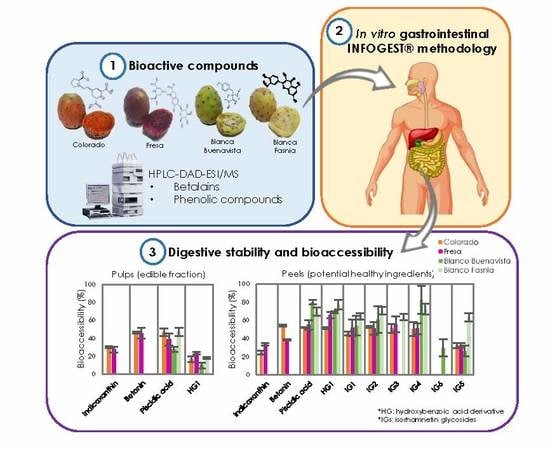
Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents, Reagents, and Standards
2.2. Prickly Pears and Physicochemical Analysis
2.3. Prickly Pear Extract Obtention for Characterization
2.4. In Vitro Digestion Assay
2.5. Betalain and Phenolic Content by High Performance Liquid Chromatography
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics
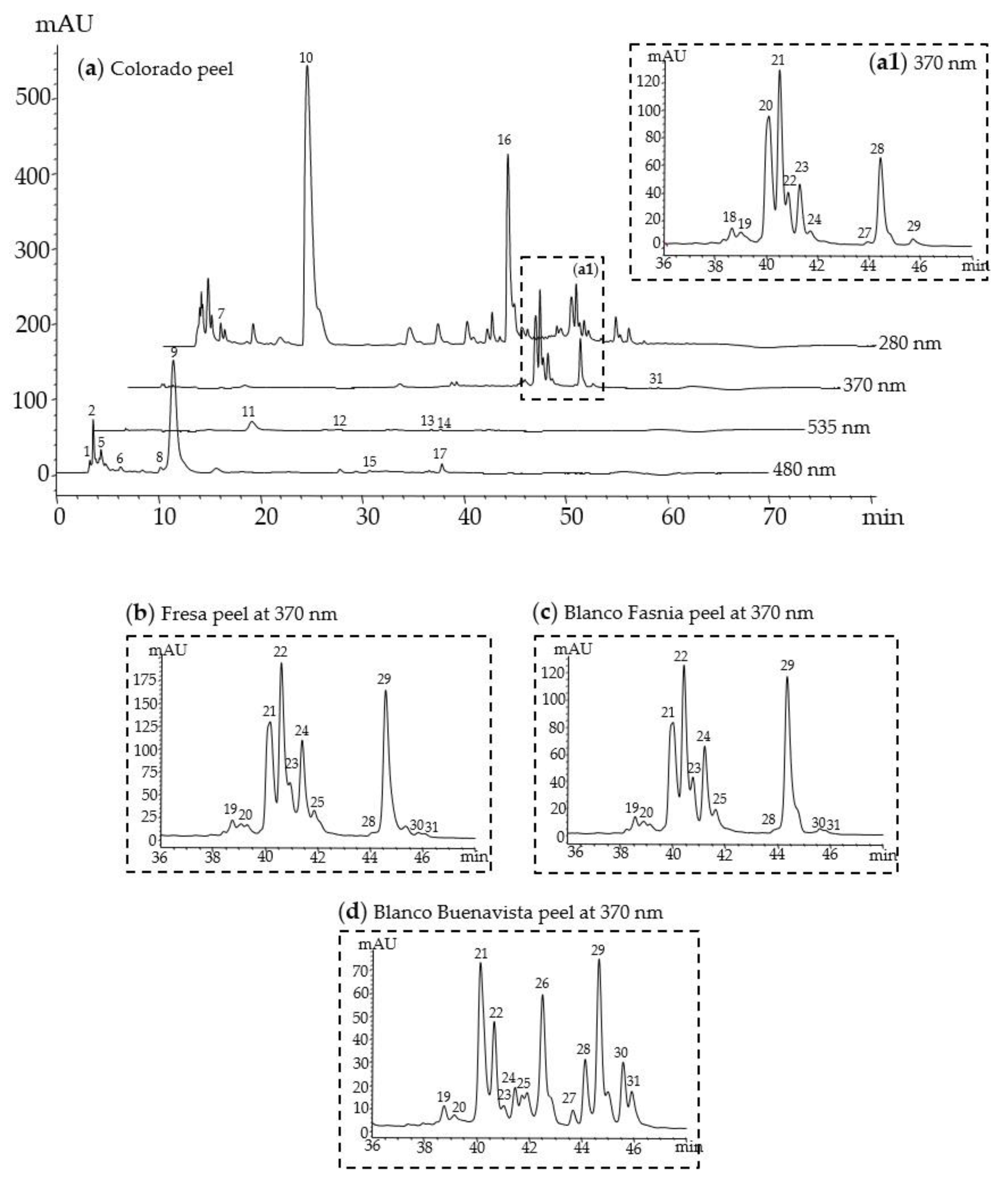
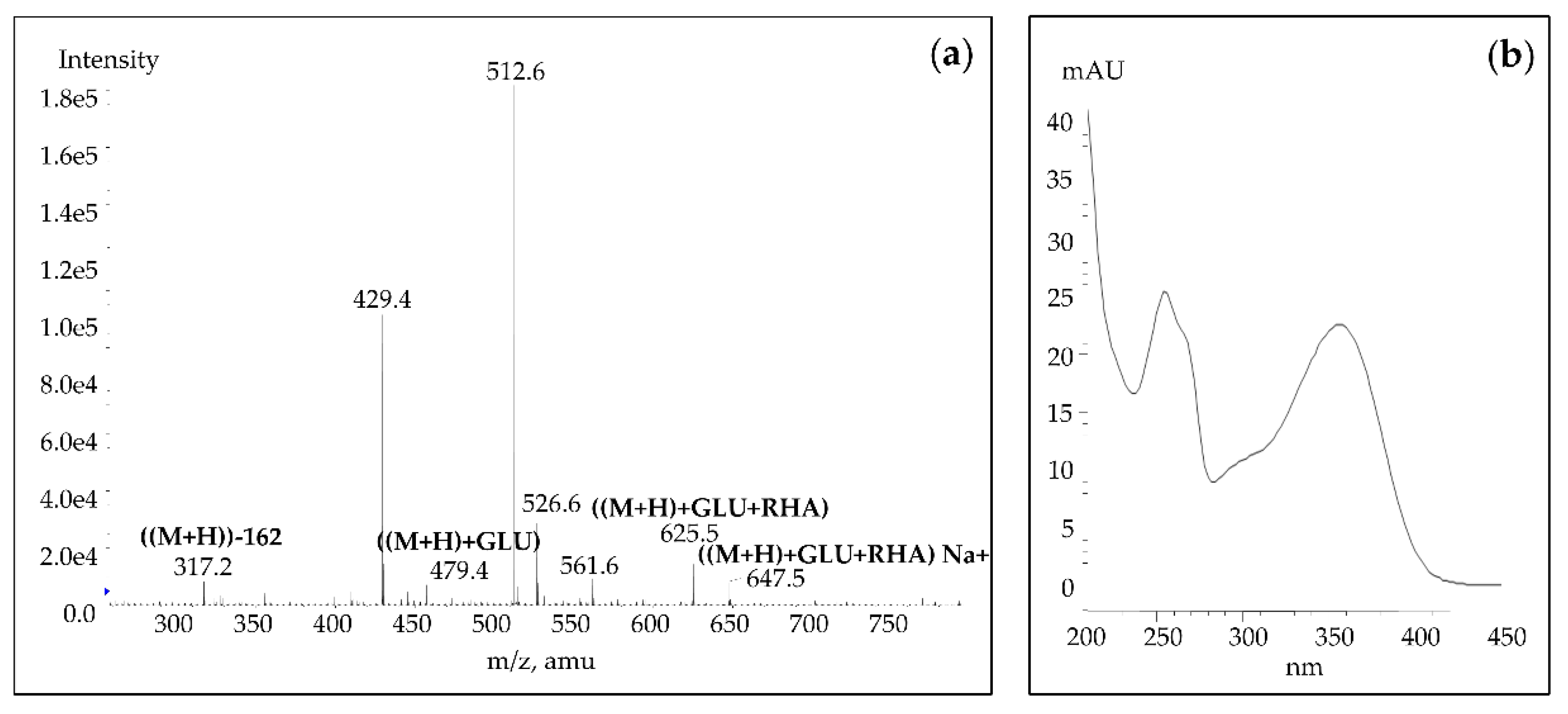
