Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells
Abstract
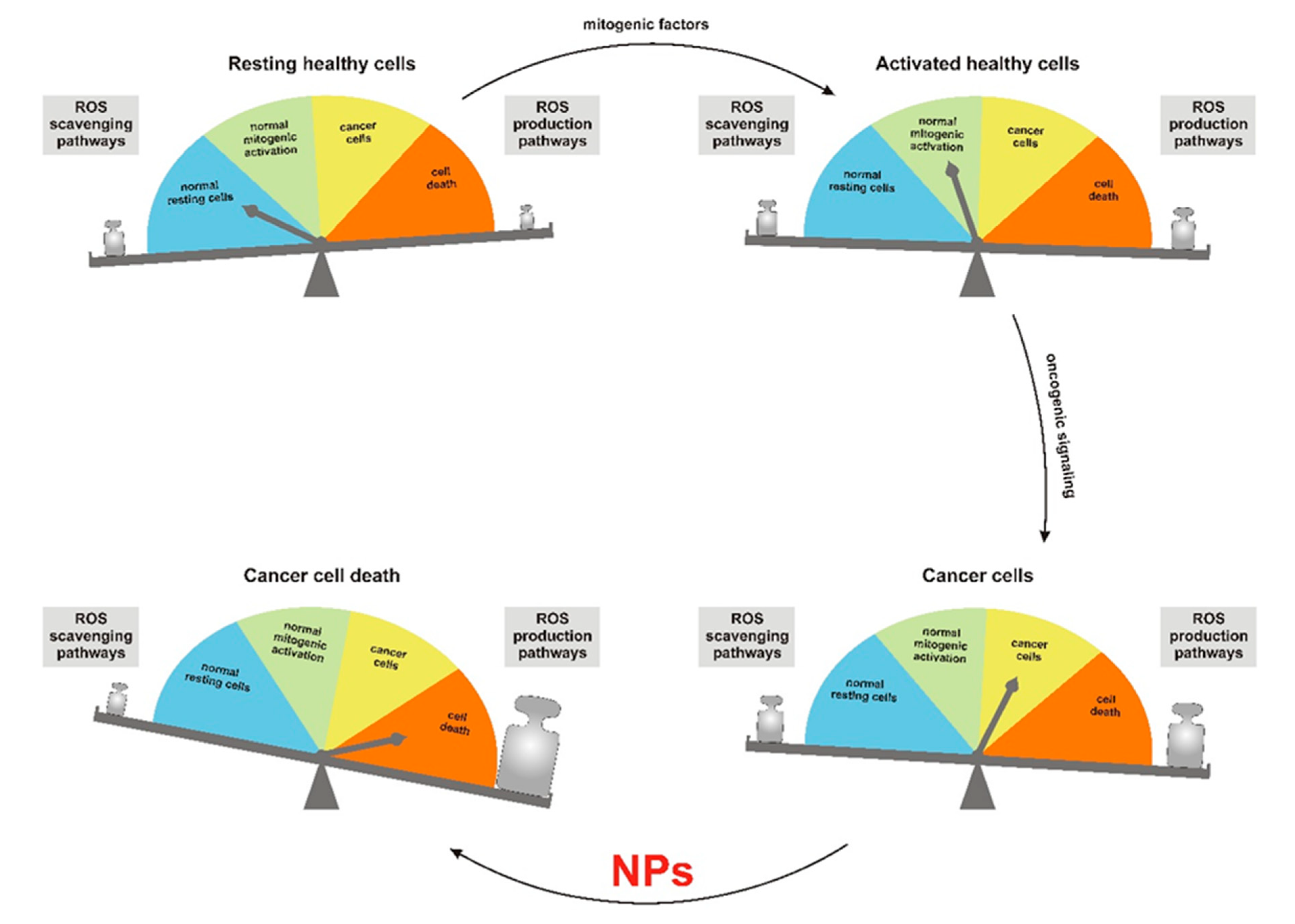
1. Importance of Reactive Oxygen Species in Cancer Therapy
2. Modulation of Redox Homeostasis by Nanoparticles
3. Nanoparticles in Photodynamic Therapy (PDT)
4. Use of Nanoparticles for Redox-Controlled Drug Delivery
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vega-Stromberg, T. Chemotherapy-induced secondary malignancies. J. Infus. Nurs. 2003, 26, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018, 36, 85–94. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Maryanovich, M.; Gross, A. A ROS rheostat for cell fate regulation. Trends Cell Biol. 2013, 23, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Cremers, C.M.; Jakob, U. Oxidant Sensing by Reversible Disulfide Bond Formation. J. Biol. Chem. 2013, 288, 26489–26496. [Google Scholar] [CrossRef]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J. Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef]
- Silic-Benussi, M.; Scattolin, G.; Cavallari, I.; Minuzzo, S.; Del Bianco, P.; Francescato, S.; Basso, G.; Indraccolo, S.; D’Agostino, D.M.; Ciminale, V. Selective killing of human T-ALL cells: An integrated approach targeting redox homeostasis and the OMA1/OPA1 axis. Cell Death Dis. 2018, 9, 822. [Google Scholar] [CrossRef]
- Shaw, A.T.; Winslow, M.M.; Magendantz, M.; Ouyang, C.; Dowdle, J.; Subramanian, A.; Lewis, T.A.; Maglathin, R.L.; Tolliday, N.; Jacks, T. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl. Acad. Sci. USA 2011, 108, 8773–8778. [Google Scholar] [CrossRef]
- Iskandar, K.; Rezlan, M.; Yadav, S.K.; Foo, C.H.; Sethi, G.; Qiang, Y.; Bellot, G.L.; Pervaiz, S. Synthetic Lethality of a Novel Small Molecule Against Mutant KRAS-Expressing Cancer Cells Involves AKT-Dependent ROS Production. Antioxid. Redox Signal. 2016, 24, 781–794. [Google Scholar] [CrossRef]
- Casares, C.; Ramirez-Camacho, R.; Trinidad, A.; Roldan, A.; Jorge, E.; Garcia-Berrocal, J.R. Reactive oxygen species in apoptosis induced by cisplatin: Review of physiopathological mechanisms in animal models. Eur. Arch. Otorhinolaryngol. 2012, 269, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Chau, S.P.; Kong, S.K.; Fung, K.P.; Kwok, T.T. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci. 2003, 73, 2047–2058. [Google Scholar] [CrossRef]
- Alexandre, J.; Hu, Y.; Lu, W.; Pelicano, H.; Huang, P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007, 67, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019. [Google Scholar] [CrossRef]
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef]
- Tabish, T.A. Graphene-based materials: The missing piece in nanomedicine? Biochem. Biophys. Res. Commun. 2018, 504, 686–689. [Google Scholar] [CrossRef]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6. [Google Scholar] [CrossRef]
