Nitrosative Stress Biomarkers in the Non-Stimulated and Stimulated Saliva, as well as Gingival Crevicular Fluid of Patients with Periodontitis: Review and Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Collection
2.3. Clinical Examination
2.4. Redox Assays
2.5. Statistical Analysis
3. Results
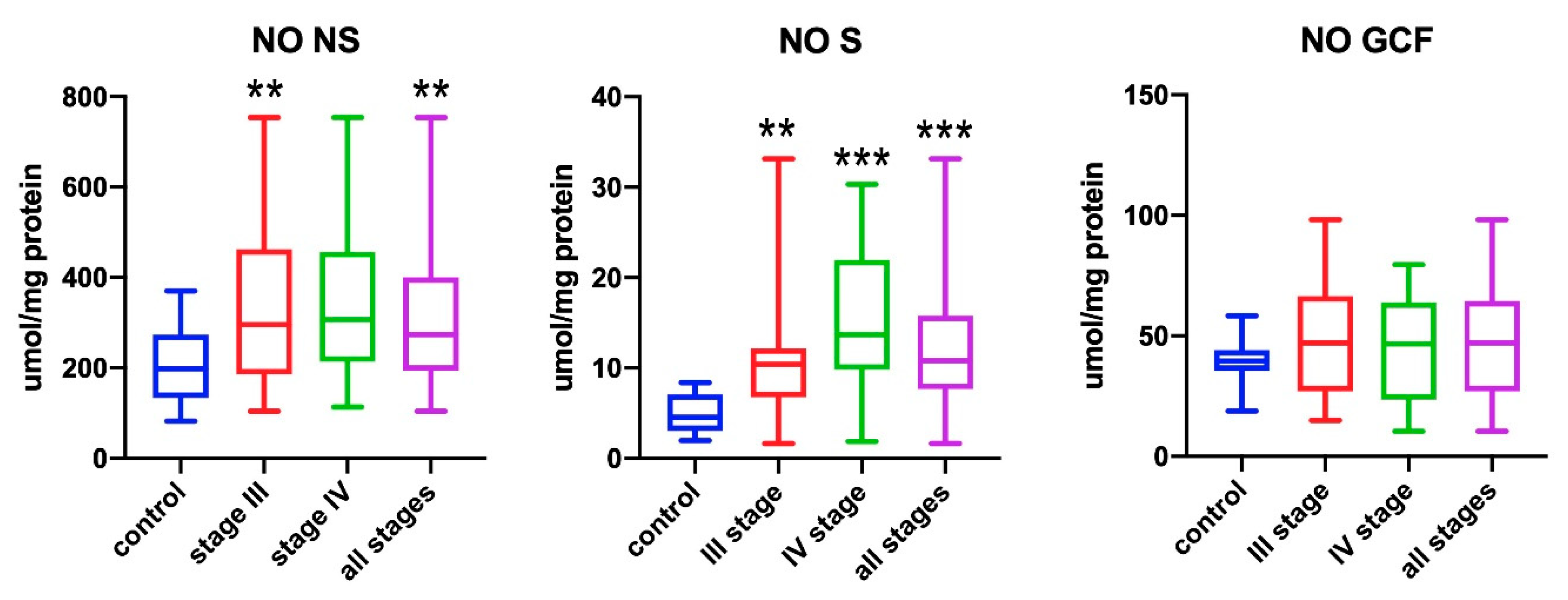
3.1. Nitric Oxide (NO)
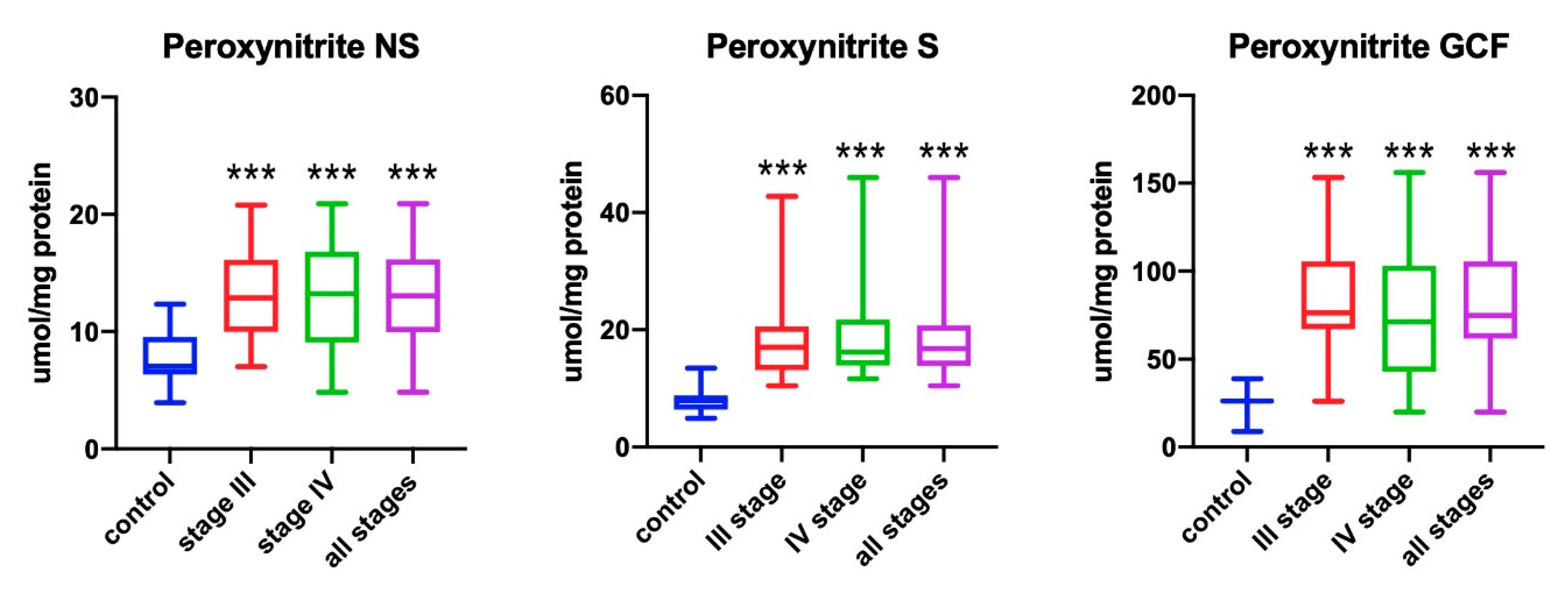
3.2. Peroxynitrite
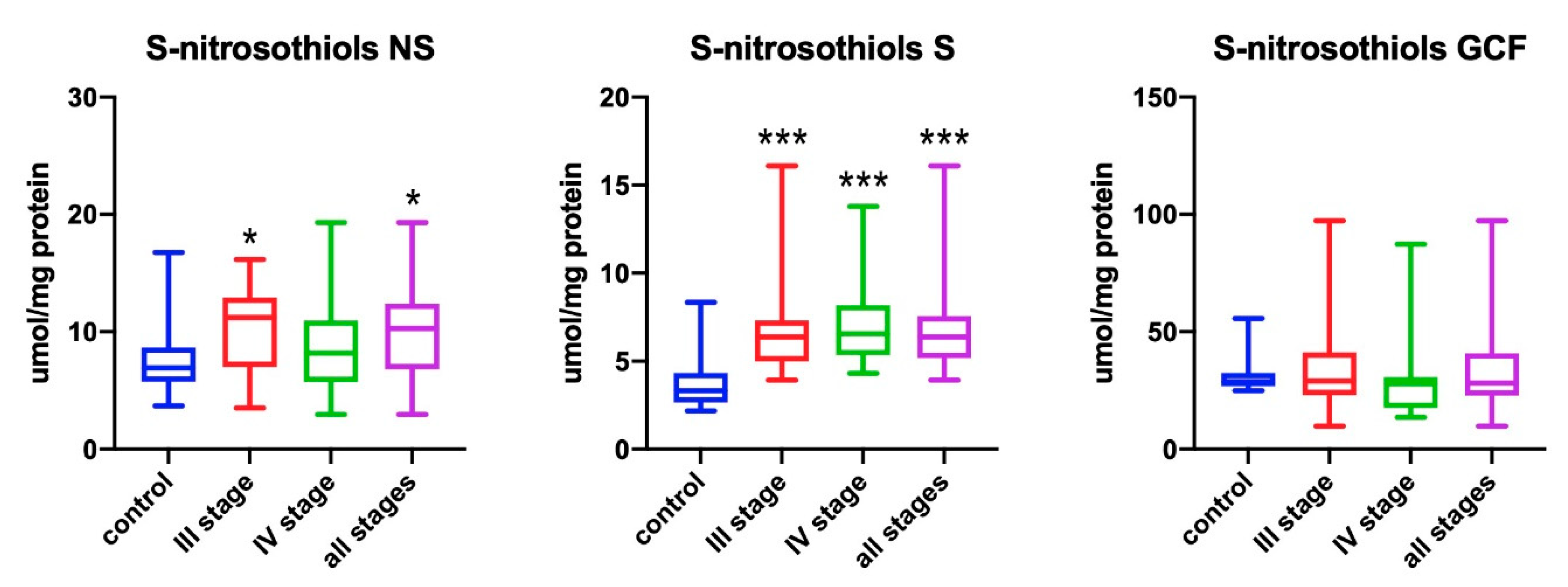
3.3. S-Nitrosothiols
3.4. Correlations
3.5. GCF Biomarkers
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.; Li, Y.R. Essentials of Free Radical Biology and Medicine; Cell Med Press AIMSCI, Inc.: Chicago, IL, USA, 2017. [Google Scholar]
- Kwon, N.S.; Nathan, C.F.; Gilker, C.; Griffith, O.W.; Matthews, D.E.; Stuehr, D.J. L-Citrulline production from L-arginine by macrophage nitric oxide synthase. The ureido oxygen derives from dioxygen. J. Biol. Chem. 1990, 265, 13442–13445. [Google Scholar] [PubMed]
- Özmeriç, N.; Elgün, S.; Uraz, A. Salivary arginase in patients with adult periodontitis. Clin. Oral Investig. 2000, 4, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; He, F. Mechanism and role of nitric oxide signaling in periodontitis. Exp. Ther. Med. 2019, 18, 3929–3935. [Google Scholar] [CrossRef] [PubMed]
- Parwani, S.R.; Chitnis, P.J.; Parwani, R.N. Salivary nitric oxide levels in inflammatory periodontal disease -A case-control and interventional study. Int. J. Dent. Hyg. 2012, 10, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Aurer, A.; Aleksić, J.; Ivić-Kardum, M.; Aurer, J.; Čulo, F. Nitric Oxide synthesis is decreased in periodontitis. J. Clin. Periodontol. 2001, 28, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int. J. Mol. Sci. 2017, 18, 2205. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Werbel, K.; Wasilewska, A.; Zalewska, A. Salivary FRAP as a marker of chronic kidney disease progression in children. Antioxidants 2019, 8, 409. [Google Scholar] [CrossRef]
