Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Production of Fucus Vesiculosus Extracts
2.3. Production of Extruded Rye Snacks
2.4. Bioactivity by Chemical Assays
2.4.1. Total Polyphenol Content
2.4.2. Oxygen Radical Absorbance Capacity
2.4.3. DPPH Radical Scavenging Activity
2.4.4. Ferrous Ion Chelating Ability
2.4.5. ABTS Assay
2.5. Bioactivity by Cell-Based Assays
2.5.1. Cellular Antioxidant Activity
2.5.2. Cell Protective Effects against Induced Oxidative Stress
2.6. Statistical Analysis
3. Results
3.1. Bioactive Properties of Fucus Vesiculosus Extracts
3.1.1. Total Polyphenol Content and Antioxidant Activity
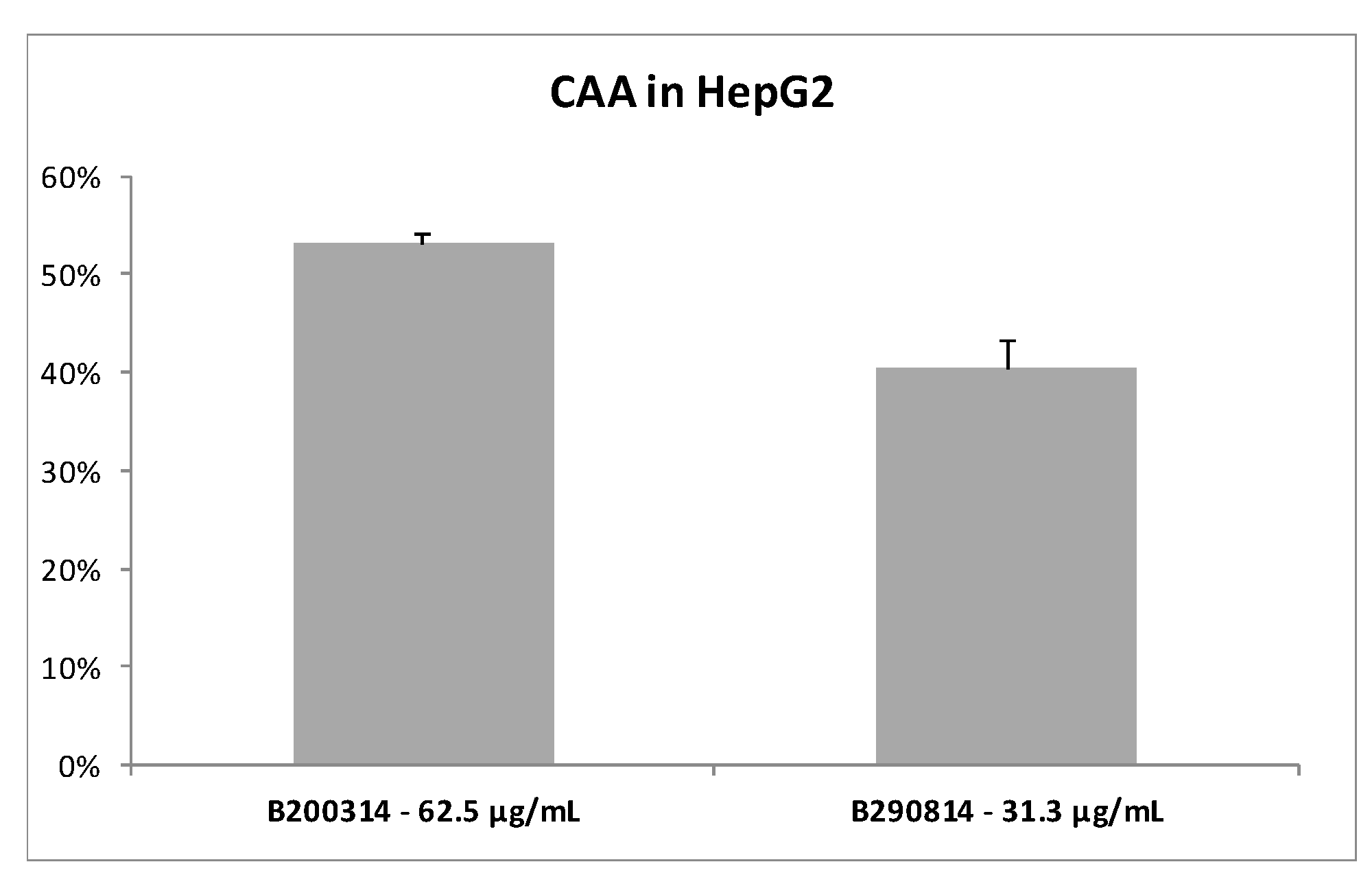
3.1.2. Cellular Antioxidant Activity of the Fucus Vesiculosus Extracts
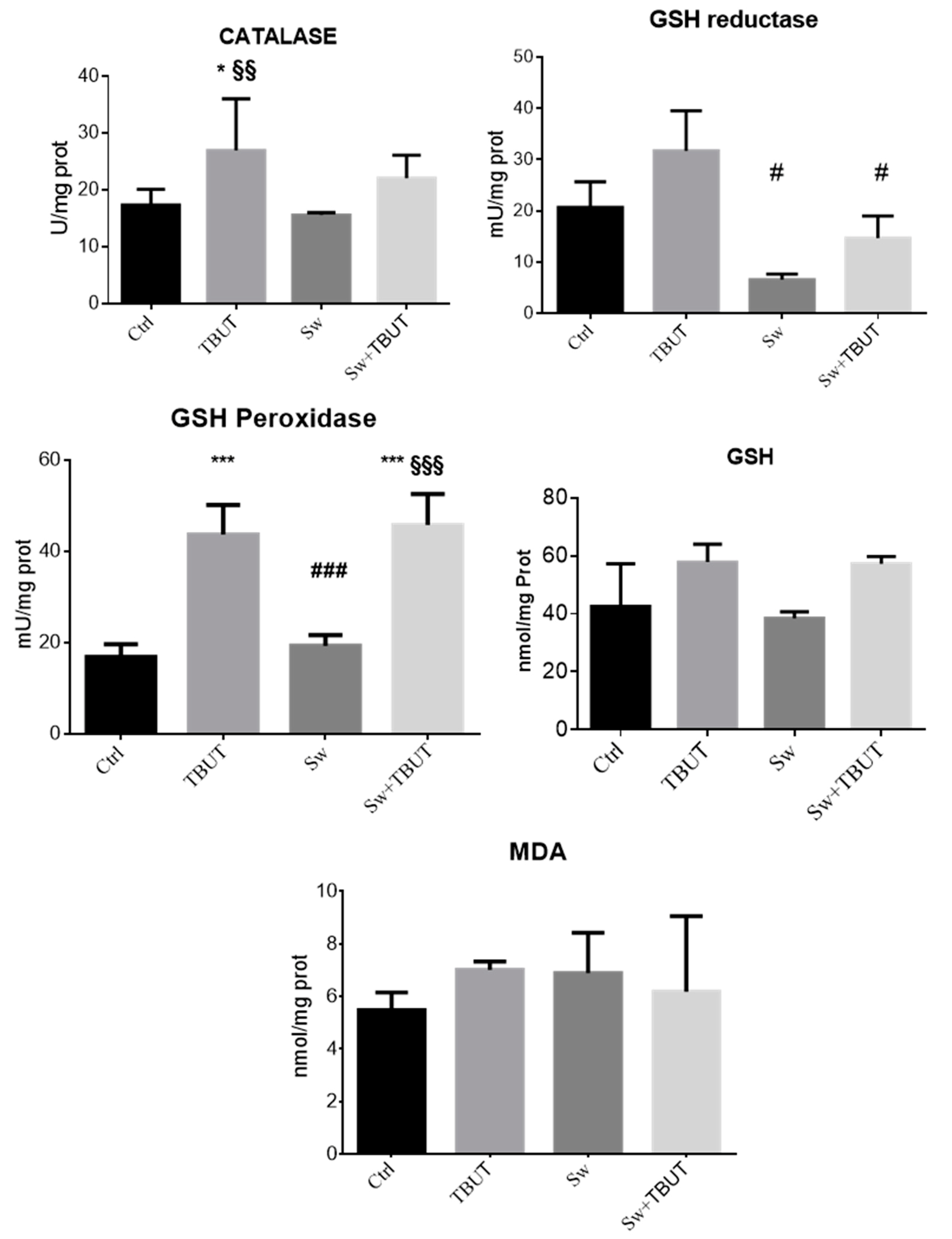
3.1.3. Cell Protective Effects of Seaweed Extract against Induced Oxidative Stress
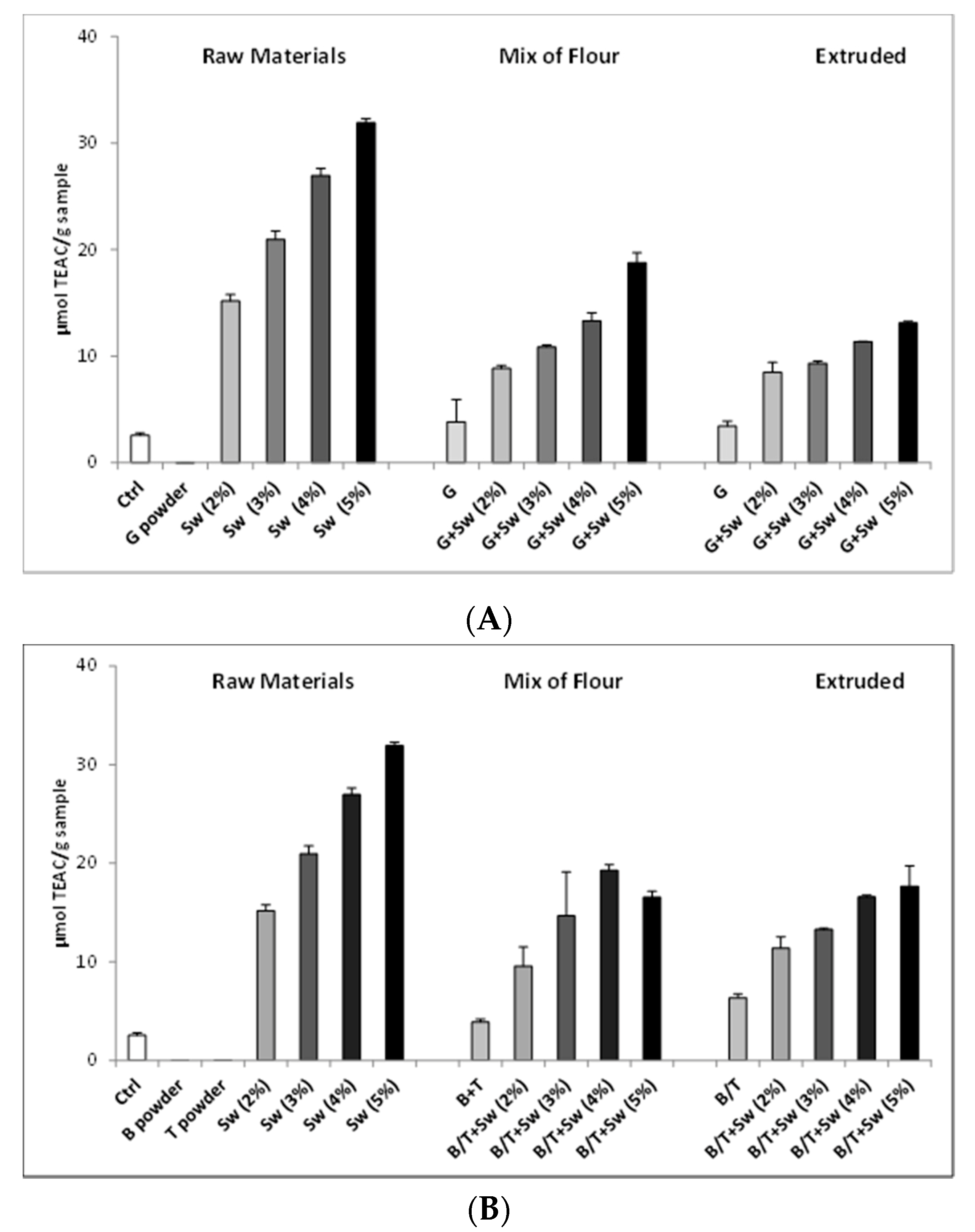
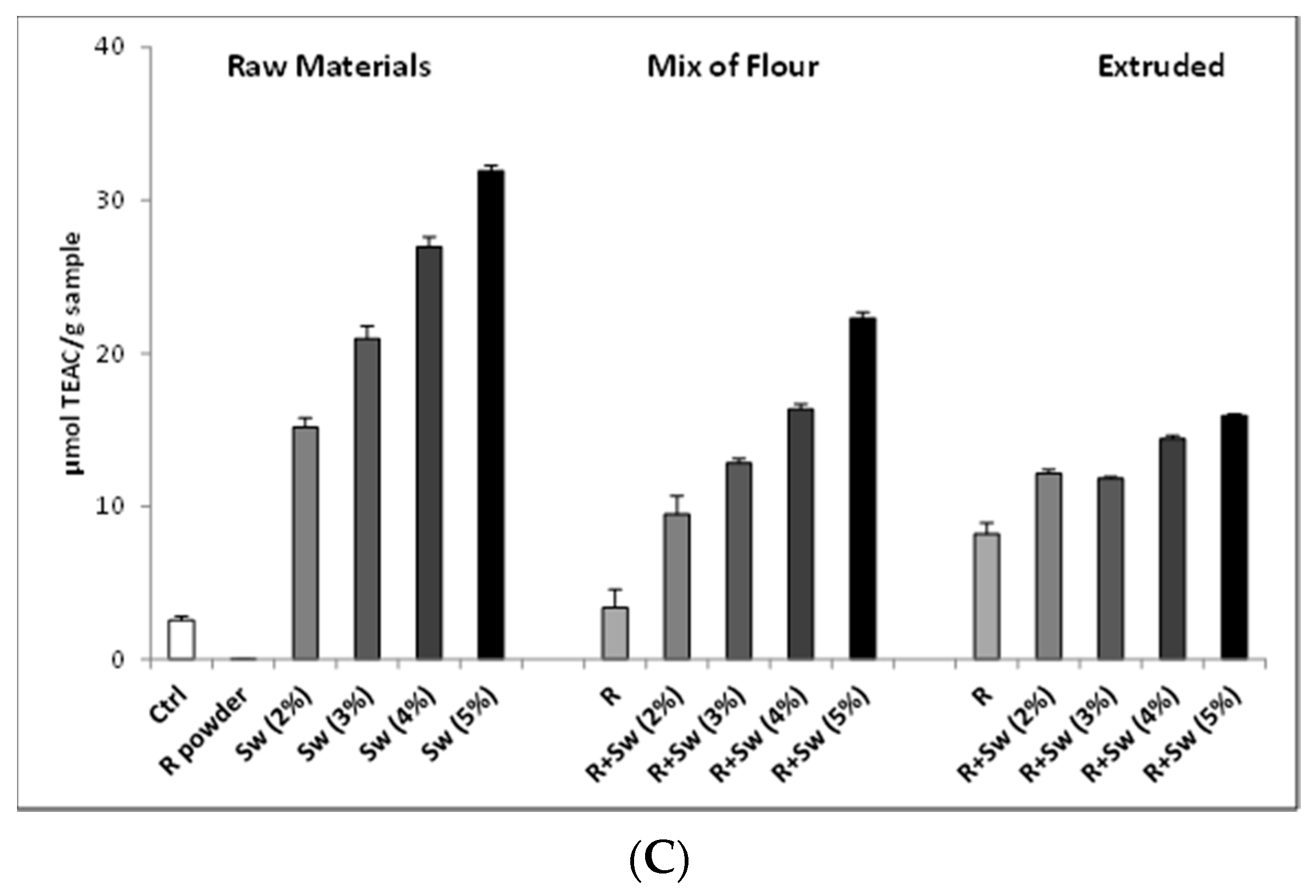
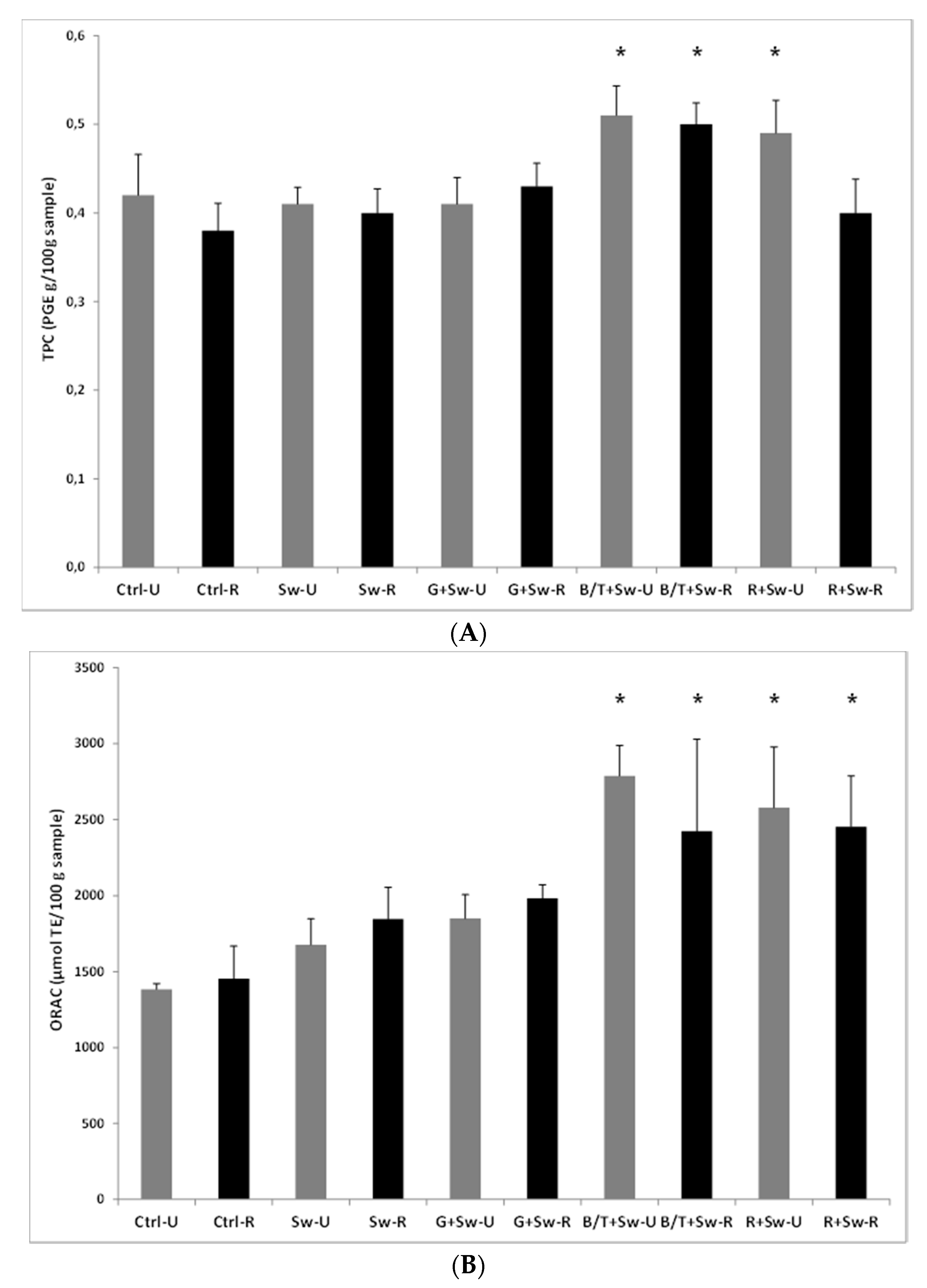
