1. Introduction
The term “oxidative stress”, describing an oxidative damage to the cells or organs, was proposed in 1985 and was originally defined as “a disturbance in the prooxidant-antioxidant balance in favor of the former” [
1]. There are many types of prooxidants, such as free radicals or reactive oxygen species (ROS). Free radicals are particles with one or more unpaired electrons and ROS are reactive compounds containing oxygen. Some of ROS, e.g., superoxide anion radical, hydroxyl radical or peroxyl radical, may be classed as free radicals as they possess unpaired electrons. Other ROS, such as hydrogen peroxide or ozone, are not free radicals, but they could also pose as prooxidants [
2,
3,
4]. Under normal physiological conditions, ROS are produced mainly in the mitochondrial respiratory chain. In smaller amounts they are also generated in endoplasmic reticulum and peroxisomes, as well as during “respiratory burst” with involvement of the NADPH oxidase complex. Moreover, ROS may arise from the autooxidation of endogenous particles such as epinephrine [
3,
5]. These endogenous ROS serve as signaling molecules in many physiological processes and cellular defense, and their level is strictly controlled by appropriate antioxidative mechanisms [
3,
4,
6].
In diabetes, excessive free radicals and ROS are generated through various pathways as a result of hyperglycemia. Glucose in enediol form may be oxidized to an enediol radical anion which is transformed into ketoaldehydes and superoxide anion radicals. The latter may lead to the production of highly reactive hydroxyl radicals or peroxynitrite radicals. The excess of glucose also promotes peroxidation of lipids in low density lipoproteins, that can consequently lead to free radicals production. Moreover, in hyperglycemic conditions glucose may interact with proteins producing advanced glycation end-products (AGEs), which could promote free radicals generation [
7,
8]. Overproduced ROS interact with numerous structures, including proteins and lipids, leading to oxidative damage [
2,
4]. Oxidative stress resulting from hyperglycemia is also a trigger for diabetic complications affecting many organs including the eye [
7,
9]. There are many types of ocular complications in diabetic patients. Such complications as glaucoma, diabetic retinopathy, keratopathy, and the inflammation of eyelids or cataract may be included. Cataract is a disease characterized by lens opacity. It causes distortions like overall worsening of the vision, blurriness, double or distorted vision, increased glare and halo around lights, changes in color vision and, eventually, it may lead to blindness [
10,
11,
12]. It is widely described that cataract is about 2–4 times more frequent and occurs earlier in diabetic patients than in healthy people of the same age. For instance, cataract formation is a problem in children and adolescents with type 1 diabetes [
11,
12,
13,
14,
15]. It is estimated that one-fifth of cataract removal surgeries are carried out in diabetic patients. Although nowadays cataract surgery is a safe procedure, diabetic patients are at higher risk of peri- and postoperative complications including higher probability of anterior segment inflammation and diabetic retinopathy or diabetic macular edema development [
13,
16].
Oxidative stress is said to be one of the major factors in cataract formation. In order to cope with free radicals and ROS, the body possesses an antioxidative system composed of enzymatic and non-enzymatic antioxidants [
2,
17]. However, when the endogenous antioxidants are insufficient to neutralize the oxidants, exogenous antioxidants can be delivered, for example with a diet or dietary supplements, to increase the antioxidative abilities of the body. Moreover, there is more and more evidence suggesting that changing lifestyle and nutrition, including appropriate supplementation, may prevent cataract formation [
11]. Among such exogenous antioxidants which can be supplemented, there are vitamins, carotenoids or polyphenols, including flavonoids [
2]. Chrysin (5,7-di-OH-flavone), a naturally occurring flavonoid belonging to the flavone subgroup, can be mainly found in the blue passion flower (
Passiflora caerulea L.) and in products made by honeybees—propolis and honey. It is also present in the roots of skullcap (
Scutellaria sp. L.) and in the pearl oyster mushroom (
Pleurotus ostreatus (Jacq. ex Fr.) P.Kumm). This flavonoid reveals many beneficial physiological effects, including antioxidative properties [
18,
19]. There are also reports on its positive effect on ocular structures, including the lenses [
20,
21,
22,
23,
24,
25], but none of these studies were conducted in vivo in diabetic rats. Therefore, our goal was to determine if chrysin administered by intragastric tube may counteract the diabetes-induced changes in the markers linked to oxidative stress in the lenses of rats.