- Sawczuk, B.; Maciejczyk, M.; Siemieniuk, S.; Posmyk, R.; Zalewska, A. Car Salivary Gland Function, Antioxidant Defence and Oxidative Damage in the Saliva of Patients with Breast Cancer: Does the BRCA1 Mutation Disturb the Salivary Redox Profile? Cancers (Basel) 2019, 11, 1501. [Google Scholar] [CrossRef] [PubMed]
- Fejfer, K.; Buczko, P.; Niczyporuk, M.; Ładny, J.R.; Hady, H.R.; Knaś, M.; Waszkiel, D.; Klimiuk, A.; Zalewska, A.; Maciejczyk, M. Oxidative Modification of Biomolecules in the Nonstimulated and Stimulated Saliva of Patients with Morbid Obesity Treated with Bariatric Surgery. Biomed. Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Klimiuk, A.; Maciejczyk, M.; Choromańska, M.; Fejfer, K.; Waszkiewicz, N.; Zalewska, A. Salivary Redox Biomarkers in Different Stages of Dementia Severity. J. Clin. Med. 2019, 8, 840. [Google Scholar] [CrossRef]
- Skutnik-Radziszewska, A.; Maciejczyk, M.; Fejfer, K.; Krahel, J.; Flisiak, I.; Kołodziej, U.; Zalewska, A. Salivary Antioxidants and Oxidative Stress in Psoriatic Patients: Can Salivary Total Oxidant Status and Oxidative Status Index Be a Plaque Psoriasis Biomarker? Oxid. Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Szulimowska, J.; Skutnik, A.; Taranta-Janusz, K.; Wasilewska, A.; Wiśniewska, N.; Zalewska, A. Salivary Biomarkers of Oxidative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2018, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Lange, D.E.; Plagmann, H.C.; Eenboom, A.; Promesberger, A. Klinische Bewertungsverahren zur Objektivierung der Mundhygiene. Dtsch. Zahnarztl. Z. 1977, 32, 44–47. [Google Scholar] [PubMed]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Newbrun, E. Indices to Measure Gingival Bleeding. J. Periodontol. 1996, 67, 555–561. [Google Scholar] [CrossRef]
- Taso, E.; Stefanovic, V.; Stevanovic, I.; Vojvodic, D.; Topic, A.; Petkovic-Curcin, A.; Obradovic-Djuricic, K.; Markovic, A.; Djukic, M.; Vujanovic, D. Influence of dental restorations on oxidative stress in gingival crevicular fluid. Oxid. Med. Cell. Longev. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R. Quantitation of nitrate and nitrite in extracellular fluids. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1996; Volume 268, pp. 237–246. [Google Scholar]