3.2. Identification of Betalains and Phenolic Compounds
3.3. Quantification of Betalains
3.4. Quantification of Phenolic Compounds
3.5. Digestive Stability of Betalains and Phenolic Compounds
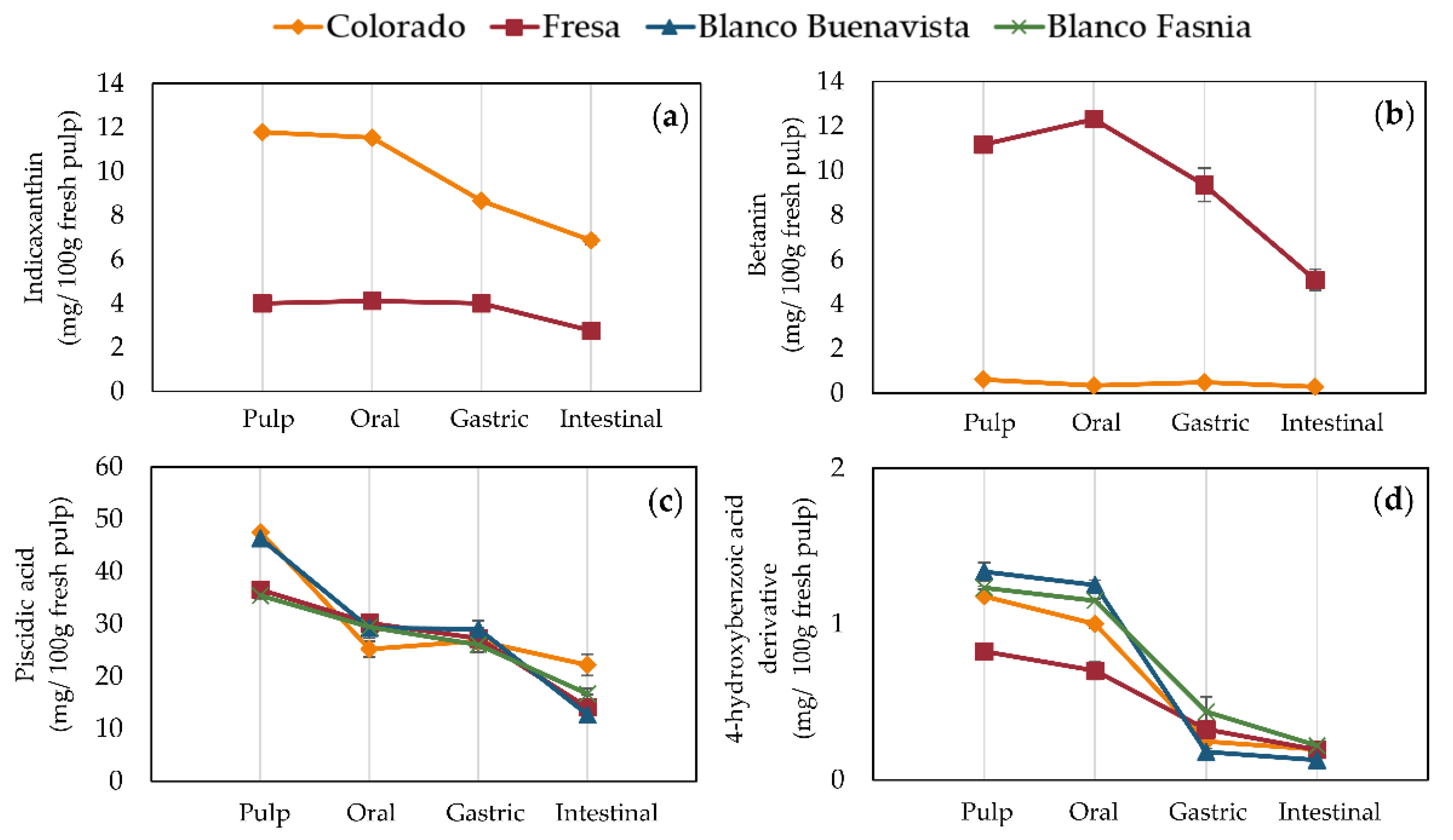
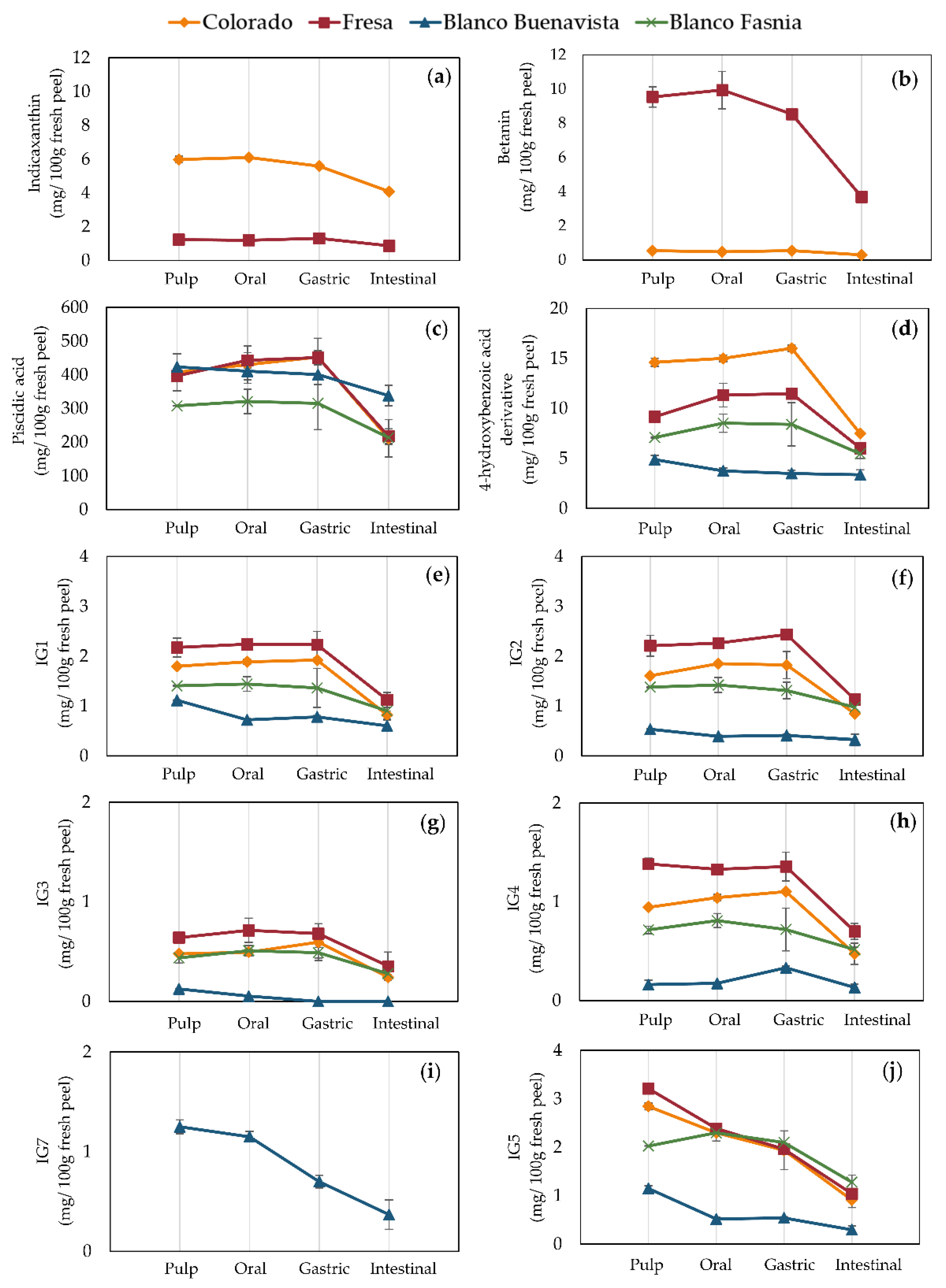
3.5.1. Digestive Stability in Pulps (Edible Fraction)
3.5.2. Digestive Stability in Peels (by-Products to Obtain Potential Healthy Ingredients)
3.6. Bioaccessiblity of Betalains and Phenolic Compounds
3.6.1. Bioaccessibility in Pulps (Edible Fraction)
3.6.2. Bioaccessibility in Peels (by-Products to Obtain Potential Healthy Ingredients)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Statista. Número de turistas que visitaron Canarias en 2018, por isla de destino. Available online: https://es.statista.com/estadisticas/526731/numero-de-turistas-que-visitaron-canarias-por-isla-de-destino/ (accessed on 26 January 2020).
