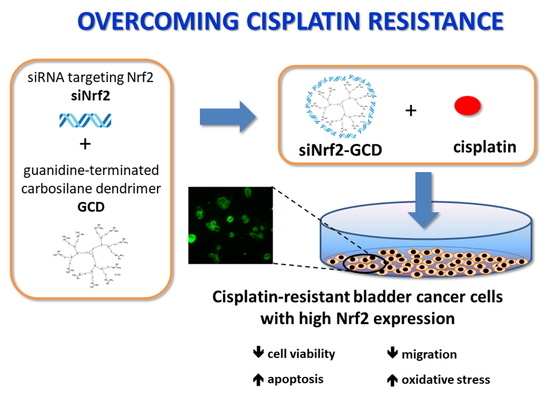
Carbosilane Dendrimers Loaded with siRNA Targeting Nrf2 as a Tool to Overcome Cisplatin Chemoresistance in Bladder Cancer Cells
Abstract
1. Introduction
2. Material and Methods
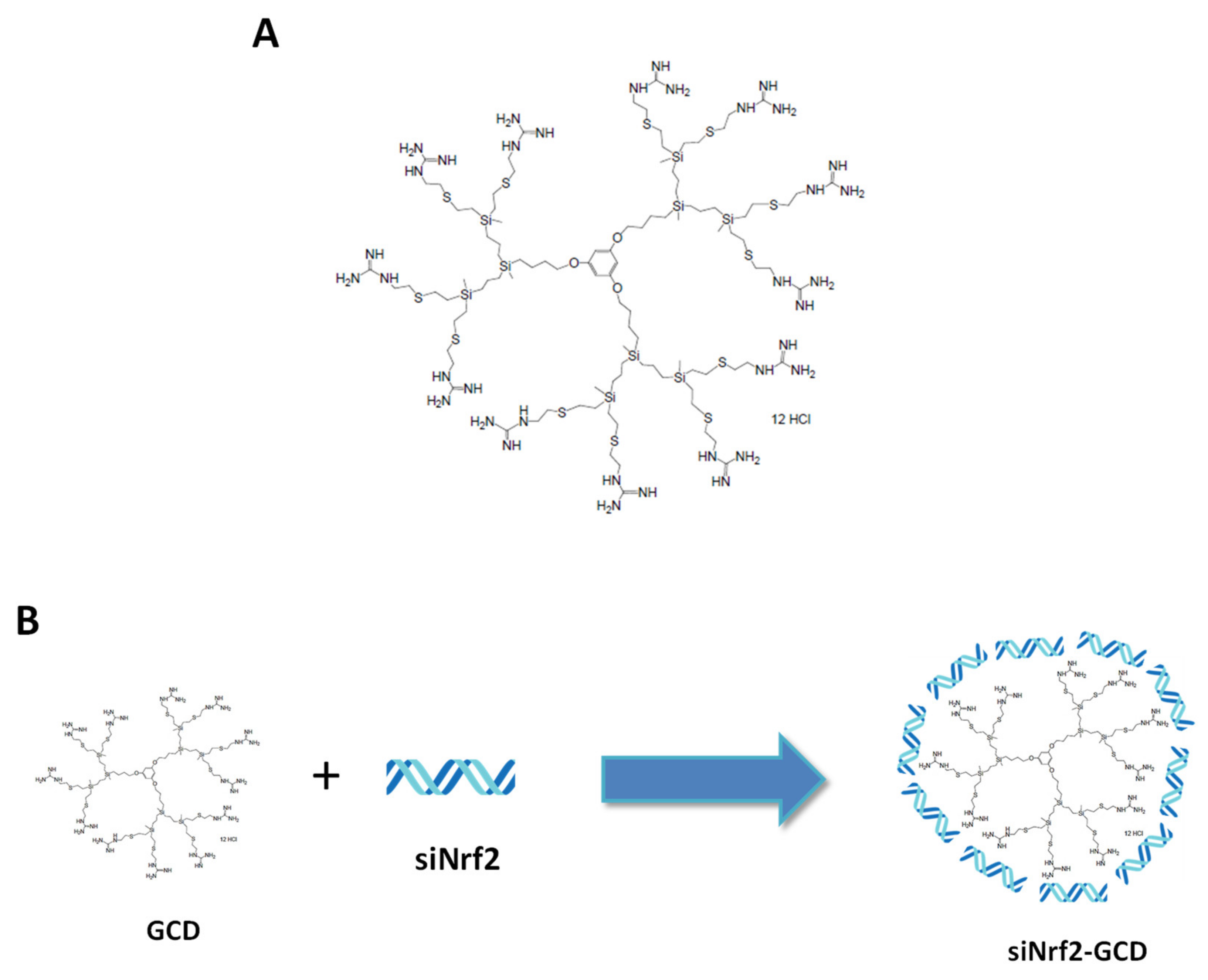
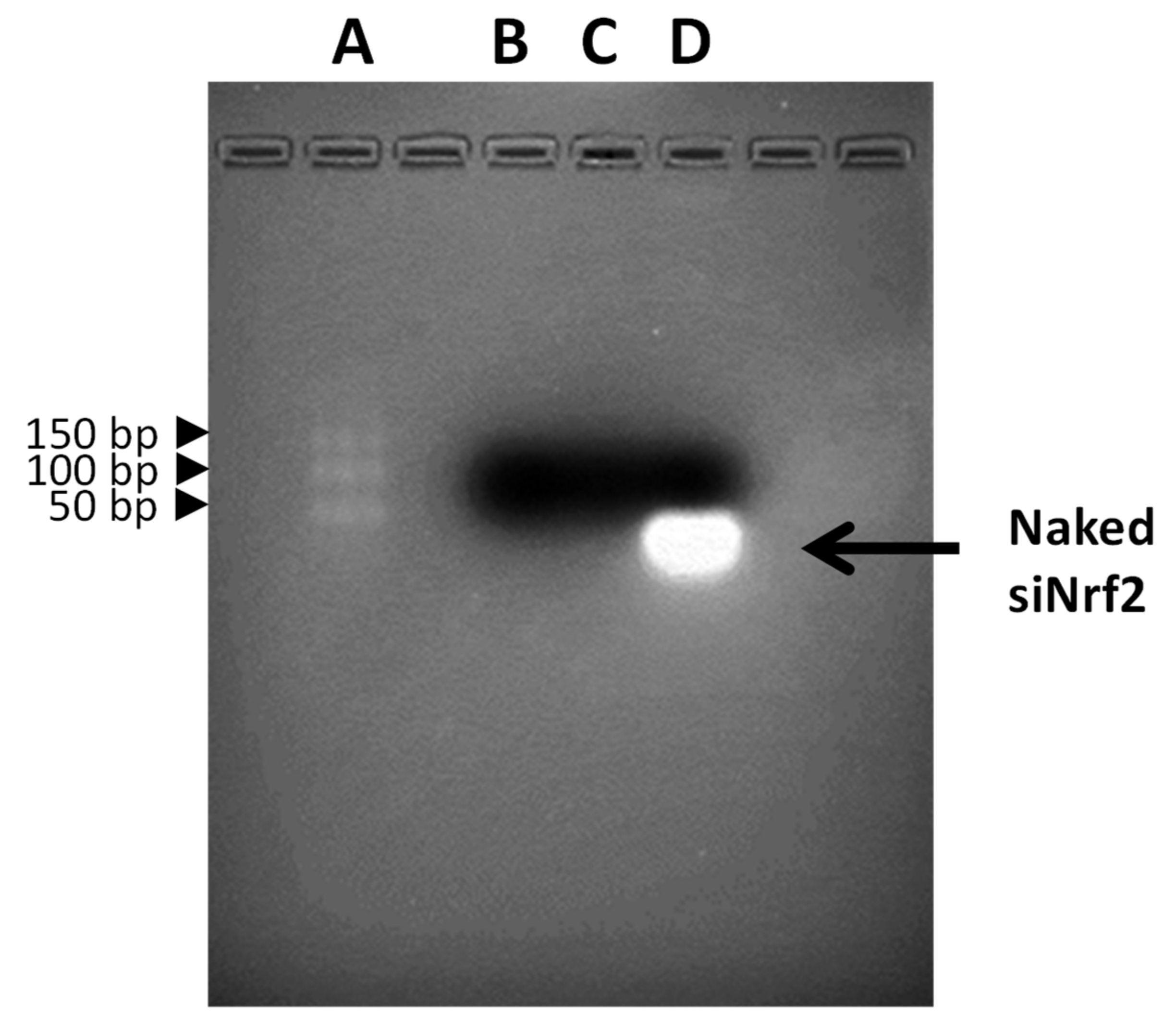
2.1. Preparation and Physico-Chemical Characterization of Guanidine-Terminated Carbosilane Dendrimers Loaded with siRNA Targeting Nrf2 (siNrf2-GCD) or 6-Coumarin
2.2. Cell Lines
2.3. MTT
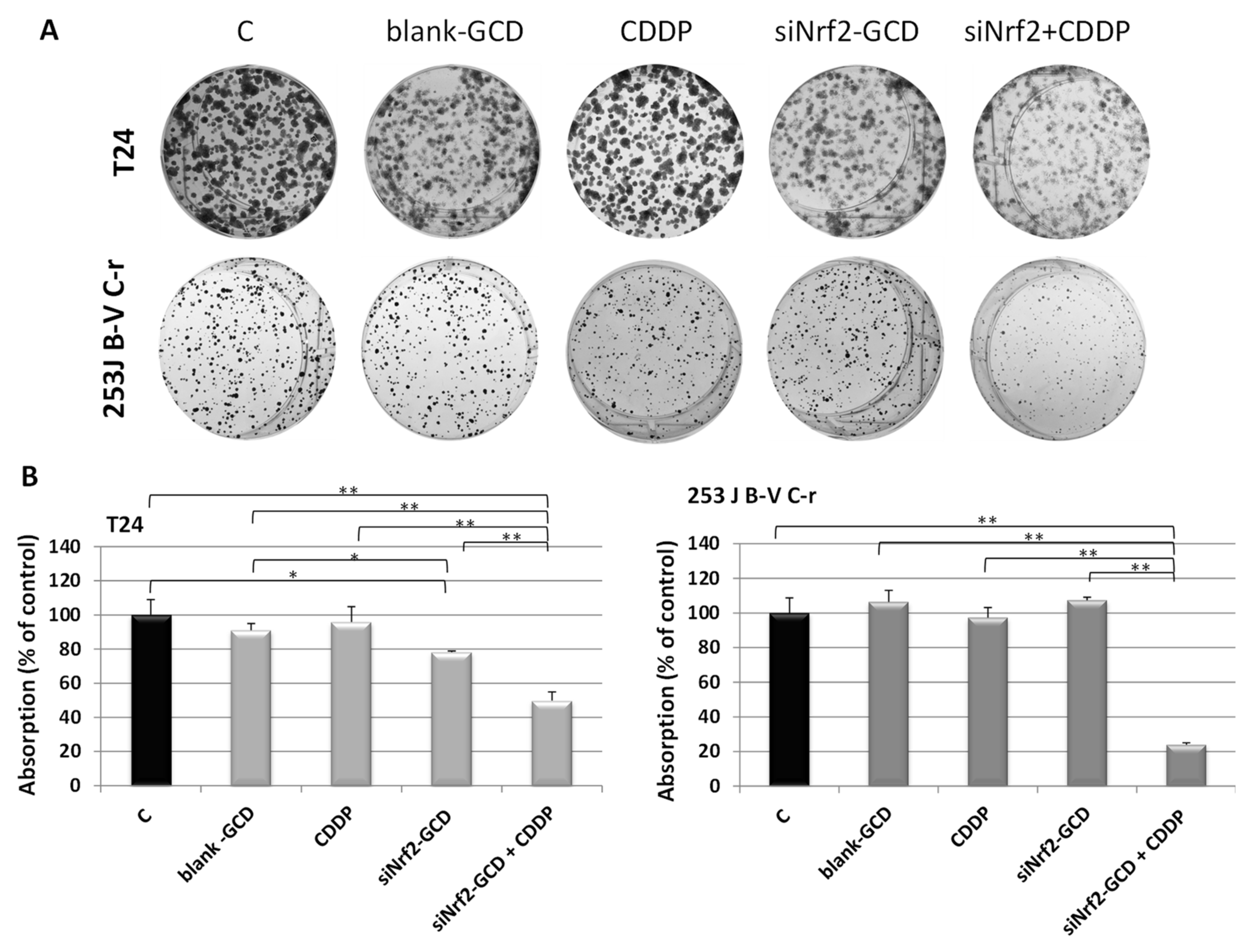
2.4. Colony Forming Assay
2.5. Apoptosis
2.6. Western Blot
2.7. siNrf2 Transfection with a Traditional Protocol
2.8. Wound-Healing Assay
2.9. Detection of the Intracellular Oxidative Stress Level
2.10. Statistical Analysis
3. Results
3.1. Preparation and Physicochemical Characterization of Guanidine-Terminated Carbosilane Dendrimer Formulations
3.2. Biological Effects of siNrf2-GCD in Bladder Cancer CDDP-Resistant Cells
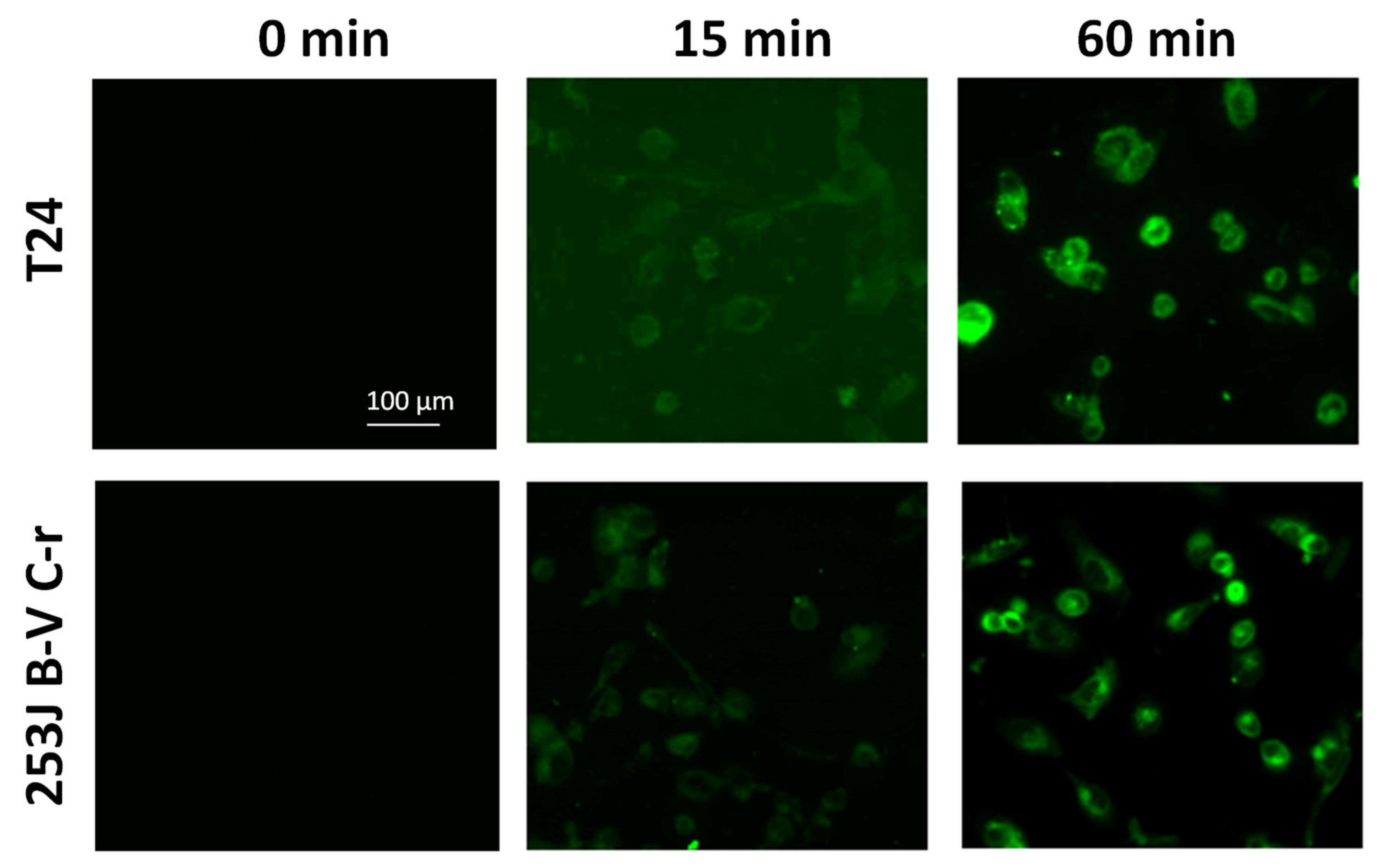
3.2.1. Cellular Penetration of GCD in CDDP-Resistant Bladder Cancer Cells
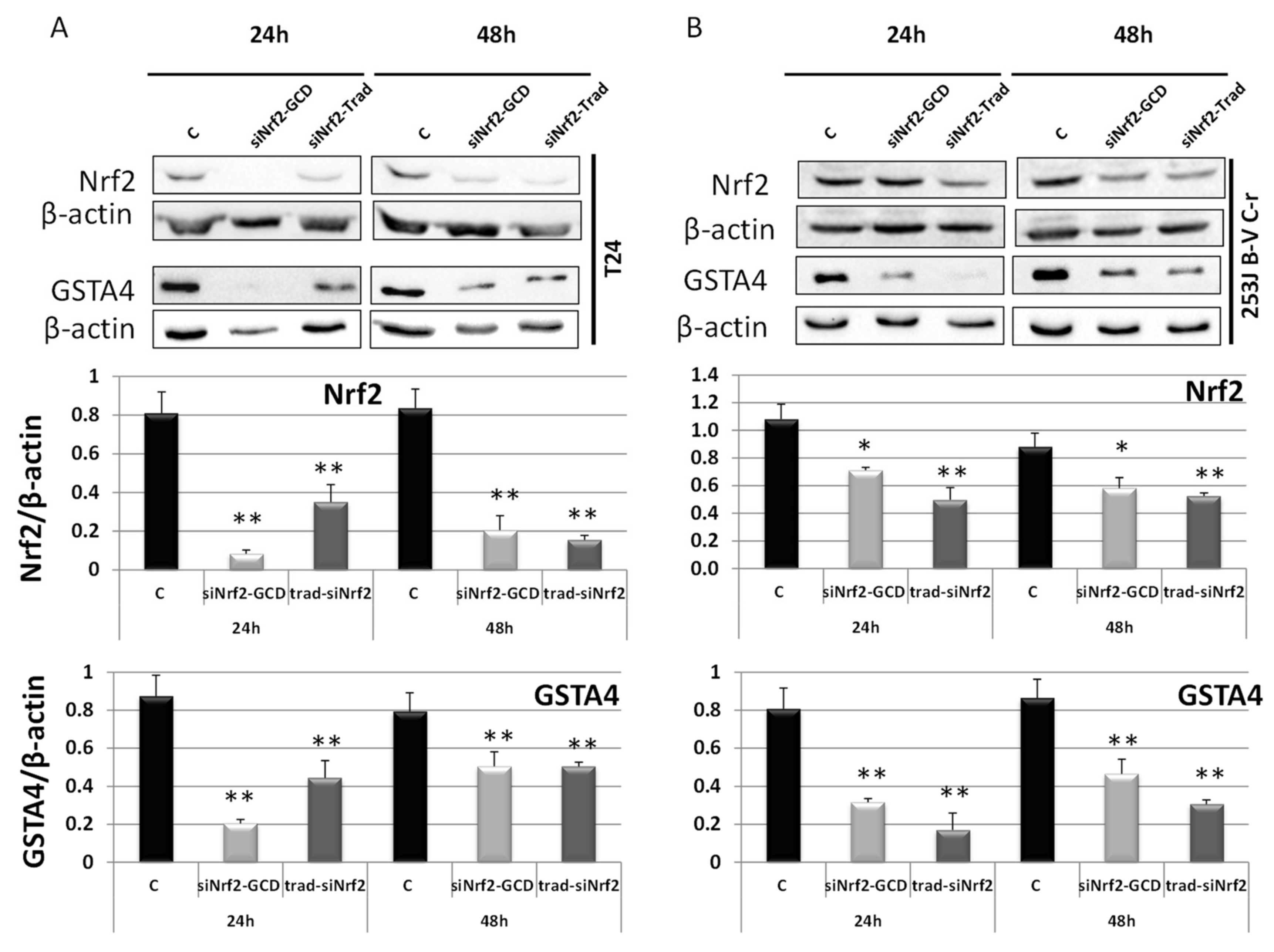
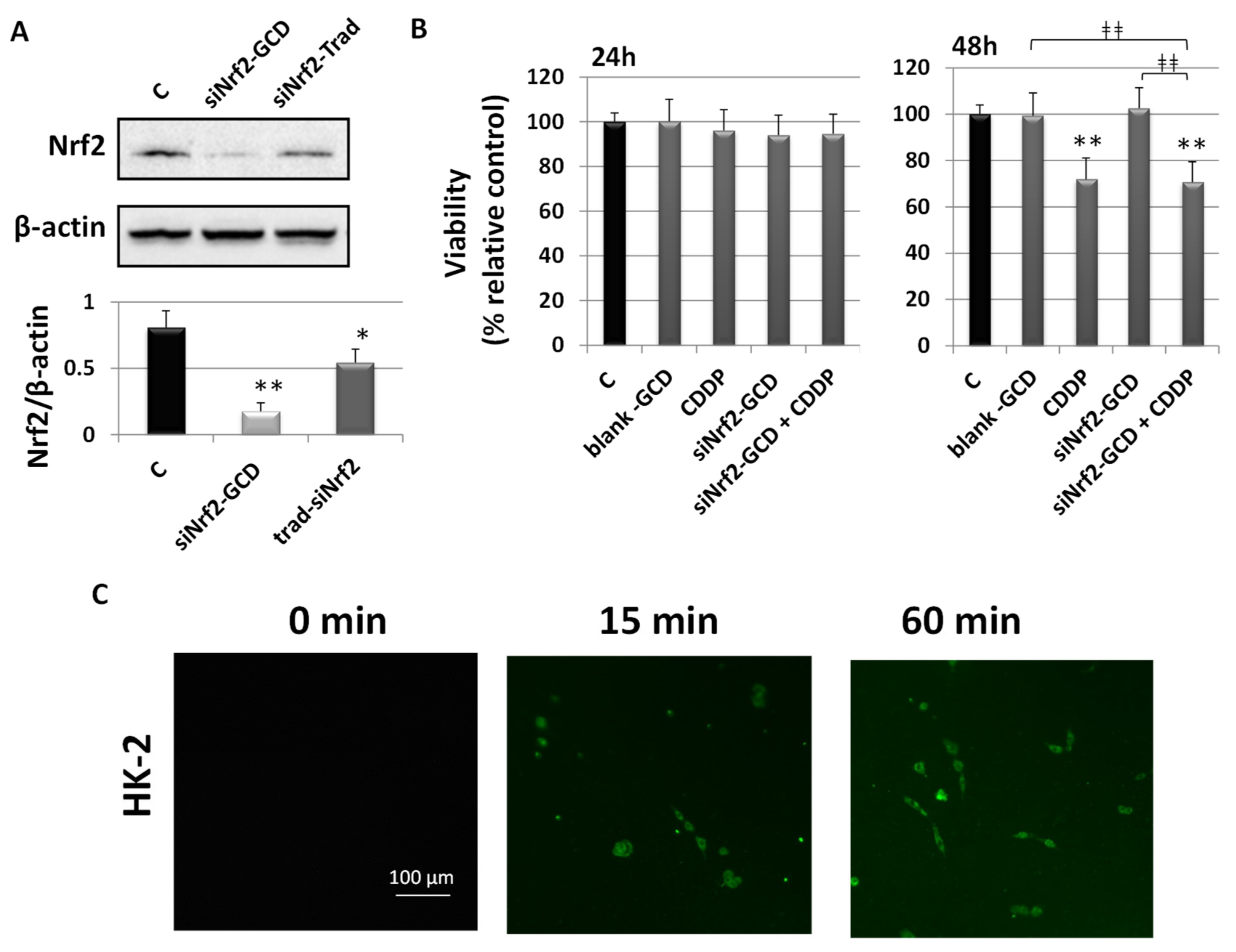
3.2.2. siNrf2-GCD Down-Regulated the Expression of Nrf2 and its Target Gene GSTA4 in CDDP-Resistant Bladder Cancer Cells
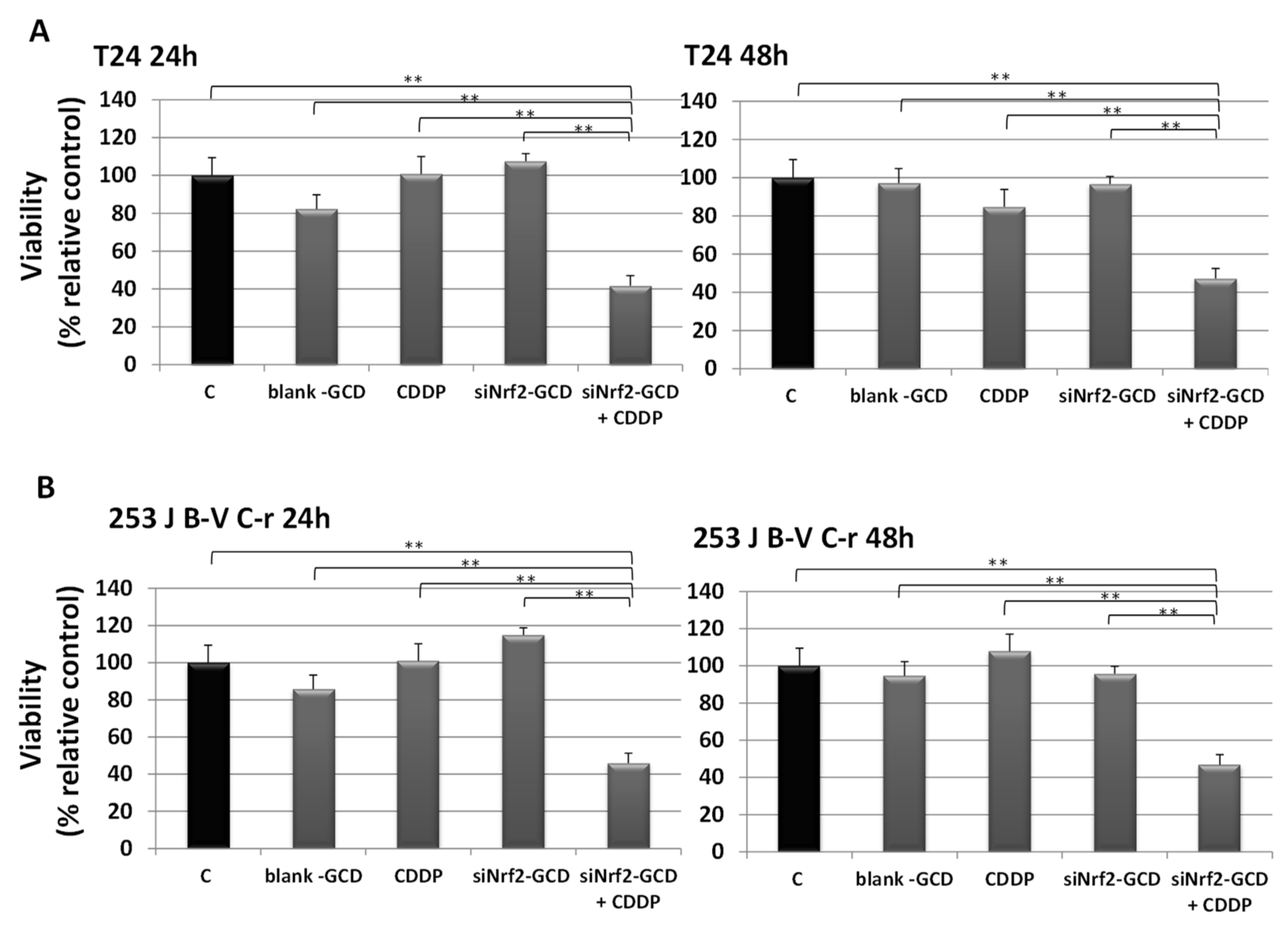
3.2.3. siNrf2-GCD Treatment Sensitized CDDP-Resistant Cells to CDDP Treatment: Effects on Viability, Proliferation, and Apoptosis
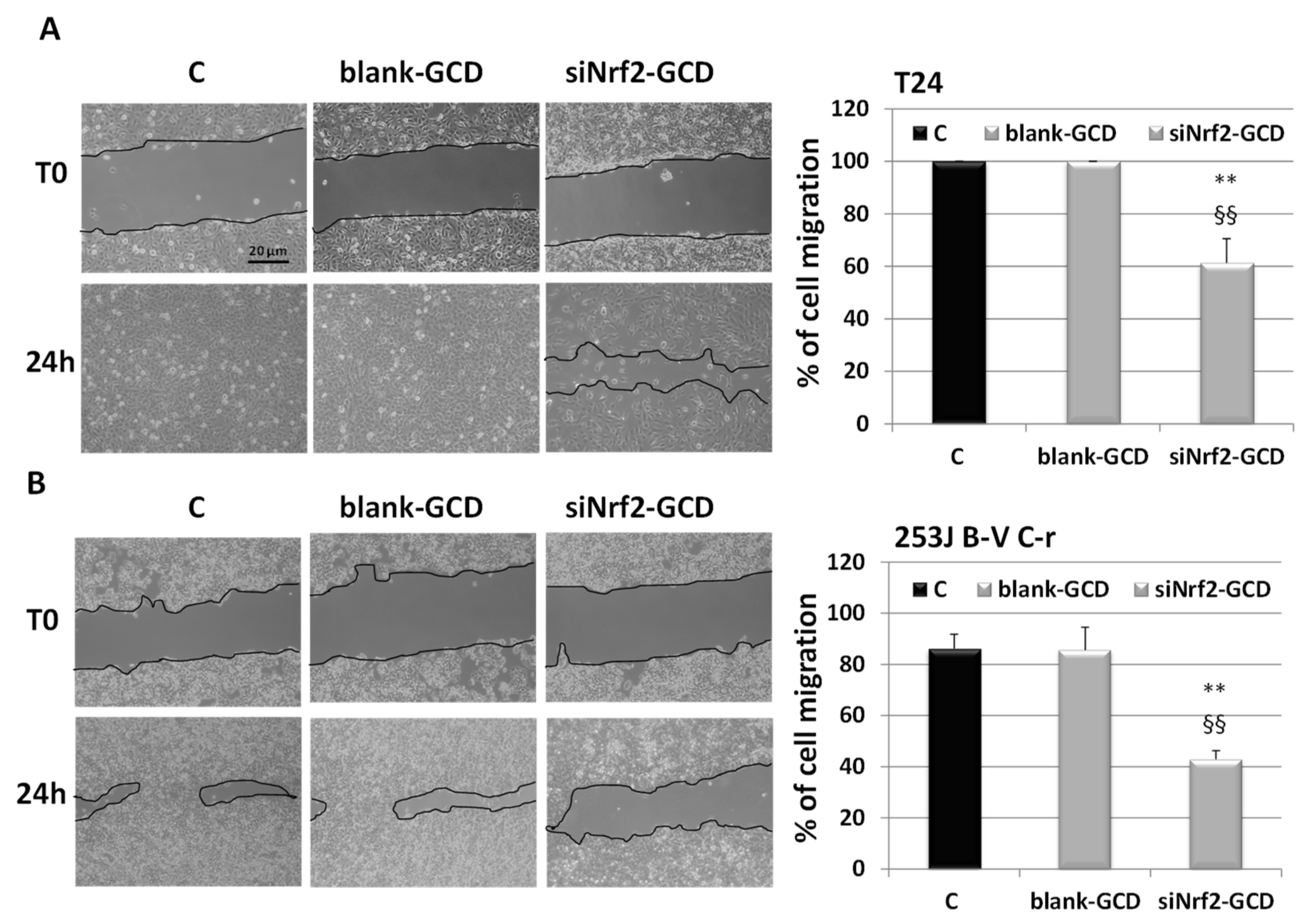