- Alili, L.; Sack, M.; von Montfort, C.; Giri, S.; Das, S.; Carroll, K.S.; Zanger, K.; Seal, S.; Brenneisen, P. Downregulation of tumor growth and invasion by redox-active nanoparticles. Antioxid. Redox Signal. 2013, 19, 765–778. [Google Scholar] [CrossRef]
- Sack-Zschauer, M.; Bader, S.; Brenneisen, P. Cerium Oxide Nanoparticles as Novel Tool in Glioma Treatment: An In vitro Study. J. Nanomed. Nanotechnol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Sulthana, S.; Banerjee, T.; Kallu, J.; Vuppala, S.R.; Heckert, B.; Naz, S.; Shelby, T.; Yambem, O.; Santra, S. Combination Therapy of NSCLC Using Hsp90 Inhibitor and Doxorubicin Carrying Functional Nanoceria. Mol. Pharm. 2017, 14, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, M.; Das, S.; Mert, I.; Gupta, A.; Al-Wahab, Z.; Tebbe, C.; Dar, S.; Chhina, J.; Giri, S.; Munkarah, A.; et al. Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- He, C.; Jiang, S.; Jin, H.; Chen, S.; Lin, G.; Yao, H.; Wang, X.; Mi, P.; Ji, Z.; Lin, Y.; et al. Mitochondrial electron transport chain identified as a novel molecular target of SPIO nanoparticles mediated cancer-specific cytotoxicity. Biomaterials 2016, 83, 102–114. [Google Scholar] [CrossRef]
- Ashrafi Hafez, A.; Naserzadeh, P.; Mortazavian, A.M.; Mehravi, B.; Ashtari, K.; Seydi, E.; Salimi, A. Comparison of the effects of MnO2-NPs and MnO2-MPs on mitochondrial complexes in different organs. Toxicol. Mech. Methods 2019, 29, 86–94. [Google Scholar] [CrossRef]
- Amara, N.; Bachoual, R.; Desmard, M.; Golda, S.; Guichard, C.; Lanone, S.; Aubier, M.; Ogier-Denis, E.; Boczkowski, J. Diesel exhaust particles induce matrix metalloprotease-1 in human lung epithelial cells via a NADP(H) oxidase/NOX4 redox-dependent mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L170–L181. [Google Scholar] [CrossRef]
- Pisanic, T.R., II; Sungho, J.; Shubayev, V.I. Iron Oxide Magnetic Nanoparticle Nanotoxicity: Incidence and Mechanisms—Nanotoxicity—Wiley Online Library. In Nanotoxicity: From In Vivo and In Vitro Models to Health Risks; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Subastri, A.; Arun, V.; Sharma, P.; Preedia Babu, E.; Suyavaran, A.; Nithyananthan, S.; Alshammari, G.M.; Aristatile, B.; Dharuman, V.; Thirunavukkarasu, C. Synthesis and characterisation of arsenic nanoparticles and its interaction with DNA and cytotoxic potential on breast cancer cells. Chem. Biol. Interact. 2018, 295, 73–83. [Google Scholar] [CrossRef]
- Hosseini, M.J.; Shaki, F.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of Arsenic (III) on Isolated Liver Mitochondria: A New Mechanistic Approach. Iran. J. Pharm. Res. 2013, 12, 121–138. [Google Scholar]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef]
- Chen, C.; Xing, G.; Wang, J.; Zhao, Y.; Li, B.; Tang, J.; Jia, G.; Wang, T.; Sun, J.; Xing, L.; et al. Multihydroxylated [Gd@C82(OH)22]n nanoparticles: Antineoplastic activity of high efficiency and low toxicity. Nano Lett. 2005, 5, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Z.; Xu, J.; Zhao, Y. Therapeutic applications of low-toxicity spherical nanocarbon materials. NPG Asia Mater. 2014, 6. [Google Scholar] [CrossRef]
