Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Cell Lines
2.2. Plant Material and Extraction Procedure
2.3. Determination of Total Phenolic Compounds
2.4. Determination of Flavonoids
2.5. LC-MS Analysis
2.6. DPPH Radical Scavenging Assay
2.7. In Vitro Cytotoxicity Assay
2.8. Mushroom Tyrosinase Inhibitory Assay
2.9. Determination of the Sun Protection Factor (SPF)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Content of Total Phenolics, Flavonoids, and Antioxidant Activity of C. incanus and C. ladanifer Extracts
3.2. Qualitative and Quantitative Analyses of C. incanus and C. ladanifer Extracts
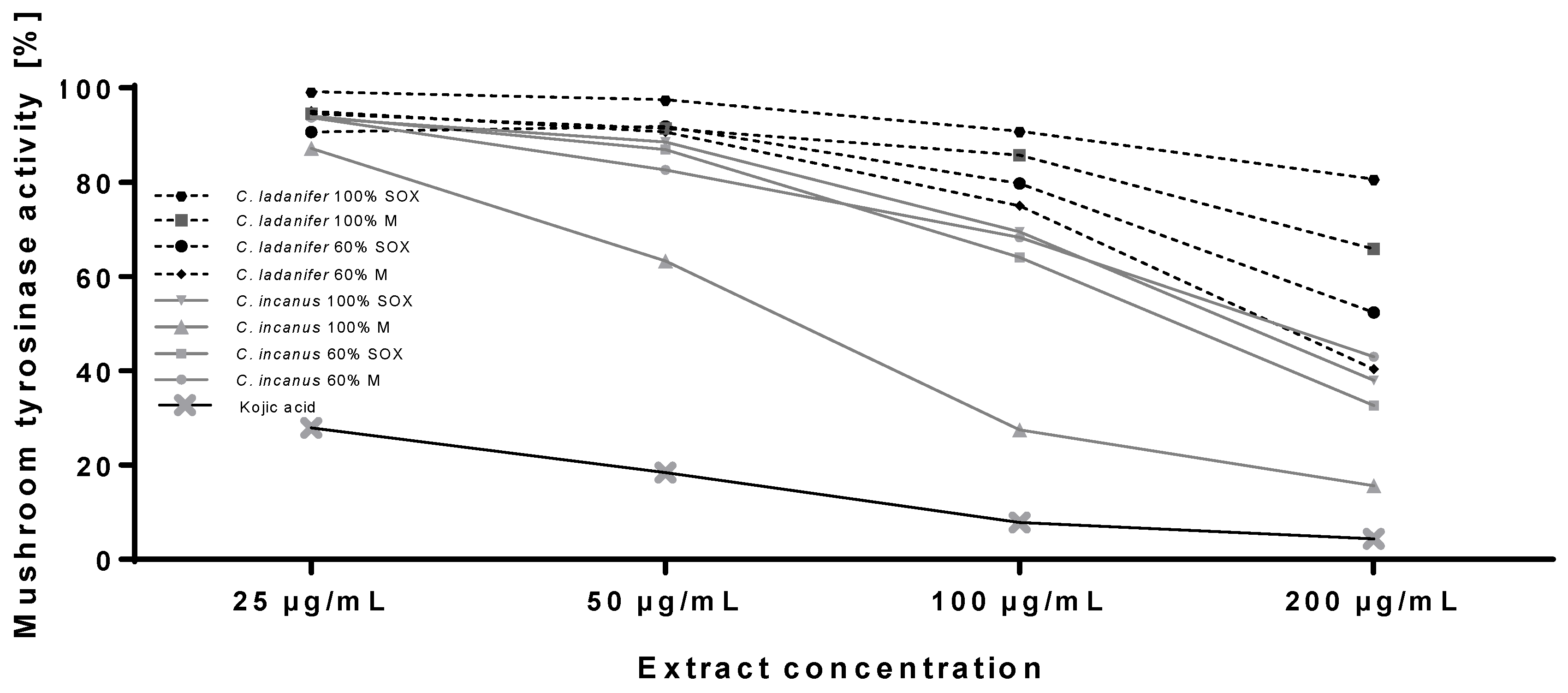
3.3. Tyrosinase Inhibitory Properties of Cistus Extracts
3.4. In Vitro Cytotoxicity Against Human Skin Cancer and Noncancerous Skin Cells
3.5. In Vitro Sun Protection Factor (SPF) of Cistus Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Uliasz, A.; Spencer, J.M. Chemoprevention of skin cancer and photoaging. Clin. Dermatol. 2004, 22, 178–182. [Google Scholar] [CrossRef]
- Gupta, S.; Mukhtar, H. Chemoprevention of skin cancer: Current status and future prospects. Cancer Metastasis Rev. 2002, 21, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed]
- Kubica, P.; Ekiert, H.; Ekiert, R.J.; Szopa, A. Species of the genus Cistus sp.—Taxonomy, distribution, chemical composition, therapeutic applications and biotechnological studies. Borgis Postępy Fitoter. 2016, 3, 179–188. [Google Scholar]
- Kalus, U.; Grigorov, A.; Kadecki, O.; Jansen, J.P.; Kiesewetter, H.; Radtke, H. Cistus incanus (CYSTUS052) for treating patients with infection of the upper respiratory tract. A prospective, randomised, placebo-controlled clinical study. Antivir. Res. 2009, 84, 267–271. [Google Scholar] [CrossRef]
- Droebner, K.; Ehrhardt, C.; Poetter, A.; Ludwig, S.; Planz, O. CYSTUS052, a polyphenol-rich plant extract, exerts anti-influenza virus activity in mice. Antivir. Res. 2007, 76, 1–10. [Google Scholar] [CrossRef]
- Chinou, I.; Demetzos, C.; Harvala, C.; Roussakis, C.; Verbist, J.F. Cytotoxic and antibacterial labdane-type diterpenes from the aerial parts of Cistus incanus subsp. Creticus. Planta Med. 1994, 60, 34–36. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Szeremeta, D.; Knaś, M.; Długosz, E.; Ott, P.G.; Kowalska, T.; Sajewicz, M. Antibacterial potential of the Cistus incanus L. Phenolics as studied with use of thin-layer chromatography combined with direct bioautography and in situ hydrolysis. J. Chromatogr. A 2018, 1534, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and antioxidant activity of crude extract and polyphenolic rich fractions from C. incanus leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef]
- Ferreira, S.; Santos, J.; Duarte, A.; Duarte, A.P.; Queiroz, J.A.; Domingues, F.C. Screening of antimicrobial activity of Cistus ladanifer and Arbutus unedo extracts. Nat. Prod. Res. 2012, 26, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Alve, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef] [PubMed]