3.1.4. Development and Test of Enriched Rye Snacks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakir, S.; Catalkaya, G.; Ceylan, F.D.; Khan, H.; Guldiken, B.; Capanoglu, E.; Kamal, M.A. Role of dietary antioxidants in neurodegenerative diseases: Where are we standing? Curr. Pharm. Des. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, A.; Raizner, A. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment. Methodist Debakey Cardiovasc. J. 2019, 15, 179–184. [Google Scholar] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.; Ferreira, I.C. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Rickert, E.; Wahl, M.; Heike, L.; Hannes, R.; Pohnert, G. Seasonal Variations in Surface Metabolite Composition of Fucus Vesiculosus and Fucus Serratus From the Baltic Sea. PLoS ONE 2016, 11, e0168196. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Brown, E.M.; Allsopp, P.J.; Magee, P.-J.; Gill, C.I.R.; Nitecki, S.; Strain, C.R.; McSrley, E.M. Seaweed and human Health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Trinchero, J.; Ponce, N.M.; Cordoba, O.L.; Flores, M.L.; Pampuro, S.; Stortz, C.A.; Salomón, H.; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother. Res. 2009, 23, 707–712. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Sardari, R.R.R.; Nordberg Karlsson, E. Marine Poly- and Oligosaccharides as Prebiotics. J. Agric. Food Chem. 2018, 66, 11544–11549. [Google Scholar] [CrossRef] [PubMed]
- Iso, H. Lifestyle and cardiovascular disease in Japan. J. Atheroscler. Thromb. 2011, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, A.; Lee, J.S.; Youn, S.; Yoo, K.Y. Dietary factors and breast cancer in Korea: An ecological study. Breast J. 2009, 15, 683–686. [Google Scholar] [CrossRef]
- Kılınç, B.; Cirik, S.; Turan, G. Seaweeds for Food and Industrial Applications. In Food Industry; IntechOpen: London, UK, 2013; pp. 735–748. [Google Scholar]
- Pereira, L.; Ribeiro-Claro, P.J.A. Analysis by vibrational spectroscopy of seaweed with potential use in food, pharmaceutical and cosmetic industries. In Marine Algae: Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; Pereira, L., Neto, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 228–250. [Google Scholar]
- Reddy, V.; Urooj, A.; Kumar, A. Evaluation of antioxidant activity of some plant extracts and their application in biscuits. Food Chem. 2005, 90, 317–321. [Google Scholar] [CrossRef]