- Kang, S.G.; Zhou, G.; Yang, P.; Liu, Y.; Sun, B.; Huynh, T.; Meng, H.; Zhao, L.; Xing, G.; Chen, C.; et al. Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82(OH)22 and its implication for de novo design of nanomedicine. Proc. Natl. Acad. Sci. USA 2012, 109, 15431–15436. [Google Scholar] [CrossRef] [PubMed]
- Skivka, L.M.; Prylutska, S.V.; Rudyk, M.P.; Khranovska, N.M.; Opeida, I.V.; Hurmach, V.V.; Prylutskyy, Y.I.; Sukhodub, L.F.; Ritter, U. C60 fullerene and its nanocomplexes with anticancer drugs modulate circulating phagocyte functions and dramatically increase ROS generation in transformed monocytes. Cancer Nanotechnol. 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Hwang, K.C. Near-Infrared-Light-Activatable Nanomaterial-Mediated Phototheranostic Nanomedicines: An Emerging Paradigm for Cancer Treatment. Adv. Mater. 2018, 30, e1706320. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I. Photodynamic therapy in oncology: Mechanisms and clinical use. J. Natl. Cancer Inst. 1993, 85, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Master, A.; Livingston, M.; Sen Gupta, A. Photodynamic nanomedicine in the treatment of solid tumors: Perspectives and challenges. J. Control. Release 2013, 168, 88–102. [Google Scholar] [CrossRef]
- Hinger, D.; Grafe, S.; Navarro, F.; Spingler, B.; Pandiarajan, D.; Walt, H.; Couffin, A.C.; Maake, C. Lipid nanoemulsions and liposomes improve photodynamic treatment efficacy and tolerance in CAL-33 tumor bearing nude mice. J. Nanobiotechnol. 2016, 14, 71. [Google Scholar] [CrossRef]
- Yan, S.; Huang, Q.; Chen, J.; Song, X.; Chen, Z.; Huang, M.; Xu, P.; Zhang, J. Tumor-targeting photodynamic therapy based on folate-modified polydopamine nanoparticles. Int. J. Nanomed. 2019, 14, 6799–6812. [Google Scholar] [CrossRef]
- van Driel, P.; Boonstra, M.C.; Slooter, M.D.; Heukers, R.; Stammes, M.A.; Snoeks, T.J.A.; de Bruijn, H.S.; van Diest, P.J.; Vahrmeijer, A.L.; van Bergen En Henegouwen, P.M.P.; et al. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control. Release 2016, 229, 93–105. [Google Scholar] [CrossRef]
- Barth, B.M.; Altinoğlu, E.I.; Shanmugavelandy, S.S.; Kaiser, J.M.; Crespo-Gonzalez, D.; DiVittore, N.A.; McGovern, C.; Goff, T.M.; Keasey, N.R.; Adair, J.H.; et al. Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia. ACS Nano 2011, 5, 5325–5337. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Yang, F.; Xi, T.; Zhang, Y.; Ho, J.S. In vivo wireless photonic photodynamic therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Reeck, G.R.; Lee, D.Y.; Lee, Y.K. Photoluminescent graphene nanoparticles for cancer phototherapy and imaging. ACS Appl. Mater. Interfaces 2014, 6, 12413–12421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, H.; Wang, F.; Ren, J.; Qu, X. How functional groups influence the ROS generation and cytotoxicity of graphene quantum dots. Chem. Commun. 