- El Youbi, A.E.; Mansouri, L.E.; Boukhira, S.; Daoudi, A.; Bousta, D. In vivo anti-inflammatory and analgesic effects of aqueous extract of Cistus ladanifer L. from Morocco. Am. J. Ther. 2016, 23, e1554–e1559. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, J.R.; Frazão, D.F.; Domingues, J.L.; Quintela-Sabarís, C.; Dentinho, T.P.; Anjos, O.; Alves, M.; Delgado, F. Neglected Mediterranean plant species are valuable resources: The example of Cistus ladanifer. Planta 2018, 248, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation Med. Aromat Plants 2015, 4, 3. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Matejić, J.S.; Džamić, A.M.; Mihajilov-Krstev, T.M.; Ranđelović, V.N.; Krivošej, Z.D.; Marin, P.D. Total phenolic and flavonoid content, antioxidant and antimicrobial activity of extracts from Tordylium maximum. J. Appl. Pharm. Sci. 2013, 3, 55–59. [Google Scholar] [CrossRef]
- Brand Williams, M.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Frątczak, A.; Bylka, W. Evaluation of properties inhibiting tyrosinase by selected plant extracts. Pol. J. Cosmetol. 2016, 19, 51–55. [Google Scholar]
- Mansur, J.S.; Breder, M.N.; Mansur, M.C.; Azulay, R.D. Determination of Sun protection factor by spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Majewska, M.; Skrzycki, M.; Podsiad, M.; Czeczot, H. Evaluation of antioxidant potential of flavonoids: An in vitro study. Acta Pol. Pharm. 2011, 68, 611–615. [Google Scholar]
- Moreira, H.; Ślęzak, A.; Szyjka, A.; Oszmiański, J.; Gąsiorowski, K. Antioxidant and cancer chemopreventive activities of cistus and pomegranate polyphenols. Acta Pol. Pharm. 2017, 74, 688–698. [Google Scholar]
- Amensour, M.; Sendra, E.; Pérez-Alvarez, J.A.; Skali-Senhaji, N.; Abrini, J.; Fernández-López, J. Antioxidant activity and chemical content of methanol and ethanol extracts from leaves of rockrose (Cistus ladaniferus). Plant Foods Hum. Nutr. 2010, 65, 170–178. [Google Scholar] [CrossRef]
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crops Prod. 2009, 30, 165–167. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Guimarãe, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C.; Hattori, Y.; Toyokuni, S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox Signal. 2004, 6, 561–570. [Google Scholar] [CrossRef]
- Schalka, S. New data on hyperpigmentation disorders. J. Eur. Acad. Dermatol. Venereol. 2017, 5, 18–21. [Google Scholar] [CrossRef]
- Kuchta, A.; Konopacka, A.; Waleron, K.; Viapiana, A.; Wesołowski, M.; Dąbkowski, K.; Ćwiklińska, A.; Mickiewicz, A.; Śledzińska, A.; Wieczorek, E.; et al. The effect of Cistus incanus herbal tea supplementation on oxidative stress markers and lipid profile in healthy adults. Cardiol. J. 2019. (ahead of print). [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Barrajón-Catalán, E.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a Cistus ladanifer aqueous extract. Phytochem. Anal. 2010, 21, 307–313. [Google Scholar] [CrossRef]