3.2.4. siNrf2-GCD Treatment Inhibited Migration In CDDP-Resistant Bladder Cancer Cells
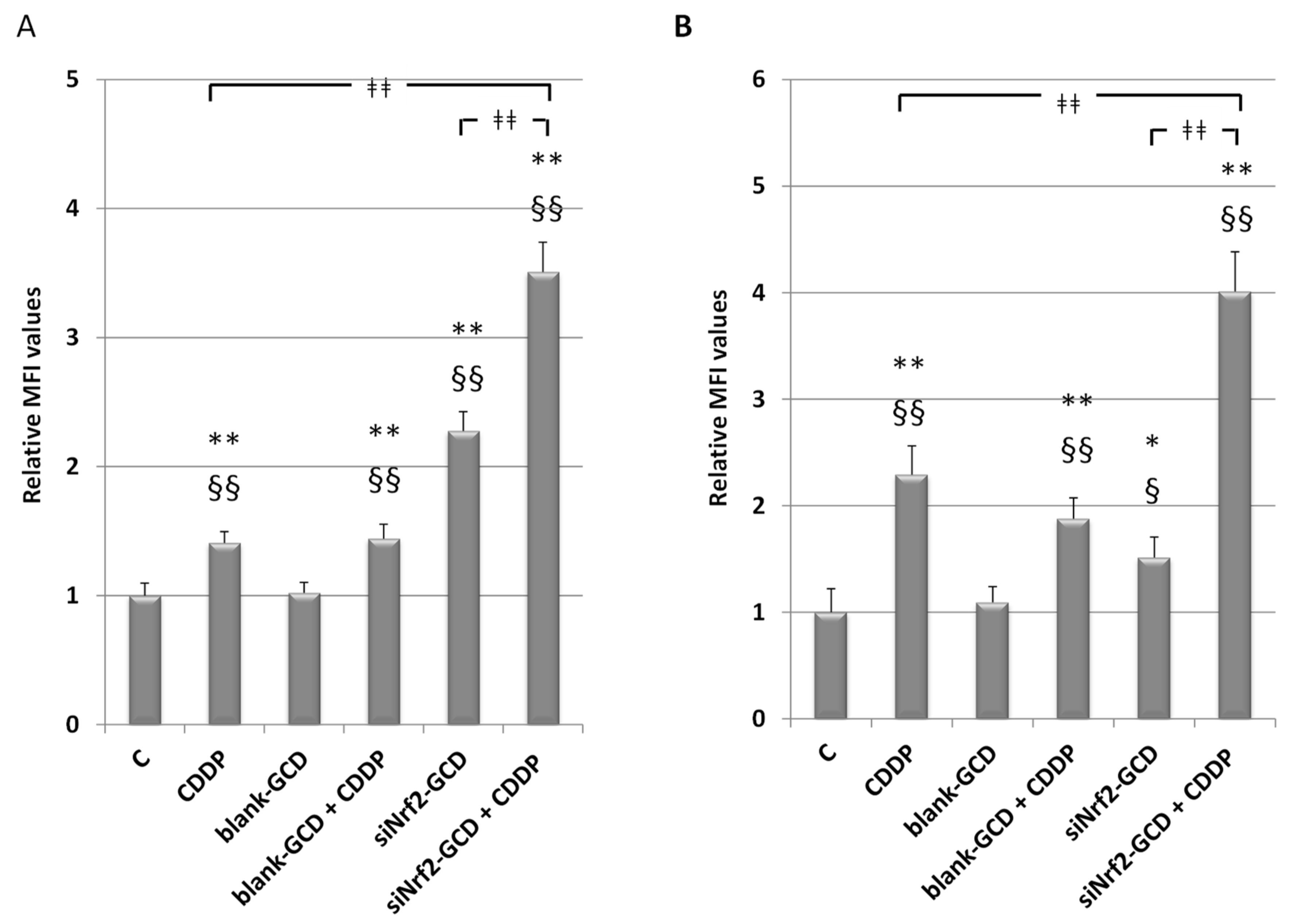
3.2.5. siNrf2-GCD Treatment Enhanced Intracellular Oxidative Stress Caused by CDDP Treatments
3.2.6. Biological Effects in Non-Cancerous Human Kidney HK-2 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Zhou, S.; Ye, W.; Zhang, M.; Liang, J. The effects of nrf2 on tumor angiogenesis: A review of the possible mechanisms of action. Crit. Rev. Eukaryot. Gene Expr. 2012, 22, 149–160. [Google Scholar] [CrossRef]
- Furfaro, A.L.; Traverso, N.; Domenicotti, C.; Piras, S.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxid. Med. Cell. Long. 2016, 2016, 1958174. [Google Scholar] [CrossRef] [PubMed]
- Ciamporcero, E.; Daga, M.; Pizzimenti, S.; Roetto, A.; Dianzani, C.; Compagnone, A.; Palmieri, A.; Ullio, C.; Cangemi, L.; Pili, R.; et al. Crosstalk between Nrf2 and YAP contributes to maintaining the antioxidant potential and chemoresistance in bladder cancer. Free. Radic. Biol. Med. 2018, 115, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.; Pizzimenti, S.; Dianzani, C.; Cucci, M.A.; Cavalli, R.; Grattarola, M.; Ferrara, B.; Scariot, V.; Trotta, F.; Barrera, G. Ailanthone inhibits cell growth and migration of cisplatin resistant bladder cancer cells through down-regulation of Nrf2, YAP, and c-Myc expression. Phytomedicine 2019, 56, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Cucci, M.A.; Grattarola, M.; Dianzani, C.; Damia, G.; Ricci, F.; Roetto, A.; Trotta, F.; Barrera, G.; Pizzimenti, S. Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism. Free Radic. Biol. Med. 2020, 150, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hayden, A.; Douglas, J.; Sommerlad, M.; Andrews, L.; Gould, K.; Hussain, S.; Thomas, G.J.; Packham, G.; Crabb, S.J. The Nrf2 transcription factor contributes to resistance to cisplatin in bladder cancer. Urol. Oncol. 2014, 32, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Calcabrini, C.; Turrini, E.; Sestili, P.; Fimognari, C. Nrf2: A potential therapeutic target for naturally occurring anticancer drugs? Expert Opin. Ther. Targets 2017, 21, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, H.; Chen, F.; Fu, J.; Xu, Y.; Hou, Y.; Kou, H.H.; Zhai, C.; Nelson, M.B.; Zhang, Q.; et al. An overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapy. Free Radic. Biol. Med. 2016, 99, 544–556. [Google Scholar] [CrossRef]
- Cai, S.J.; Liu, Y.; Han, S.; Yang, C. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell. Biosci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Harder, B.; Tian, W.; La Clair, J.J.; Tan, A.-C.; Ooi, A.; Chapman, E.; Zhang, D.D. Brusatol overcomes chemoresistance through inhibition of protein translation. Mol. Carcinog. 2017, 56, 1493–1500. [Google Scholar] [CrossRef]
- Ku, S.H.; Jo, S.D.; Lee, Y.K.; Kim, K.; Kim, S.H. Chemical and structural modifications of RNAi therapeutics. Adv. Drug Deliv. Rev. 2016, 104, 16–28. [Google Scholar] [CrossRef]