- Dellarosa, N.; Laghi, L.; Martinsdóttir, E.; Jónsdóttir, R.; Kolbrún Sveinsdóttir, K. Enrichment of convenience seafood with omega-3 and seaweed extracts: Effect on lipid oxidation. Food Sci. Technol. 2015, 62, 746–752. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Olafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Ganske, F.; Dell, E.J. ORAC Assay on the Fluorstar Optima to Determine Antioxidant Capacity; Application note of BMG Labtech; BMG Labtech: Ortenberg, Germany, 2006. [Google Scholar]
- Boyer, R.F.; Grabill, T.W.; Petrovich, R.M. Reductive release of ferritin iron: A kinetic assay. Anal. Biochem. 1988, 174, 17–22. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A.G.P.; Kitts, D.D.; Li-Chan, E.C.Y. Antioxidative and Angiotensin-1-Converting Enzyme Inhibitory Potential of a Pacific Hake (Merluccius productus) Fish Protein Hydrolysate Subjected to Simulated Gastrointestinal Digestion and Caco-2 Cell Permeation. J. Agric. Food Chem. 2010, 58, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Berselli, P.V.R.; Zava, S.; Montorfano, G.; Corsetto, P.A.; Krzyzanowska, J.; Oleszek, W.; Berra, B.; Rizzo, A.M. A