- Koch, W.; Kukula-Koch, W.; Głowniak, K. Catechin composition and antioxidant activity of black teas in relation to brewing time. J. AOAC 2017, 100, 1694–1699. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medical chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Leyden, J.J.; Shergill, B.; Micali, G.; Downie, J.; Wallo, W. Natural options for the management of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1140–1145. [Google Scholar] [CrossRef]
- No, J.K.; Soung, D.Y.; Kim, Y.J.; Shim, K.H.; Jun, Y.S.; Rhee, S.H.; Yokozawa, T.; Chung, H.Y. Inhibition of tyrosinase by green tea components. Life Sci. 1999, 65, PL241–PL246. [Google Scholar] [CrossRef]
- Sato, K.; Toriyama, M. Depigmenting effect of catechins. Molecules 2009, 14, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, S.H.; Kwon, S.B.; Li, K.; Youn, S.W.; Park, K.C. (-)-Epigallocatechin-3-gallate and hinokitiol reduce melanin synthesis via decreased MITF production. Arch. Pharm. Res. 2004, 27, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, J.; Kim, J.; Han, C.; Yoon, J.; Kim, N.; Seo, J.; Lee, C. Flavonoids as mushroom tyrosinase inhibitors: A fluorescence quenching study. J. Agric. Food Chem. 2006, 54, 935–941. [Google Scholar] [CrossRef]
- Matsuda, H.; Higashino, M.; Chen, W.; Tosa, H.; Iinuma, M.; Kubo, M. Studies of cuticle drugs from natural sources. III. Inhibitory effect of Myrica rubra on melanin biosynthesis. Biol. Pharm. Bull. 1995, 18, 1148–1150. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I. Flavonols from saffron flower: Tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef]
- Xie, L.P.; Chen, Q.X.; Huang, H.; Wang, H.Z.; Zhang, R.Q. Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry (Mosc) 2003, 68, 487–491. [Google Scholar] [CrossRef]
- Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Ahmad, N. Melanoma chemoprevention: Current status and future prospects. Photochem. Photobiol. 2017, 93, 975–989. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Madhunapantula, S.V.; Robertson, G.P. Chemoprevention of Melanoma. Adv. Pharmacol. 2012, 65, 361–398. [Google Scholar] [CrossRef]
- Jemia, M.B.; Kchouk, M.E.; Senatore, F.; Autore, G.; Marzocco, S.; De Feo, V.; Bruno, M. Antiproliferative activity of hexane extract from Tunisian Cistus libanotis, Cistus monspeliensis and Cistus villosus. Chem. Cent. J. 2013, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chang, L.; Qu, Y.; Liang, J.; Jin, W.; Xia, X. Tea polyphenols inhibit the proliferation, migration, and invasion of melanoma cells through the down-regulation of TLR4. Int. J. Immunopathol. Pharmacol. 2018, 31, 0394632017739531. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postepy Hig. Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Deguchi, A.; Lim, J.T.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (-)-Epi-gallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef]