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, phenols and antioxidant capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and determination of polyphenols and betalain pigments in the Moroccan prickly pear fruits (Opuntia ficus indica). Arab. J. Chem. 2016, 9, S278–S281. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography− electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, M.J.; Chaalal, M.; Louaileche, H.; Parrado, J.; Heredia, F.J. Betalain profile, phenolic content, and color characterization of different parts and varieties of Opuntia ficus-indica. J. Agric. Food Chem. 2014, 62, 8491–8499. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Santiago, E.; Yahia, E.M. Identification and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Cruz, D.M.; Pérez-Ramírez, I.F.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef] [PubMed]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; Del Rio, D.; Hernández, F. Phytochemical characterization of different prickly pear (Opuntia ficus-indica (L.) Mill.) cultivars and botanical parts: UHPLC-ESI-MSn metabolomics profiles and their chemometric analysis. Food Res. Int. 2018, 108, 301–308. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, A.M.; Subba Rao, G.; Arai, T.; Iida, A.; Tokuda, H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (Adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines. Anti-Cancer Agents Med. Chem. 2011, 11, 280–284. [Google Scholar] [CrossRef]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’alessio, P. Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2014, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Tan, C.; Wang, Y.; Yang, S.; Tan, D. Betanin reduces the accumulation and cross-links of collagen in high-fructose-fed rat heart through inhibiting non-enzymatic glycation. Chem.-Biol. Interact. 2015, 227, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Allegra, M.; Sardo, P.; Attanzio, A.; Tesoriere, L.; Livrea, M.A.; Ferraro, G.; Carletti, F. Brain distribution and modulation of neuronal excitability by indicaxanthin from Opuntia ficus indica administered at nutritionally-relevant amounts. Front. Aging Neurosci. 2018, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; Carletti, F.; Gambino, G.; Tutone, M.; Attanzio, A.; Tesoriere, L.; Ferraro, G.; Sardo, P.; Almerico, A.M.; Livrea, M.A. Indicaxanthin from Opuntia ficus-indica crosses the blood–brain barrier and modulates neuronal bioelectric activity in rat hippocampus at dietary-consistent amounts. J. Agric. Food Chem. 2015, 63, 7353–7360. [Google Scholar] [CrossRef]
- Ressaissi, A.; Attia, N.; Falé, P.L.; Pacheco, R.; Victor, B.L.; Machuqueiro, M.; Serralheiro, M.L.M. Isorhamnetin derivatives and piscidic acid for hypercholesterolemia: Cholesterol permeability, HMG-CoA reductase inhibition, and docking studies. Arch. Pharm. Res. 2017, 40, 1278–1286. [Google Scholar] [CrossRef]
- Matias, A.; Nunes, S.L.; Poejo, J.; Mecha, E.; Serra, A.T.; Madeira, P.J.A.; Bronze, M.R.; Duarte, C.M.M. Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food Funct. 2014, 5, 3269–3280. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Rodríguez-Rodríguez, C.; Gutiérrez-Uribe, J.A.; Cepeda-Cañedo, E.; Serna-Saldívar, S.O. Bioaccessibility, intestinal permeability and plasma stability of isorhamnetin glycosides from Opuntia ficus-indica (L.). Int. J. Mol. Sci. 2017, 18, 1816. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Fernández-López, R.; Welti-Chanes, J.; Cano, M.P. Inhibitory potential of prickly pears and their isolated bioactives against digestive enzymes linked to type 2 diabetes and inflammatory response. J. Sci. Food Agric. 2019, 99, 6380–6391. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Comparative analyses of total phenols, antioxidant activity, and flavonol glycoside profile of cladode flours from different varieties of Opuntia spp. J. Agric. Food Chem. 2011, 59, 7054–7061. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Moreno-García, B.E.; Gutiérrez-Uribe, J.A.; Aráiz-Hernández, D.; Alvarez, M.M.; Serna-Saldivar, S.O. Induction of apoptosis in colon cancer cells treated with isorhamnetin glycosides from Opuntia ficus-indica pads. Plant Food Hum. Nutr. 2014, 69, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrout, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.N.; Luu, A.Y.; Dragsted, L.O.; Arrigoni, E. Adaption of an in vitro digestion method to screen carotenoid liberation and in vitro accessibility from differently processed spinach preparations. Food Chem. 2017, 224, 407–413. [Google Scholar] [CrossRef]