- Wink, D.A.; Kim, S.; Coffin, D.; Cook, J.C.; Vodovotz, Y.; Chistodoulou, D.; Jourd’heuil, D.; Grisham, M.B. Detection of S-nitrosothiols by fluorometric and colorimetric methods. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 301, pp. 201–211. [Google Scholar]
- Wassall, R.R.; Preshaw, P.M. Clinical and technical considerations in the analysis of gingival crevicular fluid. Periodontology 2000 2016, 70, 65–79. [Google Scholar] [CrossRef]
- Menaka, K.; Ramesh, A.; Thomas, B.; Kumari, N.S. Estimation of nitric oxide as an inflammatory marker in periodontitis. J. Indian Soc. Periodontol. 2009, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Dhotre, P.S.; Suryakar, A.N.; Bhogade, R.B. Oxidative stress in periodontitis: A critical link to cardiovascular disease. Biomed. Res. 2011, 22, 178–182. [Google Scholar]
- Mani Sundar, N.; Krishnan, V.; Krishnaraj, S.; Hemalatha, V.T.; Alam, M.N. Comparison of the salivary and the serum nitric oxide levels in chronic and aggressive periodontitis: A biochemical study. J. Clin. Diagn. Res. 2013, 7, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Haririan, H.; Bertl, K.; Rausch, W.D.; Bantleon, H.P.; Moritz, A.; Rausch-Fan, X. Nitric oxide production, systemic inflammation and lipid metabolism in periodontitis patients: Possible gender aspect. J. Clin. Periodontol. 2013, 40, 916–923. [Google Scholar] [CrossRef]
- Wadhwa, D.; Bey, A.; Hasija, M.; Moin, S.; Kumar, A.; Aman, S.; Sharma, V.K. Determination of levels of nitric oxide in smoker and nonsmoker patients with chronic periodontitis. J. Periodontal Implant Sci. 2013, 43, 215–220. [Google Scholar] [CrossRef]
- Poorsattar Bejeh-Mir, A.; Parsian, H.; Akbari Khoram, M.; Ghasemi, N.; Bijani, A.; Khosravi-Samani, M. Diagnostic Role of Salivary and GCF Nitrite, Nitrate and Nitric Oxide to Distinguish Healthy Periodontium from Gingivitis and Periodontitis. Int. J. Mol. Cell. Med. 2014, 3, 138–145. [Google Scholar]