4. Discussion
Streptozotocin (STZ; 2-deoxy-2-(3-methyl-3-nitrosourea)-1-
d-glucopyranose) is a toxic glucose analog isolated from
Streptomyces achromogenes. In high doses, STZ is widely used to develop type 1 diabetes in animal research. It accumulates preferentially in pancreatic beta cells and leads to their necrosis. As a result of beta cells death, insulin-dependent diabetes develops in animals. In this model, diabetes is characterized by severe hyperglycemia, polyuria and polydipsia—the symptoms similar to human type 1 diabetes [
42,
43,
44,
45].
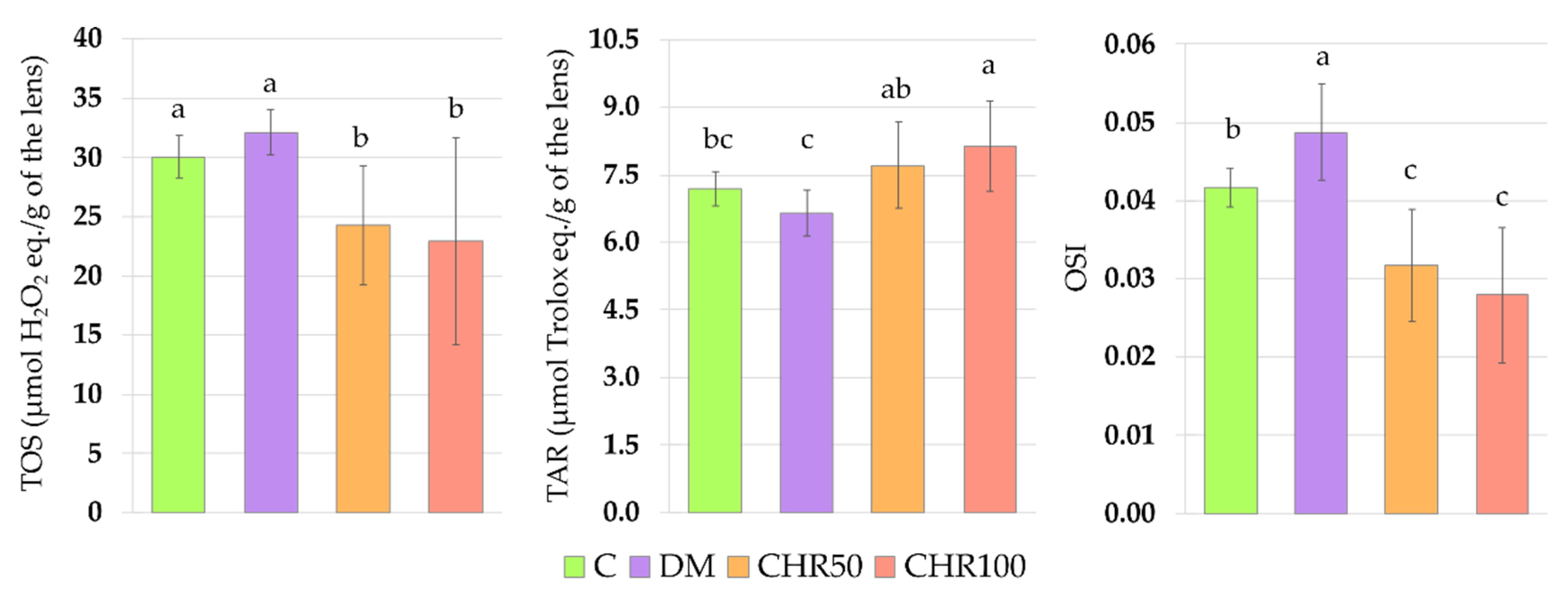
Hyperglycemia is one of the main reasons for ROS overproduction in diabetic conditions [
7]. These oxidants interact with double bonds between carbons in fatty acid chains (especially in polyunsaturated fatty acids) leading to lipid peroxidation and the generation of toxic secondary metabolites, such as malondialdehyde (MDA) [
46]. In the lenses, oxidation of lipids may cause the membrane stiffness and, in consequence, light scattering [
47]. As we observed in our study, the oxidative stress index (OSI) was significantly higher in the lenses of diabetic rats than in the lenses of healthy control rats, indicating that there was an excess of oxidants in the system. We also noted that in the lenses of the DM rats, MDA level was elevated, which may be a result of the oxidant/antioxidant imbalance. Our results overlap with other studies in which an increase in the MDA level was described in the lenses of diabetic animals [
48,
49,
50,
51]. ROS and free radicals may also interact directly with the thiol groups of amino acids in peptides and proteins, which promotes oxidation of these compounds and their structure and/or function change, and may lead to protein aggregation [
52]. In the eye lens, there are soluble proteins—crystallins—which ensure lens transparency, since they do not scatter light. The aggregation of these soluble proteins eventually leads to cataract formation [
53]. Such aggregates may be formed by an increase in disulfides and disulfide cross-links, the level of which can be measured indirectly by assaying the advanced oxidation protein products (AOPP) [
52,
54]. During oxidative stress and in the presence of reducing sugars, amino acids may transform to highly reactive carbonyl groups (PCG), which are a good marker for protein oxidative damage in the cells [
55,
56,
57]. As we observed in this study, in the lenses of diabetic rats there were no changes in the total protein level, but the level of soluble protein decreased, while the level of both AOPP and PCG increased. This suggests that, due to the oxidative stress, protein aggregates were formed in the lenses of these animals. Similar results were obtained by other authors [
51,
58,
59].