- De Ancos, B.; Cilla, A.; Barberá, R.; Sánchez-Moreno, C.; Cano, M.P. Influence of orange cultivar and mandarin postharvest storage on polyphenols, ascorbic acid and antioxidant activity during gastrointestinal digestion. Food Chem. 2017, 225, 114–124. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; El-Hady, E.S.A.A.; Omran, H.T.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Influence of cultivar and origin on the flavonol profile of fruits and cladodes from cactus Opuntia ficus-indica. Food Res. Int. 2014, 64, 864–872. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC–DAD–ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef]
- Allegra, M.; Tutone, M.; Tesoriere, L.; Almerico, A.M.; Culletta, G.; Livrea, M.A.; Attanzio, A. Indicaxanthin, a multi-target natural compound from Opuntia ficus-indica fruit: From its poly-pharmacological effects to biochemical mechanisms and molecular modelling studies. Eur. J. Med. Chem. 2019, 179, 753–764. [Google Scholar] [CrossRef]
- Choo, K.Y.; Ong, Y.Y.; Lim, R.L.H.; Tan, C.P.; Ho, C.W. Study on bioaccessibility of betacyanins from red dragon fruit (Hylocereus polyrhizus). Food Sci. Biotechnol. 2019, 28, 1163–1169. [Google Scholar] [CrossRef]
- Vieira Teixeira da Silva, D.; dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; Flosi Paschoalin, V.M. Betanin, a natural food additive: Stability, bioavailability, antioxidant and preservative ability assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Welti-Chanes, J.; Cano, M.P. Release mechanisms of bioactive compounds in fruits submitted to high hydrostatic pressure: A dynamic microstructural analysis based on prickly pear cells. Food Res. Int. 2020, 130, 108909. [Google Scholar] [CrossRef]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr. Rev. Food Sci. 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Naselli, F.; Tesoriere, L.; Caradonna, F.; Bellavia, D.; Attanzio, A.; Gentile, C.; Livrea, M.A. Anti-proliferative and pro-apoptotic activity of whole extract and isolated indicaxanthin from Opuntia ficus-indica associated with re-activation of the onco-suppressor p16INK4a gene in human colorectal carcinoma (Caco-2) cells. Biochem. Biophys. Res. Commun. 2014, 450, 652–658. [Google Scholar] [CrossRef]
- Rahimi, P.; Mesbah-Namin, S.A.; Ostadrahimi, A.; Separham, A.; Jafarabadi, M.A. Betalain-and betacyanin-rich supplements’ impacts on the PBMC SIRT1 and LOX1 genes expression and Sirtuin-1 protein levels in coronary artery disease patients: A pilot crossover clinical trial. J. Funct. Foods 2019, 60, 103401. [Google Scholar] [CrossRef]
- Rahimi, P.; Mesbah-Namin, S.A.; Ostadrahimi, A.; Abedimanesh, S.; Separham, A.; Jafarabadi, M.A. Effects of betalains on atherogenic risk factors in patients with atherosclerotic cardiovascular disease. Food Funct. 2019, 10, 8286–8297. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Constantinou, C.M.; Keane, K.M.; West, D.J.; Howatson, G.; Stevenson, E.J. The plasma bioavailability of nitrate and betanin from Beta vulgaris rubra in humans. Eur. J. Nutr. 2017, 56, 1245–1254. [Google Scholar] [CrossRef]
- Allegra, M.; Ianaro, A.; Tersigni, M.; Panza, E.; Tesoriere, L.; Livrea, M.A. Indicaxanthin from cactus pear fruit exerts anti-inflammatory effects in carrageenin-induced rat pleurisy. J. Nutr. 2014, 144, 185–192. [Google Scholar] [CrossRef]
- Ressaissi, A.; Attia, N.; Pacheco, R.; Falé, P.L.; Serralheiro, M.L.M. Cholesterol transporter proteins in HepG2 cells can be modulated by phenolic compounds present in Opuntia ficus-indica aqueous solutions. J. Funct. Foods 2020, 64, 103674. [Google Scholar] [CrossRef]
- De Santiago, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Digestibility of (Poly) phenols and Antioxidant Activity in Raw and Cooked Cactus Cladodes (Opuntia ficus-indica). J. Agric. Food Chem. 2018, 66, 5832–5844. [Google Scholar] [CrossRef] [PubMed]
- De Santiago, E.; Gill, C.I.; Carafa, I.; Tuohy, K.M.; De Peña, M.P.; Cid, C. Digestion and colonic fermentation of raw and cooked Opuntia ficus-indica cladodes impacts bioaccessibility and bioactivity. J. Agric. Food Chem. 2019, 67, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