- Wattamwar, P.P.; Kolte, R.A.; Kolte, A.P.; Shah, K.K. Influence of interventional nonsurgical periodontal treatment on levels of salivary and serum nitric oxide in smokers and nonsmokers with chronic periodontitis. J. Indian Soc. Periodontol. 2016, 20, 592–596. [Google Scholar]
- Dhotre, P.S.; Bhogade, R.B.; Shaikh, A.K. Nitric oxide levels in periodontitis with relation to obesity. Int. J. Clin. Biochem. Res. 2016, 3, 50. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Ozer, L.; Elgun, S.; Ozdemir, B.; Pervane, B.; Ozmeric, N. Arginine–Nitric Oxide–Polyamine Metabolism in Periodontal Disease. J. Periodontol. 2011, 82, 320–328. [Google Scholar] [CrossRef]
- Mazurek-Mochol, M.; Kozak, M.; Sawczuk, M.; Maciejewska, A.; Malinowski, D.; Safranow, K.; Pawlik, A. NOS3 gene rs1799983 and rs2070744 polymorphisms in patients with periodontal disease. Folia Biol. 2018, 64, 59–64. [Google Scholar]
- Topcu, A.O.; Akalin, F.A.; Sahbazoglu, K.B.; Yamalik, N.; Kilinc, K.; Karabulut, E.; Tözüm, T.F. Nitrite and Nitrate Levels of Gingival Crevicular Fluid and Saliva in Subjects with Gingivitis and Chronic Periodontitis. J. Oral Maxillofac. Res. 2014, 5, e5. [Google Scholar] [CrossRef] [PubMed]
- Meschiari, C.A.; Zuardi, L.R.; Gomes, V.A.; Costa de Almeida, G.R.; Novaes, A.B.; Gerlach, R.F.; Marcaccini, A.M. Salivary, blood and plasma nitrite concentrations in periodontal patients and healthy individuals before and after periodontal treatment. Clin. Chim. Acta 2015, 444, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Scarel-Caminaga, R.; Cera, F.; Pigossi, S.; Finoti, L.; Kim, Y.; Viana, A.; Secolin, R.; Montenegro, M.; Tanus-Santos, J.; Orrico, S.; et al. Inducible Nitric Oxide Synthase Polymorphisms and Nitric Oxide Levels in Individuals with Chronic Periodontitis. Int. J. Mol. Sci. 2017, 18, 1128. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zalewska, A.; Gerreth, A.K. Salivary Redox Biomarkers in Selected Neurodegenerative Diseases. J. Clin. Med. 2020, 9, 497. [Google Scholar] [CrossRef]