- Morré, D.J.; Morré, D.M.; Sun, H.; Cooper, R.; Chang, J.; Janle, E.M. Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX). Pharmacol. Toxicol. 2003, 92, 234–241. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, S.A. Vertuani. Herbal extracts, lichens and biomolecules as natural photo-protection alternatives to synthetic UV filters. A systematic review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Attaguile, G.; Caruso, A.; Pennisi, G.; Savoca, F. Gastroprotective effect of aqueous extract of Cistus incanus in rats. Pharmacol. Res. 1995, 31, 29–32. [Google Scholar] [CrossRef]
| Wavelenght (λ, nm) | EE × I (Normalized) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
| Total | 1 |
| Total Phenolic Content (mg GAE/g DW) | Total Flavonoid Content (mg QuE/g DW) | DPPH Scavenging (IC50, µg/mL) | ||
|---|---|---|---|---|
| C. incanus | 60% M | 331.82 ± 13.39 b,c | 44.76 ± 0.45 a | 4.05 ± 0.43 a,b |
| 60% SOX | 347.27 ± 17.03 c | 53.76 ± 0.89 b | 3.81 ± 0.36 a | |
| 100% M | 297.71 ± 13.77 a,b | 44.77 ± 1.64 a | 4.76 ± 0.18 a,b,c | |
| 100% SOX | 269.28 ± 23.62 a | 50.85 ± 0.54 b | 16.75 ± 0.47 | |
| C. ladanifer | 60% M | 267.58 ± 3.78 a | 32.35 ± 0.94 | 5.49 ± 0.48 b,c |
| 60% SOX | 301.82 ± 10.91 a,b | 42.35 ± 1.32 a | 4.08 ± 0.31 a,b | |
| 100% M | 142.81 ± 6.30 | 27.91 ± 1.02 | 10.20 ± 1.37 | |
| 100% SOX | 191.18 ± 6.79 | 37.54 ± 1.88 | 5.88 ± 0.26 c |

| No | Ion (+/−) | Rt (min) | Molecular Formula | m/z Calculated | m/z Experimental | Delta(mmu) | RDB | MS/MS Fragments | Proposed Compound | C. incanus | C. ladanifer | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [M − H]− | 19.9 | C7H6O5 | 169.0142 | 169.0151 | −5.02 | 5 | 125, 97 | Gallic acid | +++ | +++ | [12,37,38] |
| 2 | [M − H]− | 21.2 | C30H26O14 | 609.1250 | 609.1251 | −0.2 | 18 | 441, 423, 303 | Epigallocatechin dimer | +++ | +++ | [12,38] |
| 3 | [M − H]− | 23.0 | C15H14O7 | 305.0667 | 305.0658 | 2.86 | 9 | 361, 219, 167 | Epigallocatechin | +++ | + | [12,37,38] |
| 4 | [M − H]− | 24.9 | C15H14O7 | 305.0667 | 305.0665 | 0.58 | 9 | 221, 179, 166, | Gallocatechin | + | Tr | [12] |
| [M − H]− | 25.5 | C12H14O8 | 285.0616 | 285.0589 | 9.41 | 6 | 153, 108 | Uralenneoside | Tr | ++ | [15] | |
| 5 | [M − H]− | 26.8 | C15H14O6 | 289.0718 | 289.0692 | 8.83 | 9 | 247, 203, 137 | Epicatechin | ++ | Tr | [12] |
| 6 | [M − H]− | 27.7 | C16H22O8 | 341.1242 | 341.1246 | −1.19 | 6 | 179 | Caffeoyl-glucose | ++ | + | [12] |
| 7 | [M − H]− | 28.3 | C15H14O6 | 289.0718 | 289.0696 | 7.45 | 9 | - | Catechin | Tr | - | [12,38] |
| 8 | [M − H]− | 28.8 | C21H20O13 | 479.0831 | 479.0859 | −5.8 | 12 | 316, 271 | Myricetin hexoside | +++ | + | [12] |
| 9 | [M − H]− | 29.2 | C20H15O12 | 447.0558 | 447.0597 | −6.25 | 13 | 300, 175 | Ducheside A | + | + | [15,37] |
| 10 | [M − H]− | 29.5 | C27H29O16 | 609.1461 | 609.1490 | −4.74 | 13 | 301, 151 | Rutoside | + | + | [12,37] |