| Prickly Pear (Opuntia ficus-indica L. Mill.) Variety | ||||
|---|---|---|---|---|
| Characteristics | Colorada (Orange) | Fresa (Red) | Blanco Buenavista (White) | Blanco Fasnia (White) |
 |  |  |  | |
| Pulp color | Orange | Fuchsia | White | White |
| Peel color | Orange | Fuchsia | Green | Yellow/green |
| Apical caliber (cm) | 6.2 ± 0.8 a | 5.8 ± 0.5 a | 6.6 ± 0.7 a | 6.3 ± 1.0 a |
| Equatorial caliber (cm) | 4.9 ± 0.3 a | 4.5 ± 0.4 a | 5.1 ± 0.3 a | 4.6 ± 0.3 a |
| Weight (g) | 106.0 ± 9.3 a | 78.9 ± 9.6 a | 129.6 ± 23.9 a | 89.0 ± 7.0 a |
| % peel | 55.7 ± 2.6 a | 41.3 ± 11.4 a | 55.4 ± 1.8 a | 44.1 ± 4.1 a |
| % pulp | 38.2 ± 3.7 a | 51.8 ± 6.6 a | 41.1 ± 6.2 a | 50.6 ± 4.2 a |
| % seeds | 6.1 ± 0.1 a | 6.9 ± 1.4 a | 3.5 ± 0.9 a | 5.3 ± 0.8 a |
| pH | 6.1 ± 0.2 a | 6.1 ± 0.0 a | 6.6 ± 0.1 b | 6.2 ± 0.1 a |
| Titratable acidity (%) | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Soluble solids (°Brix) | 13.4 ± 0.1 a | 13.6 ± 0.1 a | 15.7 ± 0.1 b | 16.0 ± 0.2 b |
| Firmness (N) | 18.3 ± 3.6 a | 12.5 ± 3.8 a | 22.0 ± 2.6 a | 10.7 ± 1.6 a |
| Color peel (CIELAB) | ||||
| L* | 50.4 ± 1.8 ab | 41.7 ± 2.4 a | 58.9 ± 2.7 b | 60.6 ± 3.5 b |
| a* | 9.2 ± 2.4 ab | 19.4 ± 3.7 b | 1.1 ± 3.6 a | 4.3 ± 2.4 ab |
| b* | 11.0 ± 2.3 ab | −4.2 ± 2.2 a | 23.9 ± 3.7 b | 20.8 ± 5.3 b |
| Color pulp (CIELAB) | ||||
| L* | 49.8 ± 4.5 a | 38.1 ± 1.3 a | 58.5 ± 2.5 a | 55.6 ± 4.7 a |
| a* | 12.5 ± 2.9 b | 11.9 ± 3.8 b | −1.7 ± 0.7 a | −1.7 ± 0.6 a |
| b* | 11.3 ± 6.6 a | −7.5 ± 1.1 a | 7.3 ± 1.7 a | 4.9 ± 1.9 a |
| No 1 | Rt (min) | Compound Identity | UV λmax (nm) | [M+H] + | MS/MS (m/z) |
|---|---|---|---|---|---|
| 1 | 3.1 | Bx-hydroxyproline (Portulacaxanthin I) | 479 | 325.11 | 307.13, 220.10, 191.14 |
| 2 | 3.3 | Bx-glycine (Portulacaxanthin III) a | 471 | 269.11 | 225.14, 136.06 |
| 3 | 3.7 | Bx-unknown | 475 | 307.08 | 116.07, 84.08, 76.02 |
| 4 | 3.9 | Bx-asparagine (Vulgaxanthin III) | 474 | 326.14 | 325.14, 307.13, 220.10 |
| 5 | 4.0 | Bx-glutamine (Vulgaxanthin I) a | 470 | 340.11 | 308.09, 116.07, 84.04, 76.02 |
| 6 | 5.5 | Bx-glutamic acid (Vulgaxanthin II) a | 474 | 341.10 | 292.20, 147.04, 72.08 |
| 7 | 6.1 | Piscidic acid derivative | 228, 275 | 322.21 | 147.04, 119.05, 107.05, 91.05 |
| 8 | 9.2 | Bx-amino butyric acid | 469 | 297.11 | 286.09, 153.04, 86.09 |
| 9 | 10.5 | Bx-proline (Indicaxanthin) a | 478 | 309.11 | 263.10, 217.10, 70.06 |
| 10 | 14.0 | Piscidic acid a | 232, 275 | 257.07 | 191.07, 147.04, 119.05, 107.05 |
| 11 | 15.7 | Betanin a | 534 | 551.15 | 390.10, 389.10 |
| 12 | 20.7 | Isobetanin | 534 | 551.15 | 390.10, 389.10 |
| 13 | 27.3 | Betanidin a | 540 | 389.10 | 345.09, 150.05 |
| 14 | 28.0 | Gomphrenin I | 536 | 551.15 | 389.10 |
| 15 | 32.3 | Neobetanin | 476 | 549.13 | 387.08 |
| 16 | 33.7 | 4-hydroxybenzoic acid derivative | 274 | 205.05 | 161.06, 131.05, 115.05, 105.07 |
| 17 | 37.9 | Bx-tryptophan | 473 | 398.25 | 307.26, 219.05 |
| 18 | 38.7 | Quercetin glycoside I (QG1) | 266, 351 | 426.24 | 303.05, 191.07, 120.08 |
| 19 | 39.1 | Quercetin glycoside II (QG2) | 269, 350 | 653.28 | 303.05, 177.05 |
| 20 | 40.0 | Isorhamnetin glucosyl-rhamnosyl-rhamnoside (IG1) a | 254, 354 | 771.23 | 625.18, 317.07, 85.03 |
| 21 | 40.4 | Isorhamnetin glucosyl-rhamnosyl-pentoside (IG2) a | 253, 354 | 757.22 | 317.07, 167.07, 86.10 |
| 22 | 40.8 | Isorhamnetin hexosyl-hexosyl-pentoside (IG3) a | 253, 354 | 757.22 | 317.06 |
| 23 | 41.2 | Isorhamnetin glucosyl-pentoside (IG4) a | 254, 354 | 611.16 | 479.12, 317.07, 177.05 |
| 24 | 41.6 | Quercetin-3-rutinoside (Rutin) a | 250, 343 | 611.23 | 303.05, 229.11, 137.07 |
| 25 | 42.5 | Isorhamnetin hexosyl-rhamnoside (IG7) a | 255, 355 | 625.53 | 479.12, 317.07 |
| 26 | 43.3 | Isorhamnetin glycoside | 254, 353 | 581.15 | 317.07 |
| 27 | 44.1 | Kaempferol glucosyl-rhamnoside (KG1) a | 262, 351 | 595.17 | 287.06 |
| 28 | 44.5 | Isorhamnetin glucosyl-rhamnoside (IG5) a | 253, 354 | 625.18 | 317.07, 85.03 |
| 29 | 45.5 | Isorhamnetin-hexosyl-pentoside (IG6) a | 253, 354 | 611.07 | 317.07 |
| 30 | 45.8 | Isorhamnetin glycoside | 232, 330 | 814.58 | 641.37, 317.07, 169.09 |
| 31 | 50.0 | Isorhamnetin a | 370 | 317.07 | 317.07 |
| Prickly Pear Variety | ||||||
|---|---|---|---|---|---|---|
| No. | Compound | Tissue | Colorado (Orange) | Fresa (Red) | Blanco Buenavista (White) | Blanco Fasnia (White) |