- Cavalli, R.; Primo, L.; Sessa, R.; Chiaverina, G.; di Blasio, L.; Alongi, J.; Manfredi, A.; Ranucci, E.; Ferruti, P. The AGMA1 polyamidoamine mediates the efficient delivery of siRNA. J. Drug Target 2017, 25, 891–898. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Argenziano, M.; Marsollier, A.C.; Mariot, V.; Rossi, D.; Selvatici, R.; Dumonceaux, J.; Cavalli, R.; Ferlini, A. Chitosan shelled nanobubbles irreversibly encapsulate morpholino conjugate antisense oligonucleotides and are ineffective for PMO-mediated gene silencing. Nucleic Acid Ther. 2020, in press. [Google Scholar] [CrossRef]
- Cavalli, R.; Soster, M.; Argenziano, M. Nanobubbles: A promising efficient tool for therapeutic delivery. Ther. Deliv. 2016, 7, 117–138. [Google Scholar] [CrossRef]
- Leiro, V.; Santos, S.D.; Pego, A.P. Delivering siRNA with Dendrimers: In Vivo Applications. Curr. Gene Ther. 2017, 17, 105–119. [Google Scholar] [CrossRef]
- Weber, N.; Ortega, P.; Clemente, M.I.; Shcharbin, D.; Bryszewska, M.; de la Mata, F.J.; Gómez, R.; Muñoz-Fernández, M.A. Characterization of arbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J. Control. Release 2008, 132, 55–64. [Google Scholar] [CrossRef]
- Sánchez-Nieves, J.; Fransen, P.; Pulido, D.; Lorente, R.; Muñoz-Fernández, M.A.; Albericio, F.; Royo, M.; Gómez, R.; de la Mata, J.F. Amphiphilic cationic carbosilane-PEG dendrimers: Synthesis and application in gene therapy. Eur. J. Med. Chem. 2014, 76, 43–52. [Google Scholar] [CrossRef]