- Klimiuk, A.; Zalewska, A.; Sawicki, R.; Knapp, M.; Maciejczyk, M. Salivary Oxidative Stress Increases with the Progression of Chronic Heart Failure. J. Clin. Med. 2020, 9, 769. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Muraglie, S.; Leonardi, R.; Lo Giudice, A. Assessment of Vitamin C and Antioxidant Profiles in Saliva and Serum in Patients with Periodontitis and Ischemic Heart Disease. Nutrients 2019, 11, 2956. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Williams, R.C.; Siciliano, V.I.; Alibrandi, A.; Cordasco, G.; Ramaglia, L. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 791–800. [Google Scholar] [CrossRef]
- Toczewska, J.; Konopka, T. Activity of enzymatic antioxidants in periodontitis: A systematic overview of the literature. Dent. Med. Probl. 2019, 56, 419–426. [Google Scholar] [CrossRef]

| Control | Stage III | Stage IV | All Stages | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max | ||
| age | 40.3 | 9.58 | 39 | 20 | 55 | 43.3 | 8.99 | 45 | 20 | 55 | 44 | 8.03 | 45 | 29 | 55 | 43.6 | 8.56 | 45 | 20 | 55 | |
| sex | women | 17 (57%) | 17 (47%) | 13 (54%) | 30 (50%) | ||||||||||||||||
| men | 13 (43%) | 19 (53%) | 11 (46%) | 30 (50%) | |||||||||||||||||
| non-stimulated saliva flow (mL/min) | 0.42 | 0.19 | 0.4 | 0.2 | 1 | 0.41 | 0.22 | 0.375 | 0.1 | 1 | 0.5 | 0.3 | 0.45 | 0.1 | 1.3 | 0.45 | 0.26 | 0.4 | 0.1 | 1.3 | |
| stimulated saliva flow (mL/min) | 1.74 | 0.75 | 1.55 | 0.4 | 3.4 | 1.43 | 0.66 | 1.4 | 0.3 | 3 | 1.47 | 0.67 | 1.4 | 0.6 | 3 | 1.45 | 0.66 | 1.4 | 0.3 | 3 | |
| protein in non-stimulated saliva (µg/mL) | 633.13 | 189.88 | 624.16 | 300.45 | 1101 | 865.87 | 237.05 | 824.55 | 481.23 | 1387 | 898.13 | 451.84 | 839.62 | 23.48 | 1847.1 | 879.15 | 338.4 | 827.95 | 23.48 | 1847.1 | |
| protein in stimulated saliva (µg/mL) | 592.16 | 160.87 | 599.74 | 235.74 | 946.29 | 594.85 | 179.63 | 626.28 | 28.91 | 926.41 | 554.59 | 217.35 | 634.78 | 42.74 | 811.92 | 577.99 | 193.06 | 634.78 | 28.91 | 926.41 | |
| protein in gingival fluid (µg/mL) | 39.23 | 21.56 | 31.22 | 8.44 | 91.66 | 132.30 | 69.83 | 130.43 | 36.8 | 336.97 | 178.78 | 109.71 | 134.24 | 45.5 | 445.64 | 151.82 | 90.75 | 131.13 | 36.8 | 445.64 | |