| Betaxanthins (mg/100 g fresh weight) | ||||||
| 1 | Bx-hydroxyproline (Portulacaxanthin I) | Peel | 0.09 ± 0.00 b | 0.01 ± 0.00 a | n.d. a | n.d. a |
| Pulp | 0.06 ± 0.00 c | 0.01 ± 0.00 b | n.d. a | n.d. a | ||
| 2 | Bx-glycine (Portulacaxanthin III) | Peel | 0.94 ± 0.02 c | 0.11 ± 0.01 b | 0.02 ± 0.00 a | 0.01 ± 0.00 a |
| Pulp | 1.16 ± 0.02 c | 0.30 ± 0.01 b | 0.03 ± 0.00 a | 0.02 ± 0.00 a | ||
| 3 | Bx-unknown | Peel | 0.19 ± 0.00 c | 0.03 ± 0.00 b | tr. a | 0.01 ± 0.00 a |
| Pulp | 0.27 ± 0.00 d | 0.06 ± 0.00 c | n.d. a | 0.02 ± 0.00 b | ||
| 4 | Bx-asparagine (Vulgaxanthin III) | Peel | 0.67 ± 0.01 c | 0.02 ± 0.00 b | tr. a | n.d. a |
| Pulp | 0.24 ± 0.01 c | 0.08 ± 0.02 b | n.d. a | n.d. a | ||
| 5 | Bx-glutamine (Vulgaxanthin I) | Peel | 0.21 ± 0.00 c | 0.02 ± 0.00 b | tr. a | n.d. a |
| Pulp | 0.29 ± 0.01 c | 0.06 ± 0.00 b | n.d. a | n.d. a | ||
| 6 | Bx-glutamic acid (Vulgaxanthin II) | Peel | 0.20 ± 0.00 c | 0.02 ± 0.00 b | n.d. a | n.d. a |
| Pulp | 0.09 ± 0.00 c | 0.02 ± 0.00 b | n.d. a | n.d. a | ||
| 9 | Bx-amino butyric acid | Peel | 0.12 ± 0.00 c | 0.01 ± 0.00 b | n.d. a | n.d. a |
| Pulp | 0.16 ± 0.02 c | 0.04 ± 0.00 b | n.d. a | n.d. a | ||
| 10 | Bx-proline (Indicaxanthin) | Peel | 5.98 ± 0.19 c | 1.24 ± 0.06 b | 0.03 ± 0.00 a | 0.02 ± 0.00 a |
| Pulp | 11.79 ± 0.15 c | 4.01 ± 0.14 b | 0.05 ± 0.00 a | 0.09 ± 0.01 a | ||
| 18 | Bx-tryptophan | Peel | 0.20 ± 0.00 c | 0.02 ± 0.00 b | 0.02 ± 0.00 b | n.d. a |
| Pulp | 0.20 ± 0.00 c | 0.05 ± 0.00 b | tr. a | n.d. a | ||
| Betacyanins (mg/100 g fresh weight) | ||||||
| 12 | Betanin | Peel | 0.54 ± 0.02 a | 9.54 ± 0.59 b | 0.02 ± 0.00 a | 0.01 ± 0.00 a |
| Pulp | 0.62 ± 0.02 b | 11.17 ± 0.28 c | 0.03 ± 0.00 a | 0.01 ± 0.00 a | ||
| 13 | Isobetanin | Peel | 0.07 ± 0.00 a | 0.51 ± 0.09 b | n.d. a | n.d. a |
| Pulp | 0.05 ± 0.01 a | 1.02 ± 0.12 b | n.d. a | n.d. a | ||
| 14 | Betanidin | Peel | 0.02 ± 0.00 a | 0.18 ± 0.01 b | tr. a | n.d. a |
| Pulp | 0.02 ± 0.00 a | 0.33 ± 0.02 b | tr. a | n.d. a | ||
| 15 | Gomphrenin I | Peel | 0.02 ± 0.00 a | 0.31 ± 0.02 b | tr. a | n.d. a |
| Pulp | 0.02 ± 0.00 b | 0.31 ± 0.00 c | tr. a | n.d. a | ||
| 16 | Neobetanin | Peel | 0.04 ± 0.00 b | 0.17 ± 0.00 c | n.d. a | n.d. a |
| Pulp | n.d. a | tr. a | n.d. a | n.d. a | ||
| Total betaxanthins 1 | Peel | 8.61 ± 0.24 c | 1.48 ± 0.07 b | 0.07 ± 0.00 a | 0.04 ± 0.00 a | |
| Pulp | 14.26 ± 0.14 c | 4.63 ± 0.13 b | 0.08 ± 0.00 a | 0.13 ± 0.01 a | ||
| Total betacyanins 1 | Peel | 0.68 ± 0.03 a | 10.71 ± 0.72 b | 0.02 ± 0.00 a | 0.01 ± 0.00 a | |
| Pulp | 0.71 ± 0.02 b | 12.84 ± 0.18 c | 0.03 ± 0.00 a | 0.01 ± 0.00 a | ||
| Total betalains 1 | Peel | 9.29 ± 0.27 b | 12.19 ± 0.80 c | 0.09 ± 0.00 a | 0.05 ± 0.00 a | |
| Pulp | 14.97 ± 0.12 b | 17.47 ± 0.31 c | 0.11 ± 0.00 a | 0.14 ± 0.01 a | ||
| Prickly Pear Variety | ||||||
|---|---|---|---|---|---|---|
| No. | Compound | Tissue | Colorada (Orange) | Fresa (Red) | Blanco Buenavista (White) | Blanco Fasnia (White) |
| Phenolic acids (mg/100 g fresh weight) | ||||||
| 7 | Piscidic acid derivative | Peel | 15.85 ± 0.70 c | 10.55 ± 0.74 b | 14.92 ± 0.38 c | 4.94 ± 0.02 a |
| Pulp | 13.49 ± 0.17 b | 13.42 ± 0.69 b | 14.04 ± 0.40 b | 1.33 ± 0.11 a | ||
| 10 | Piscidic acid | Peel | 407.35 ± 13.44 b | 396.88 ± 9.99 b | 423.61 ± 37.96 b | 307.94 ± 1.26 a |
| Pulp | 47.49 ± 0.05 b | 36.53 ± 0.20 a | 46.33 ± 0.58 b | 35.41 ± 0.55 a | ||
| 16 | 4-hydroxybenzoic acid derivative | Peel | 14.62 ± 0.41 d | 9.17 ± 0.16 c | 4.88 ± 0.42 a | 7.10 ± 0.06 b |
| Pulp | 1.18 ± 0.01 b | 0.83 ± 0.03 a | 1.34 ± 0.06 c | 1.23 ± 0.01 ab | ||
| Flavonoids (mg/100 g fresh weight) | ||||||
| 18 | Quercetin glycoside (QG1) | Peel | 0.79 ± 0.00 b | 0.86 ± 0.06 b | 0.53 ± 0.01 a | 0.53 ± 0.01 a |
| Pulp | tr. a | tr. a | tr. a | tr. a | ||
| 19 | Quercetin glycoside (QG2) | Peel | 0.73 ± 0.01 c | 0.78 ± 0.02 d | 0.51 ± 0.01 b | 0.34 ± 0.01 a |
| Pulp | tr. a | tr. a | tr. a | tr. a | ||
| 20 | Isorhamnetin glucosyl-rhamnosyl-rhamnoside (IG1) | Peel | 1.80 ± 0.03 b | 2.17 ± 0.19 c | 1.11 ± 0.02 a | 1.40 ± 0.01 a |
| Pulp | 0.01 ± 0.00 a | 0.02 ± 0.00 b | 0.03 ± 0.00 bc | 0.03 ± 0.00 d | ||
| 21 | Isorhamnetin glucosyl-rhamnosyl-pentoside (IG2) | Peel | 1.07 ± 0.03 b | 2.21 ± 0.21 c | 0.53 ± 0.01 a | 1.38 ± 0.01 b |
| Pulp | 0.01 ± 0.00 a | 0.02 ± 0.00 c | 0.01 ± 0.00 b | 0.02 ± 0.00 c | ||