- Ortega, P.; Sánchez-Nieves, J.; Martínez-Bonet, M.; Perisé-Barrios, J.; Gómez, R.; Muñoz-Martínez, M.A.; de la Mata, F.J. Cationic Dendritic Systems as Non-viral Vehicles for Gene Delivery Applications. In Cationic Polymers in Regenerative Medicine; Sangram, K.S., Dubruelr, P., Eds.; The Royal Society of Chemistry: London, UK, 2015; Chapter 25; pp. 321–355. [Google Scholar]
- Heredero-Bermejo, I.; Hernández-Ros, J.M.; Sánchez-García, L.; Maly, M.; Verdú-Expósito, C.; Soliveri, J.; de la Mata, F.J.; Copa-Patiño, J.L.; Pérez-Serrano, J.; Sánchez-Nieves, J.; et al. Ammonium and guanidine carbosilane dendrimers and dendrons as microbicides. Eur. Polym. J. 2018, 101, 159–168. [Google Scholar] [CrossRef]
- Clemente, N.; Argenziano, M.; Gigliotti, C.L.; Ferrara, B.; Boggio, E.; Chiocchetti, A.; Caldera, F.; Trotta, F.; Benetti, E.; Annaratone, L.; et al. Paclitaxel-Loaded Nanosponges Inhibit Growth and Angiogenesis in Melanoma Cell Models. Front. Pharmacol. 2019, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Met. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Cucci, M.A.; Compagnone, A.; Daga, M.; Grattarola, M.; Ullio, C.; Roetto, A.; Palmieri, A.; Rosa, A.C.; Argenziano, M.; Cavalli, R.; et al. Post-translational inhibition of YAP oncogene expression by 4-hydroxynonenal in bladder cancer cells. Free Radic. Biol. Med. 2019, 141, 205–219. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager, R.A.; Torok-Storb, B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Na+ dependent/phlorizin sensitive sugar transport; adenylate cyclase responsiveness to parathyroid, but not to antidiuretic, hormone. Kidney Int. 1994, 4, 48–57. [Google Scholar] [CrossRef]
