative Damage and NF-κB-Mediated Inflammation. Oxid. Med. Cell Longev. 2018, 2018, 9086747. [Google Scholar] [CrossRef]
- Wu, T.; Peng, Y.; Yan, S.; Li, N.; Chen, Y.; Lan, T. Andrographolide Ameliorates Atherosclerosis by Suppressing Pro-Inflammation and ROS Generation-Mediated Foam Cell Formation. Inflammation 2018, 41, 1681–1689. [Google Scholar] [CrossRef]
- Li, B.; Jiang, T.; Liu, H.; Miao, Z.; Fang, D.; Zheng, L.; Zhao, J. Andrographolide protects chondrocytes from oxidative stress injury by activation of the Keap1-Nrf2-Are signaling pathway. J. Cell Physiol. 2018, 234, 561–571. [Google Scholar] [CrossRef]
- Zhan, J.Y.X.; Wang, X.F.; Liu, Y.H.; Zhang, Z.B.; Wang, L.; Chen, J.N.; Lai, X.P. Andrographolide Sodium Bisulfate Prevents UV-Induced Skin Photoaging through Inhibiting Oxidative Stress and Inflammation. Med. Inflamm. 2016, 2016, 3271451. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem. Pharmacol. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Smith, R.S.; Smith, T.J.; Blieden, T.M.; Phipps, R.P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am. J. Pathol. 1997, 151, 317–322. [Google Scholar] [PubMed]
- Hwang, E.; Kim, S.H.; Lee, S.; Lee, C.H.; Do, S.G.; Kim, J.; Kim, S.Y. A comparative study of baby immature and adult shoots of Aloe vera on UVB-induced skin photoaging in vitro. Phytother. Res. PTR 2013, 27, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, M.J.; Ahn, J.; Kim, S.; Kim, H.H.; Kim, K.H.; Chung, J.H. Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J. Investig. Dermatol. 2004, 123, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Park, B.; Seo, S.A.; Cho, J.G.; Yi, T.H. Dietary Foeniculum vulgare Mill extract attenuated UVB irradiation-induced skin photoaging by activating of Nrf2 and inhibiting MAPK pathways. Phytomedicine 2016, 23, 1273–1284. [Google Scholar] [CrossRef]
- Zhong, J.; Hu, N.; Xiong, X.; Lei, Q.; Li, L. A novel promising therapy for skin aging: Dermal multipotent stem cells against photoaged skin by activation of TGF-β/Smad and p38 MAPK signaling pathway. Med. Hypotheses 2011, 76, 343–346. [Google Scholar] [CrossRef]
- Sheeja, K.; Kuttan, G. Andrographis paniculata downregulates proinflammatory cytokine production and augments cell mediated immune response in metastatic tumor-bearing mice. Asian Pac. J. Cancer Prev. APJCP 2010, 11, 723–729. [Google Scholar]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, C.; Chen, L.; Ma, P.; Li, K.; Jin, J.; Li, A. Effects of andrographolide on postoperative cognitive dysfunction and the association with NF-κB/MAPK pathway. Oncol. Lett. 2017, 14, 7367–7373. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tan, M.G.K.; Banks, W.A.; Wong, W.S.F.; Wong, P.T.-H.; Lai, M.K.P. Andrographolide attenuates LPS-stimulated up-regulation of C-C and C-X-C motif chemokines in rodent cortex and primary astrocytes. J. Neuroinflamm. 2016, 13, 34. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, H.; Wang, J.; Wang, W.; Gao, J. Synthesis of andrographolide analogues and their neuroprotection and neurite outgrowth-promoting activities. Bioorg. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Lee, K.-C.; Chang, H.-H.; Chung, Y.-H.; Lee, T.-Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Meng, W.; Liao, W.; Lian, S. Andrographolide Antagonizes TNF-α-Induced IL-8 via Inhibition of NADPH Oxidase/ROS/NF-κB and Src/MAPKs/AP-1 Axis in Human Colorectal Cancer HCT116 Cells. J. Agric. Food Chem. 2018, 66, 5139–5148. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. JDD 2008, 7 (Suppl. 2), s12–s16. [Google Scholar] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and Its Bioactive Diterpenoids Protect Dermal Fibroblasts against Inflammation and Oxidative Stress. Antioxidants 2020, 9, 432. https://doi.org/10.3390/antiox9050432
Mussard E, Jousselin S, Cesaro A, Legrain B, Lespessailles E, Esteve E, Berteina-Raboin S, Toumi H. Andrographis paniculata and Its Bioactive Diterpenoids Protect Dermal Fibroblasts against Inflammation and Oxidative Stress. Antioxidants. 2020; 9(5):432. https://doi.org/10.3390/antiox9050432
Chicago/Turabian StyleMussard, Eugenie, Sundy Jousselin, Annabelle Cesaro, Brigitte Legrain, Eric Lespessailles, Eric Esteve, Sabine Berteina-Raboin, and Hechmi Toumi. 2020. "Andrographis paniculata and Its Bioactive Diterpenoids Protect Dermal Fibroblasts against Inflammation and Oxidative Stress" Antioxidants 9, no. 5: 432. https://doi.org/10.3390/antiox9050432
APA StyleMussard, E., Jousselin, S., Cesaro, A., Legrain, B., Lespessailles, E., Esteve, E., Berteina-Raboin, S., & Toumi, H. (2020). Andrographis paniculata and Its Bioactive Diterpenoids Protect Dermal Fibroblasts against Inflammation and Oxidative Stress. Antioxidants, 9(5), 432. https://doi.org/10.3390/antiox9050432