| number of teeth | 26.1 | 2.63 | 27.5 | 19 | 28 | 26.86 | 1.53 | 27.5 | 21 | 28 | 22.21 | 4 | 23 | 15 | 28 | 25 | 3.59 | 26 | 15 | 28 | |
| PI | 22.22 | 16.16 | 20.5 | 0 | 79 | 45.25 | 25.59 | 43 | 9 | 100 | 49.79 | 32.14 | 43.5 | 0 | 100 | 47.07 | 28.21 | 43.5 | 0 | 100 | |
| API | 36.87 | 16.27 | 32 | 7 | 68 | 64.06 | 21.26 | 65 | 29 | 100 | 77.54 | 23.41 | 86.5 | 22 | 100 | 69.45 | 23.14 | 72.5 | 22 | 100 | |
| BoP | 11.8 | 7.34 | 9.5 | 0.7 | 26 | 45.12 | 27.66 | 41 | 4 | 100 | 63.38 | 28.70 | 61 | 17 | 100 | 52.42 | 29.26 | 46.5 | 4 | 100 | |
| PD | 1.74 | 0.32 | 1.7 | 1.2 | 2.3 | 3.19 | 0.66 | 3.15 | 2.1 | 5.3 | 4 | 0.61 | 4.1 | 2.7 | 5.4 | 3.51 | 0.75 | 3.5 | 2.1 | 5.4 | |
| mean CAL > 0 | 2.26 | 1.17 | 2.1 | 1 | 5.2 | 4.74 | 1.4 | 4.65 | 2.4 | 8.1 | 6.1 | 1.75 | 6.05 | 3 | 10.1 | 5.29 | 1.68 | 5.4 | 2.4 | 10.1 | |
| Author, Year and Country | Fluid Method | Study Group Size and Age | p for Perio | Other Data |
|---|---|---|---|---|
| Aurer et al. [7], 2001, Croatia | NS saliva S saliva Griess reaction | AgP, 25 (19–35) CP, 25 (39–59) HP, 25 (21–42) | NS saliva ↓ NO p < 0.001 S saliva ↓ NO p < 0.05 | Decrease depends on periodontitis severity |
| Menaka et al. [24], 2009, India | Serum Griess reaction | CP, 30 (18–45) HP, 30 (18–45) | ↑ NO p = 0.000 | |
| Dhotre et al. [25], 2011, India | NS saliva Serum Cortas and Wakid method | P, 100 (mean 52.7) HP, 100 (mean 50.3) | Saliva ↑ NO p < 0.001 serum ↑ NO p < 0.001 | |
| Parwani et al. [6], 2012, India | NS saliva Griess reaction | CP, 30 (20–60) HP, 30 (20–60) | ↑ NO p = 0.000 | Positive correlation with PD |
| Sundar et al. [26], 2013, India | NS saliva Serum Griess reaction | AgP, 20 (25–35) CP, 20 (35–55) HP, 20 (25–55) | Saliva ↑ NO p < 0.001 serum ↑ NO p < 0.001 | Positive correlation for both with plaque, GI, PD, CAL, and concentration for saliva and serum |
| Andrukhov et al. [27], 2013, Austria | NS saliva (nitrite) Serum Griess reaction | Severe P, 89 (mean 34.3) HP, 54 (mean 42.2) | Saliva ↓ NO p < 0.01 serum ↓ NO p < 0.01 | No correlation between saliva and serum |