2017, 53, 10588–10591. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.; Li, H. Induction of Endogenous Reactive Oxygen Species in Mitochondria by Fullerene-Based Photodynamic Therapy. J. Nanosci. Nanotechnol. 2016, 16, 5592–5597. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Eng. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef]
- Tang, Q.; Yu, B.; Gao, L.; Cong, H.; Song, N.; Lu, C. Stimuli Responsive Nanoparticles for Controlled Anti-cancer Drug Release. Curr. Med. Chem. 2018, 25, 1837–1866. [Google Scholar] [CrossRef]
- Hou, L.; Yang, X.; Ren, J.; Wang, Y.; Zhang, H.; Feng, Q.; Shi, Y.; Shan, X.; Yuan, Y.; Zhang, Z. A novel redox-sensitive system based on single-walled carbon nanotubes for chemo-photothermal therapy and magnetic resonance imaging. Int. J. Nanomed. 2016, 11, 607–624. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xie, Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J. Control. Release 2017, 251, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Rezaeifard, S.; Naeimi, M.; Ghasemi, S.; Mohammadi-Samani, S.; Welland, M.E.; Tayebi, L. Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly (vinylcaprolactam). Int. J. Nanomed. 2019, 14, 6901–6915. [Google Scholar] [CrossRef]
- Dvorak, P.; Hlavac, V.; Mohelnikova-Duchonova, B.; Liska, V.; Pesta, M.; Soucek, P. Downregulation of ABC Transporters in Non-neoplastic Tissues Confers Better Prognosis for Pancreatic and Colorectal Cancer Patients. J. Cancer 2017, 8, 1959–1971. [Google Scholar] [CrossRef]
- Lu, Y.L.; Ma, Y.B.; Feng, C.; Zhu, D.L.; Liu, J.; Chen, L.; Liang, S.J.; Dong, C.Y. Co-delivery of Cyclopamine and Doxorubicin Mediated by Bovine Serum Albumin Nanoparticles Reverses Doxorubicin Resistance in Breast Cancer by Down-regulating P-glycoprotein Expression. J. Cancer 2019, 10, 2357–2368. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, W.; Tan, W.; Lai, Z.; Fang, D.; Jiang, L.; Zuo, C.; Yang, N.; Lai, Y. An Efficient Cell-Targeting Drug Delivery System Based on Aptamer-Modified Mesoporous Silica Nanoparticles. Nanoscale Res. Lett. 2019, 14, 390. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Asharani, P.V.; Xinyi, N.; Hande, M.P.; Valiyaveettil, S. DNA damage and p53-mediated growth arrest in human cells treated with platinum nanoparticles. Nanomedicine 2010, 5, 51–64. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Chaplin, D.J.; Olive, P.L.; Durand, R.E. Intermittent blood flow in a murine tumor: Radiobiological effects. Cancer Res. 1987, 47, 597–601. [Google Scholar]
- Jain, R.K. Barriers to drug delivery in solid tumors. Sci. Am. 1994, 271, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Tan, S.; Zhuang, X.; Guo, Y.; Zhao, Y.; Wu, T.; Ye, Q.; Si, L.; Zhang, Z. Nitric oxide releasing d-alpha-tocopheryl polyethylene glycol succinate for enhancing antitumor activity of doxorubicin. Mol. Pharm. 2014, 11, 4118–4129. [Google Scholar] [CrossRef] [PubMed]