| 11 | [M − H]− | 29.9 | C23H26O12 | 493.1351 | 493.1385 | −6.78 | 11 | 313, 179 | Kaempferol dimethylether glucoside | ++ | + | [15,37] |
| 12 | [M − H]− | 30.3 | C20H18O12 | 449.0725 | 449.0703 | 5.00 | 12 | 316 | Myricetin pentoside | ++ | + | |
| 13 | [M − H]− | 30.7 | C21H20O12 | 463.0882 | 463.0877 | 1.08 | 12 | 316, 271 | Myricitrin | +++ | +++ | [12] |
| [M − H]− | 31.5 | C16H24O7 | 327.1449 | 327.1428 | 6.48 | 5 | 228, 165 | Betuloside | Tr | ++ | [15] | |
| 14 | [M − H]− | 32.1 | C20H15O12 | 447.0933 | 447.0937 | −0.93 | 12 | 284, 201 | Kaempferol glycoside | + | + | [12,37] |
| 15 | [M − H]− | 32.5 | C20H18O11 | 433.0776 | 433.0772 | 1.00 | 12 | 345, 300, 151 | Quercetin pentoside | ++ | + | [12] |
| 16 | [M − H]− | 32.7 | C20H15O12 | 447.0933 | 447.0927 | 1.31 | 12 | 403, 284, 174 | Kaempferol glycoside | + | Tr | [12,37] |
| 17 | [M − H]− | 33.01 | C21H20O11 | 447.0933 | 447.0956 | −5.17 | 12 | 393, 301, 179 | Quercetrine | ++ | ++ | [12] |
| 18 | [M − H]− | 36.1 | C23H20O9 | 439.1035 | 439.1037 | −0.55 | 14 | 421, 409, 393, 287, 260 | Unknown compound | + | - | |
| 19 | [M − H]− | 36.4 | C14H6O8 | 300.999 | 300.9986 | 1.29 | 12 | 283, 229 | Ellagic acid | Tr | Tr | [37] |
| 20 | [M − H]− | 36.8 | C15H10O8 | 317.0303 | 317.0305 | −0.66 | 11 | 255, 179 | Myricetin | +++ | Tr | [12] |
| 21 | [M − H]− | 38.4 | C30H26O13 | 593.1301 | 593.1340 | 1.79 | 18 | 447, 285, 223 | Kaempferol diglycoside | +++ | +++ | [15,37] |
| 22 | [M − H]− | 39.3 | C30H26O13 | 593.1301 | 593.1315 | −2.42 | 18 | 447, 285 | Kaempferol diglycoside | ++ | ++ | [15,37] |
| 23 | [M − H]− | 43.9 | C39H32O15 | 739.1668 | 739.1677 | −1.16 | 24 | 593, 453, 285 | Kaempferol dicoumaroyl glucose | +++ | +++ | [12] |
| 24 | [M − H]− | 44.8 | C39H32O15 | 739.1668 | 739.1660 | 1.14 | 24 | 593, 453, 285 | Kaempferol dicoumaroyl glucose | +++ | +++ | [12] |
| 25 | [M − H]− | 48.3 | C17H26O4 | 293.1758 | 293.1751 | 2.49 | 5 | 249, 236, 221 | Unknown compound | ++ | ++ | |
| 26 | [M − H]− | 48.6 | C16H11O5 | 283.0612 | 283.0589 | 8.09 | 11 | 268, 257 | Apigenin methylether | ++ | ++ | [15,37] |
| 27 | [M − H]− | 49.3 | C17H13O6 | 313.0718 | 313.0722 | −1.4 | 11 | 298, 283, 255 | Kaempferol dimethylether | +++ | +++ | [15,37] |
| C. incanus | C. ladanifer | |||||||
|---|---|---|---|---|---|---|---|---|
| 60% M | 60% SOX | 100% M | 100% SOX | 60% M | 60% SOX | 100% M | 100% SOX | |
| Ellagic acid | 41.39 a ± 0.83 | 55.15 b ± 1.10 | 88.66 ± 2.50 | 656.37 ± 5.20 | 44.84 a,b ± 0.64 | 609.89 ± 6.10 | 264.36 ± 8.80 | 199.55 ± 1.20 |
| Gallic acid | 278.86 c ± 10.00 | 298.49 c ± 17.00 | 283.39 c ± 6.30 | 772.90 ± 11.00 | 301.98 c ± 12.00 | - | 614.18 ± 38.00 | 541.61 ± 16.00 |
| Quercetrine | 687.27 ± 22.00 | 547.40 ±34.00 | 1275.09 ± 75.00 | 2473.45 ± 64.00 | - | 46.63 ± 0.25 | - | - |
| Epicatechin | 100.49 d ± 8.60 | 67.34 e ± 3.40 | 104.07 d ± 6.20 | 59.93 e ± 0.99 | trace | - | trace | trace |