| 22 | Isorhamnetin hexosyl-hexosyl-pentoside (IG3) | Peel | 0.48 ± 0.01 b | 0.64 ± 0.03 c | 0.13 ± 0.00 a | 0.44 ± 0.00 b |
| Pulp | tr. a | 0.01 ± 0.00 b | tr. b | 0.01 ± 0.00 c | ||
| 23 | Isorhamnetin glucosyl-pentoside (IG4) | Peel | 0.95 ± 0.01 c | 1.38 ± 0.06 d | 0.16 ± 0.05 a | 0.72 ± 0.04 b |
| Pulp | tr. a | 0.01 ± 0.00 a | 0.01 ±0.00 a | 0.01 ± 0.00 b | ||
| 24 | Quercetin-3-rutinoside (Rutin) | Peel | 1.33 ± 0.19 b | 1.22 ± 0.14 b | 0.45 ± 0.04 a | 0.75 ± 0.01 a |
| Pulp | tr. a | tr. a | 0.01 ± 0.00 a | 0.03 ± 0.00 b | ||
| 25 | Isorhamnetin hexosyl-rhamnoside (IG7) | Peel | n.d. a | n.d. a | 1.25 ± 0.07 a | n.d. a |
| Pulp | n.d. a | n.d. a | 0.01 ± 0.00 a | n.d. a | ||
| 26 | Isorhamnetin glycoside | Peel | tr. a | 0.07 ± 0.01 b | 0.14 ± 0.02 c | n.d. a |
| Pulp | tr. a | tr. a | tr. a | n.d. a | ||
| 27 | Kaempferol glucosyl-rhamnoside (KG1) | Peel | 0.27 ± 0.04 a | 0.36 ± 0.00 a | 0.94 ± 0.19 b | 0.15 ± 0.01 a |
| Pulp | 0.01 ± 0.00 b | 0.02 ± 0.00 b | 0.08 ± 0.00 c | 0.01 ± 0.00 a | ||
| 28 | Isorhamnetin glucosyl-rhamnoside (IG5) | Peel | 2.85 ± 0.07c | 3.21 ± 0.07 d | 1.15 ± 0.05 a | 2.03 ± 0.00 b |
| Pulp | 0.01 ± 0.00 a | 0.02 ± 0.00 b | 0.05 ± 0.00 d | 0.03 ± 0.00 c | ||
| 29 | Isorhamnetin hexosyl-pentoside (IG6) | Peel | 0.37 ± 0.06 c | 0.23 ± 0.01 b | 0.45 ± 0.01 c | 0.12 ± 0.01 a |
| Pulp | tr. a | 0.01 ± 0.00 a | tr. a | n.d. a | ||
| 30 | Isorhamnetin glycoside | Peel | n.d. a | n.d. a | 0.37 ± 0.03 a | n.d. a |
| Pulp | n.d. a | n.d. a | tr. a | n.d. a | ||
| 31 | Isorhamnetin | Peel | 0.33 ± 0.01 b | 0.62 ± 0.03 d | 0.53 ± 0.02 c | n.d. a |
| Pulp | tr. a | tr. a | tr. a | tr. a | ||
| Total phenolic acids 1 | Peel | 437.82 ± 14.55 b | 416.60 ± 11.65 b | 443.41 ± 38.41 b | 319.98 ± 13.01 a | |
| Pulp | 62.16 ± 0.21 c | 50.78 ± 0.46 b | 61.71 ± 0.93 c | 37.98 ± 0.67 a | ||
| Total flavonoids 1 | Peel | 11.49 ± 0.17 b | 13.76 ± 0.75 b | 8.25 ± 0.40 a | 7.84 ± 0.07 a | |
| Pulp | 0.04 ± 0.00 a | 0.09 ± 0.01 c | 0.19 ± 0.01 d | 0.14 ± 0.01 c | ||
| Total phenolic compounds 1 | Peel | 449.31 ± 14.38 a | 430.36 ± 11.65 a | 451.66 ± 38.02 a | 327.82 ± 1.37 b | |
| Pulp | 62.20 ± 0.21 c | 50.87 ± 0.45 b | 61.90 ± 0.93 c | 38.12 ± 0.68 a | ||
| Compound Identity | Colorado (Orange) | Fresa (Red) | Blanco Buenavista (White) | Blanco Fasnia (White) |
|---|---|---|---|---|
| Indicaxanthin | 58.2 ± 5.0 b | 68.9 ± 5.1 b | n.d. a | n.d. a |
| Betanin | 46.2 ± 1.1 b | 45.6 ± 5.9 b | n.d. a | n.d. a |
| Piscidic acid | 46.7 ± 4.2 b | 38.6 ± 6.9 b | 27.4 ± 2.7 a | 47.1 ± 4.8 b |
| 4-hydroxybenzoic acid derivative | 16.6 ± 3.1 b | 23.4 ± 1.3 c | 9.6 ± 3.1 a | 17.8 ± 1.0 b |
| Compound Identity | Colorado (Orange) | Fresa (Red) | Blanco Buenavista (White) | Blanco Fasnia (White) |
|---|---|---|---|---|
| Indicaxanthin | 68.2 ± 4.0 b | 70.4 ± 4.5 b | n.d. a | n.d. a |
| Betanin | 54.1 ± 1.1 c | 38.6 ± 0.8 b | n.d. a | n.d. a |
| Piscidic acid | 51.8 ± 0.4 a | 54.8 ± 5.1 a | 79.9 ± 2.3 b | 69.4 ± 4.6 b |
| 4-hydroxybenzoic acid derivative | 52.3 ± 0.9 a | 65.8 ± 3.4 b | 68.9 ± 0.9 b | 77.1 ± 5.0 b |
| Isorhamnetin glucosyl-rhamnosyl-rhamnoside (IG1) | 45.5 ± 2.2 a | 51.6 ± 9.2 a | 54.1 ± 11.0 a | 64.2 ± 3.5 a |
| Isorhamnetin glucosyl-rhamnosyl-pentoside (IG2) | 52.6 ± 1.0 a | 51.1 ± 6.7 a | 60.9 ± 14.8 a | 70.6 ± 4.1 a |
| Isorhamnetin hexosyl-hexosyl-pentoside (IG3) | 51.0 ± 5.1 b | 55.3 ± 8.8 b | n.d. a | 63.6 ± 3.2 b |
| Isorhamnetin glucosyl-pentoside (IG4) | 50.2 ± 7.8 a | 50.6 ± 6.0 a | 82.5 ± 15.2 a | 72.1 ± 6.1 a |
| Isorhamnetin hexosyl-rhamnoside (IG7) | n.a. a | n.a. a | 29.5 ± 9.5 b | n.a. a |
| Isorhamnetin glucosyl-rhamnoside (IG5) | 32.1 ± 2.8 a | 32.4 ± 2.2 a | 25.9 ± 5.9 a | 63.2 ± 4.7 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164. https://doi.org/10.3390/antiox9020164
Gómez-Maqueo A, Antunes-Ricardo M, Welti-Chanes J, Cano MP. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants. 2020; 9(2):164. https://doi.org/10.3390/antiox9020164
Chicago/Turabian StyleGómez-Maqueo, Andrea, Marilena Antunes-Ricardo, Jorge Welti-Chanes, and M. Pilar Cano. 2020. "Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients" Antioxidants 9, no. 2: 164. https://doi.org/10.3390/antiox9020164
APA StyleGómez-Maqueo, A., Antunes-Ricardo, M., Welti-Chanes, J., & Cano, M. P. (2020). Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants, 9(2), 164. https://doi.org/10.3390/antiox9020164