- Fraszczak, J.; Trad, M.; Janikashvili, N.; Cathelin, D.; Lakomy, D.; Granci, V.; Morizot, A.; Audia, S.; Micheau, O.; Lagrost, L.; et al. Peroxynitrite-dependent killing of cancer cells and presentation of released tumor antigens by activated dendritic cells. J. Immunol. 2010, 184, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.M.; Shroff, E.H.; Liu, J.; Chandel, N.S. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS ONE 2009, 4, e7059. [Google Scholar] [CrossRef]
- Deepagan, V.G.; Ko, H.; Kwon, S.; Rao, N.V.; Kim, S.K.; Um, W.; Lee, S.; Min, J.; Lee, J.; Choi, K.Y.; et al. Intracellularly Activatable Nanovasodilators To Enhance Passive Cancer Targeting Regime. Nano Lett. 2018, 18, 2637–2644. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Tai, L.A.; Wang, Y.C.; Yang, C.S. Heat-activated sustaining nitric oxide release from zwitterionic diazeniumdiolate loaded in thermo-sensitive liposomes. Nitric Oxide 2010, 23, 60–64. [Google Scholar] [CrossRef]
- Fan, W.; Lu, N.; Huang, P.; Liu, Y.; Yang, Z.; Wang, S.; Yu, G.; Hu, J.; He, Q.; Qu, J.; et al. Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew. Chem. Int. Ed. Eng. 2017, 56, 1229–1233. [Google Scholar] [CrossRef]
| Nanoparticle Systems | Nanoparticle Composition | Mechanism of Action | Model Used for Validation | References |
|---|---|---|---|---|
| ROS Modulators | ||||
| CeO-NPs | Cerium oxide | ROS scavenging or generation depending on pH | In vitro cell lines and xenograft animal model | [19,20,21,22,23] |
| SPIONs | Super-paramagnetic iron oxide | Generation of O2•− by the ETC through the release of iron ions | SG-7701, Raw264.7, NIH3T3, HUVEC and HK2 normal cells; N2a, GY7703, HepG2, CNE1 and CNE2 cancer cells | [25] |
| MnO2-NPs | Manganese oxide | Impairment of the ETC and increased ROS production | Evaluation of pharmacokinetics and toxicity of NPs in C57 mice organs | [26] |
| IOMNPs | Magnetic iron oxide | Activation of NADPH oxidases and generation of O2•− | Several human cancer cell lines and in vivo animal models | [28] |
| As-NPs | Arsenic | Inhibition of complex I and II of the ETC | MDA-MB-231, MCF-7 (human breast cancer) cell lines in vitro; isolated rat liver mitochondria | [29,30] |
| Carbon-NPs | Fullerenes, carbon nanotubes, carbon nanodots | Transition metal-catalyzed generation of ROS and activation of NADPH oxidases and TLRs in professional phagocytes | Human hepatoma (H22) murine model; human pancreatic tumor xenografts mice; U937 (human myeloid lineage cells), nonsensitized human peripheral blood phagocytes | [32,34,35] |
| PDT Mediators | ||||
| Ce6 | Cerium 6 activated by LED-equipped microdevice | Wireless-activated infrared irradiation | Bladder cancer mouse xenograft and adult pig | [43] |