| Gallocatechin | 3.06 ± 0.17 | 115.35 ± 5.20 | - | - | - | - | - | - |
| Epigallocatechin | 891.87 f ± 4.90 | 591.42 f ± 22.00 | 7271.59 ± 300.00 | 1384.63 ± 34.0 | 164.33 g ± 4.40 | 3.22 ± 0.15 | 191.46 g ± 6.20 | 38.93 ± 1.10 |
| Epigallocatechin dimer | 298.96 ± 7.00 | 83.08 ± 3.60 | 67.44 ± 1.50 | 25.76 ± 0.52 | 17.56 h ± 1.10 | 2.22 I,j ± 0.16 | 9.50 h,I ± 0.27 | 0.48 j ± 0.02 |
| Rutoside | 61.02 ± 3.90 | 40.17 ± 1.20 | 20.28 ± 0.79 | 171.54 ± 3.10 | 5.40 k ± 0.32 | 1.44 k ± 0.08 | 4.09 k ± 0.25 | 2.66 k ± 0.15 |
| Kaempferol glucoside | 103.62 l ± 3.20 | 112.07 l ± 7.70 | 211.11 n ± 7.30 | 638.59 ± 51.0 | 84.38 l,m ± 1.00 | 44.44 m ± 2.00 | 204.48 n ± 6.20 | 211.27 n ± 4.80 |
| Kaempferol diglycoside | 246.10 ±7.10 | 414.49 ± 8.80 | 625.44 ± 14.00 | 5889.89 ± 9.80 | 290.66 ± 7.30 | 522.16 ± 13.00 | 851.36 ± 22.00 | 777.14 ± 9.30 |
| Kaempferol diglycoside | 79.38 o ± 3.30 | 123.73 o,p ± 7.50 | 244.77 ± 61.00 | 442.52 r ± 11.00 | 90.24 o ± 1.50 | 168.82 p ± 6.40 | 379.65 r ± 9.20 | 700.03 ± 12.00 |
| Myricetin | 73.43 s ± 0.18 | 236.33 ± 5.60 | 38.48 s ± 1.00 | 1076.44 ± 39.00 | - | - | - | 343.53 ±8.80 |
| Myricitine pentoside | 621.04 t ± 13.00 | 440.80 ± 9.50 | 636.38 t ± 11.00 | 1808.26 ± 67.00 | - | - | - | - |
| Myricitrin | 1310.94 ± 74.00 | 1079.80 ± 66.00 | 1795.36 ± 43.00 | 604.22 ± 15.00 | 62.89 u ± 2.60 | 23.00 u ± 0.81 | 41.04 u ± 1.80 | 23.13 u ± 0.69 |
| Quercetin pentoside | 246.01 ± 6.70 | 136.85 ± 4.10 | 427.72 ± 17.00 | 860.18 ± 9.20 | trace | - | - | - |
| IC50 (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| C. incanus | C. ladanifer | |||||||
| 60% M | 60% SOX | 100% M | 100% SOX | 60% M | 60% SOX | 100% M | 100% SOX | |
| A375 | 60.41 | 84.62 | 57.80 | 109.41 | 95.30 | 138.61 | 199.01 | 164.91 |
| SCC-15 | >500 | >500 | 383.61 | >500 | >500 | >500 | >500 | >500 |
| HaCaT | 319.71 | >500 | 343.40 | >500 | >500 | >500 | >500 | >500 |
| Sun Protection Factor (100 µg/mL Extract) | ||
|---|---|---|
| C. incanus | C. ladanifer | |
| 60% M | 3.42 ± 0.13 a | 3.33 ± 0.15 a |
| 60% SOX | 3.77 ± 0.28 a,b | 3.50 ± 0.52 a |
| 100% M | 3.64 ± 0.08 a,b | 3.71 ± 0.30 a,b |
| 100% SOX | 4.13 ± 0.19 a,b | 4.37 ± 0.42 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. https://doi.org/10.3390/antiox9030202
Gaweł-Bęben K, Kukula-Koch W, Hoian U, Czop M, Strzępek-Gomółka M, Antosiewicz B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants. 2020; 9(3):202. https://doi.org/10.3390/antiox9030202
Chicago/Turabian StyleGaweł-Bęben, Katarzyna, Wirginia Kukula-Koch, Uliana Hoian, Marcin Czop, Marcelina Strzępek-Gomółka, and Beata Antosiewicz. 2020. "Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics" Antioxidants 9, no. 3: 202. https://doi.org/10.3390/antiox9030202
APA StyleGaweł-Bęben, K., Kukula-Koch, W., Hoian, U., Czop, M., Strzępek-Gomółka, M., & Antosiewicz, B. (2020). Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants, 9(3), 202. https://doi.org/10.3390/antiox9030202