mint purified extract protects human keratinocytes from short-term, chemically induced oxidative stress. J. Agric. Food Chem. 2010, 58, 11428–11434. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Pinto, M.C.; Mata, A.M.; Lopez-barea, J. Reversible inactivation of Saccharomyces cerevisiae glutathione reductase under reducing conditions. Arch. Biochem. Biophys. 1984, 228, 1–12. [Google Scholar] [CrossRef]
- Prohaska, J.R.; Ganther, H.E. Selenium and glutathione peroxidase in developing rat brain. J. Neurochem. 1976, 27, 1379–1387. [Google Scholar] [CrossRef]
- Griffith, O.W. Glutathione and Glutathione Disulphide. Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1984; pp. 521–529. [Google Scholar]
- Aoun, M.; Corsetto, P.A.; Nugue, G.; Montorfano, G.; Ciusani, E.; Crouzier, D.; Hogarth, P.; Gregory, A.; Hayflick, S.; Zorzi, G.; et al. Changes in Red Blood Cell membrane lipid composition: A new perspective into the pathogenesis of PKAN. Mol. Genet. Metab. 2017, 121, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Karatas, F.; Karatepe, M.; Baysar, A. Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Anal. Biochem. 2002, 311, 76–79. [Google Scholar] [CrossRef]
- Holdt, S.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Khemka, V.K.; Chatterjee, G.; Ganguly, A.; Mukhopadhyay, S.; Chakrabarti, S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol. Cell. Biochem. 2015, 399, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Gupta, R.; Maurya, P.K. Oxidative stress and accelerated aging in neurodegenerative and neuropsychiatric disorder. Curr. Pharm. Des. 2019, 24, 4711–4725. [Google Scholar] [CrossRef]
- Liguori, L.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Draper, H.H.; Csallany, A.S.; Hadley, M. Urinary aldehydes as indicators of lipid peroxidation In Vivo. Free Radic. Biol. Med. 2000, 29, 1071–1077. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Pinto, E.; Sigaud-kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal–induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef] [PubMed]
| Extract | TPC 1 | ORAC 2 | DPPH IC50 3 | FCA 1.0 mg/mL | FCA 5.0 mg/mL | FCA 10 mg/mL |