| GQNs | Graphene quantum nanodots | Generation of singlet oxygen and heat upon irradiation with 670 nm photons | MDA-MB-231 breast cancer cells, breast cancer xenograft mouse model | [45,46] |
| Redox-Based Delivery Systems | ||||
| SWCNTs | Carbon nanotubes + hyaluronic acid + doxorubicin | Release of doxorubicin in the presence of high levels of hyaluronidase and glutathione | MCF-7 (human breast cancer cells), breast cancer xenograft mouse model | [51] |
| BSA-NPs | Bovine serum albumin + doxorubicin + cyclopamine | Release of doxorubicin and decreased expression of ABC proteins | MDA-MB-231 and MCF-7 (breast cancer cells) in vitro, breast cancer xenograft mouse model | [56] |
| MSNPs | Mesoporous silica + doxorubicin + Sgc8 aptamer | Specific release of doxorubicin in T-ALL cells after PTK-7 binding | CEM (T-ALL), Ramos (Burkitt lymphoma), Lo2 (normal liver), 293T (human embryonic kidney) cell lines in vitro | [57] |
| Pt-NPs | Water-soluble platinum NPs capped with polyvinyl alcohol | Release of Pt2+ ions at low endosomal pH | Human glioblastoma U251 cell line | [59] |
| TNO3 | d-α-tocopheryl/polyethylene glycol succinate micelles + doxorubicin | Release of NO in the presence of high levels of glutathione and synergistic anticancer effect with doxorubicin | Hepatocellular carcinoma cells in vivo | [64] |
| NO-NPs | Hydrophilic polyethylene glycol + hydrophobic nitrated dextran | Boosted EPR effect by releasing NO upon reduction by glutathione | In vitro release of NO in the presence of glutathione, HT29 human colon carcinoma cell line in vitro, HT29 tumor-bearing mice | [67] |
| NONOate-loaded liposomes | Liposomes + zwitterionic diazeniumdiolate | Release of NO in tumor microenvironment due to low pH | Acellular system with controlled pH | [69] |
| Mesoporous organosilica NPs | Mesoporous organosilica + glucose oxidase + l-arginine | Release of high amount of NO in the presence of glucose and consequent glucose starvation of cancer cells | U87MG mouse xenograft model | [70] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccarese, F.; Raimondi, V.; Sharova, E.; Silic-Benussi, M.; Ciminale, V. Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells. Antioxidants 2020, 9, 211. https://doi.org/10.3390/antiox9030211
Ciccarese F, Raimondi V, Sharova E, Silic-Benussi M, Ciminale V. Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells. Antioxidants. 2020; 9(3):211. https://doi.org/10.3390/antiox9030211
Chicago/Turabian StyleCiccarese, Francesco, Vittoria Raimondi, Evgeniya Sharova, Micol Silic-Benussi, and Vincenzo Ciminale. 2020. "Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells" Antioxidants 9, no. 3: 211. https://doi.org/10.3390/antiox9030211
APA StyleCiccarese, F., Raimondi, V., Sharova, E., Silic-Benussi, M., & Ciminale, V. (2020). Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells. Antioxidants, 9(3), 211. https://doi.org/10.3390/antiox9030211