- Karleta, V.; Andrlik, I.; Braunmüller, S.; Franke, T.; Wirth, M.; Gabor, F. Poloxamer 188 supplemented culture medium increases the vitality of Caco-2 cells after subcultivation and freeze/thaw cycles. ALTEX 2010, 27, 191–197. [Google Scholar] [CrossRef]
- Ortega, P.; Bermejo, J.F.; Chonco, L.; de Jesús, E.; de la Mata, F.J.; Fernández, G.; Flores, J.C.; Gómez, R.; Serramía, M.J.; Muñoz-Fernández, M.A. Novel Water-Soluble Carbosilane Dendrimers: Synthesis and Biocompatibility. Eur. J. Inorg. Chem. 2006, 7, 1388–1396. [Google Scholar] [CrossRef]
- Gonzalo, T.; Clemente, M.I.; Chonco, L.; Weber, N.D.; Díaz, L.; Serramía, M.J.; Gras, R.; Ortega, P.; de la Mata, F.J.; Gómez, R.; et al. Gene Therapy in HIV-infected Cells to Decrease Viral Impact by Using an Alternative Delivery Method. ChemMedChem 2010, 5, 921–929. [Google Scholar] [CrossRef]
- Serramía, M.J.; Álvarez, S.; Fuentes-Paniagua, E.; Clemente, M.I.; Sánchez-Nieves, J.; Gómez, R.; de la Mata, F.J.; Muñoz-Fernández, M.Á. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J. Control. Release 2015, 200, 60–70. [Google Scholar] [CrossRef]
- Cavalli, R.; Bisazza, A.; Trotta, M.; Argenziano, M.; Civra, A.; Donalisio, M.; Lembo, D. New chitosan nanobubbles for ultrasound-mediated gene delivery: Preparation and in vitro characterization. Int. J. Nanomed. 2012, 7, 3309–3318. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Chartoumpekis, D.V.; Kensler, T.W. Crosstalk between Nrf2 and Notch signaling. Free Radic. Biol. Med. 2015, 88, 158–167. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Q.; He, X.; Yuan, X.; Chen, Y.; Chu, Q.; Wu, K. Emerging roles of Nrf2 signal in non-small cell lung cancer. J. Hematol. Oncol. 2016, 9, 14. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Design and development of Nrf2 modulators for cancer chemoprevention and therapy: A review. Drug Des. Devel. Ther. 2018, 12, 3181–3197. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef]
- Patravale, V.; Dandekar, P.; Jain, R. Nanoparticulate Drug Delivery: Perspectives on the Transition from Laboratory to Market, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–244. [Google Scholar]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Lamberti, G. Lipid Delivery Systems for Nucleic-Acid-Based-Drugs: From Production to Clinical Applications. Pharmaceutics 2019, 11, 360. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Formulation | Average Diameter (nm) a ± SD | Polydispersity Index a ± SD | Zeta Potential (mV) a ± SD | pH |
|---|---|---|---|---|
| blank-GCD | 346.8 ± 22.3 | 0.05 ± 0.005 | 12.10 ± 0.13 | 6.0 |
| 600 μg/mL GCD | ||||
| siNrf2-GCD | 128.1 ± 10.1 ** | 0.09 ± 0.004 ** | 2.72 ± 0.28 ** | 6.0 |
| 6 µM siNrf2 in 600 μg/mL GCD | ||||
| 6-coumarin-GCD | 325.6 ± 20.34 ** | 0.110 ± 0.02 ** | 10.34 ± 2.11 | 6.0 |
| 0.06 μg/mL 6-coumarin | ||||