|---|---|---|---|---|---|---|
| B200314 | 0.26 ± 0.02 | 1545 ± 220 | 0.614 | 15.0 ± 4.5 | 49.7 ± 5.3 | 59.2 ± 2.1 |
| B290814 | 0.30 ± 0.01 | 1840 ± 3 | 0.608 | 21.5 ± 2.8 | 50.4 ± 6.7 | 53.0 ± 3.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food. Antioxidants 2020, 9, 249. https://doi.org/10.3390/antiox9030249
Corsetto PA, Montorfano G, Zava S, Colombo I, Ingadottir B, Jonsdottir R, Sveinsdottir K, Rizzo AM. Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food. Antioxidants. 2020; 9(3):249. https://doi.org/10.3390/antiox9030249
Chicago/Turabian StyleCorsetto, Paola Antonia, Gigliola Montorfano, Stefania Zava, Irma Colombo, Bergros Ingadottir, Rosa Jonsdottir, Kolbrun Sveinsdottir, and Angela Maria Rizzo. 2020. "Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food" Antioxidants 9, no. 3: 249. https://doi.org/10.3390/antiox9030249
APA StyleCorsetto, P. A., Montorfano, G., Zava, S., Colombo, I., Ingadottir, B., Jonsdottir, R., Sveinsdottir, K., & Rizzo, A. M. (2020). Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food. Antioxidants, 9(3), 249. https://doi.org/10.3390/antiox9030249