| Wadhwa et al. [28], 2013, India | NS saliva Serum Griess reaction | CP, 20 (no data) HP, 20 (no data) | Saliva ↑ NO p < 0.05 serum ↑ NO p < 0.05 | |
| Poorsattar Bejeh- Mir [29], 2014, Iran | GCF NS saliva ELISA | P, 14 (mean 38.3) HP, 14 (mean 37.7) | GCF ↓ NO p < 0.001 saliva ↑ NO p = 0.007 | Sensitivity and specificity for NO in saliva in periodontitis is 0.93 and 0.96 |
| Wattamwar et al. [30], 2016, India | NS saliva Serum Griess reaction | CP, 20 (30–55) | saliva ↑ NO p < 0.001 versus serum | Significant correlation for both with plaque, GI, PD, CAL, and between saliva and serum |
| Own study | GCF NS saliva S saliva Griess reaction | P, 60 (20–55) HP, 30 (20–55) | GCF, n.s. serum ↑ NO p < 0.001 | In severe P in GCF positive correlation with interproximal CAL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toczewska, J.; Konopka, T.; Zalewska, A.; Maciejczyk, M. Nitrosative Stress Biomarkers in the Non-Stimulated and Stimulated Saliva, as well as Gingival Crevicular Fluid of Patients with Periodontitis: Review and Clinical Study. Antioxidants 2020, 9, 259. https://doi.org/10.3390/antiox9030259
Toczewska J, Konopka T, Zalewska A, Maciejczyk M. Nitrosative Stress Biomarkers in the Non-Stimulated and Stimulated Saliva, as well as Gingival Crevicular Fluid of Patients with Periodontitis: Review and Clinical Study. Antioxidants. 2020; 9(3):259. https://doi.org/10.3390/antiox9030259
Chicago/Turabian StyleToczewska, Joanna, Tomasz Konopka, Anna Zalewska, and Mateusz Maciejczyk. 2020. "Nitrosative Stress Biomarkers in the Non-Stimulated and Stimulated Saliva, as well as Gingival Crevicular Fluid of Patients with Periodontitis: Review and Clinical Study" Antioxidants 9, no. 3: 259. https://doi.org/10.3390/antiox9030259
APA StyleToczewska, J., Konopka, T., Zalewska, A., & Maciejczyk, M. (2020). Nitrosative Stress Biomarkers in the Non-Stimulated and Stimulated Saliva, as well as Gingival Crevicular Fluid of Patients with Periodontitis: Review and Clinical Study. Antioxidants, 9(3), 259. https://doi.org/10.3390/antiox9030259