| in 600 μg/mL GCD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, L.; Argenziano, M.; Cucci, M.A.; Grattarola, M.; de Graaf, I.A.M.; Dianzani, C.; Barrera, G.; Sánchez Nieves, J.; Gomez, R.; Cavalli, R.; et al. Carbosilane Dendrimers Loaded with siRNA Targeting Nrf2 as a Tool to Overcome Cisplatin Chemoresistance in Bladder Cancer Cells. Antioxidants 2020, 9, 993. https://doi.org/10.3390/antiox9100993
Ambrosio L, Argenziano M, Cucci MA, Grattarola M, de Graaf IAM, Dianzani C, Barrera G, Sánchez Nieves J, Gomez R, Cavalli R, et al. Carbosilane Dendrimers Loaded with siRNA Targeting Nrf2 as a Tool to Overcome Cisplatin Chemoresistance in Bladder Cancer Cells. Antioxidants. 2020; 9(10):993. https://doi.org/10.3390/antiox9100993
Chicago/Turabian StyleAmbrosio, Leanne, Monica Argenziano, Marie Angèle Cucci, Margherita Grattarola, Inge A.M. de Graaf, Chiara Dianzani, Giuseppina Barrera, Javier Sánchez Nieves, Rafael Gomez, Roberta Cavalli, and et al. 2020. "Carbosilane Dendrimers Loaded with siRNA Targeting Nrf2 as a Tool to Overcome Cisplatin Chemoresistance in Bladder Cancer Cells" Antioxidants 9, no. 10: 993. https://doi.org/10.3390/antiox9100993
APA StyleAmbrosio, L., Argenziano, M., Cucci, M. A., Grattarola, M., de Graaf, I. A. M., Dianzani, C., Barrera, G., Sánchez Nieves, J., Gomez, R., Cavalli, R., & Pizzimenti, S. (2020). Carbosilane Dendrimers Loaded with siRNA Targeting Nrf2 as a Tool to Overcome Cisplatin Chemoresistance in Bladder Cancer Cells. Antioxidants, 9(10), 993. https://doi.org/10.3390/antiox9100993