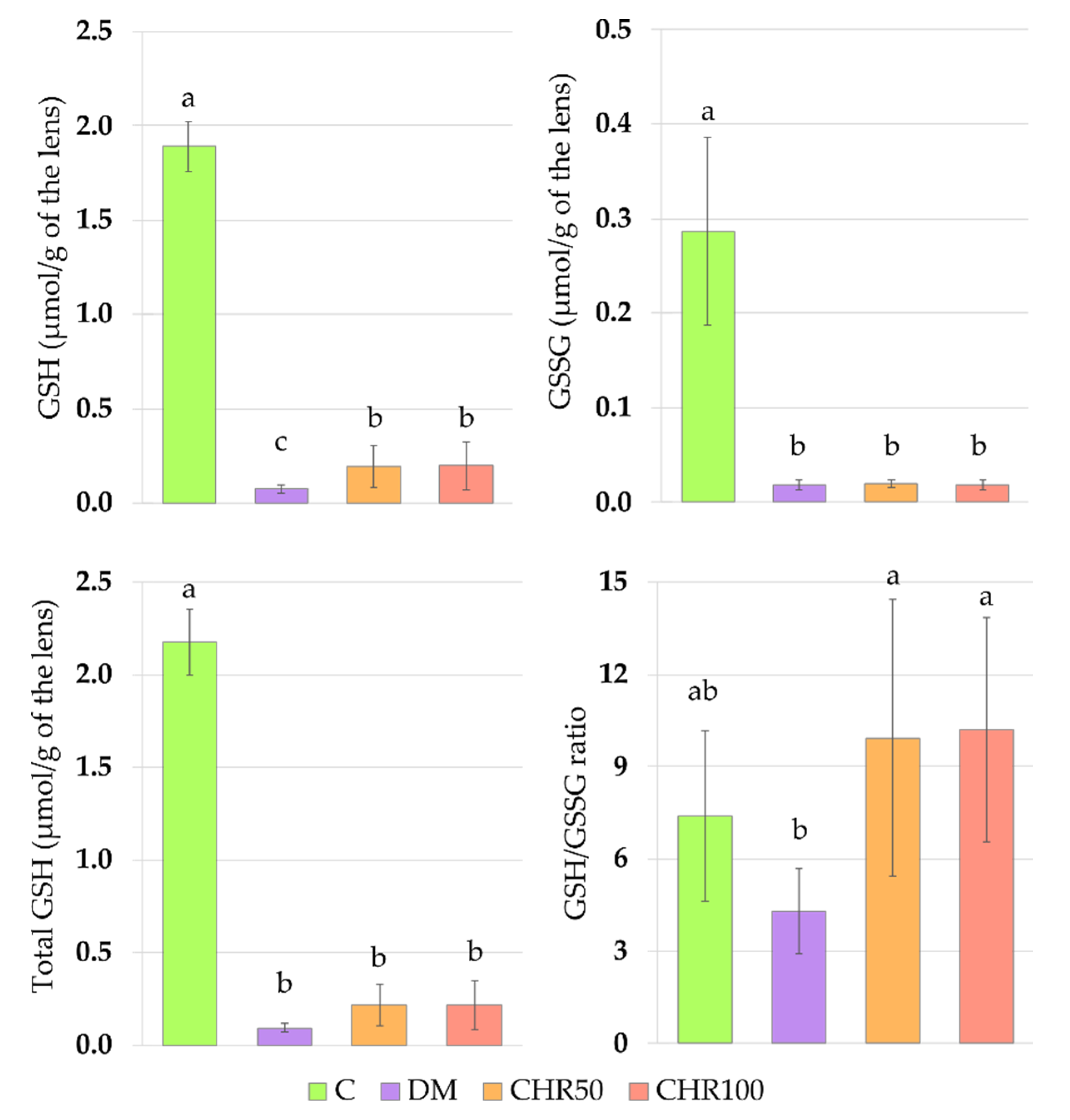
The main non-enzymatic antioxidant responsible for ROS scavenging in the lenses is glutathione (GSH) [
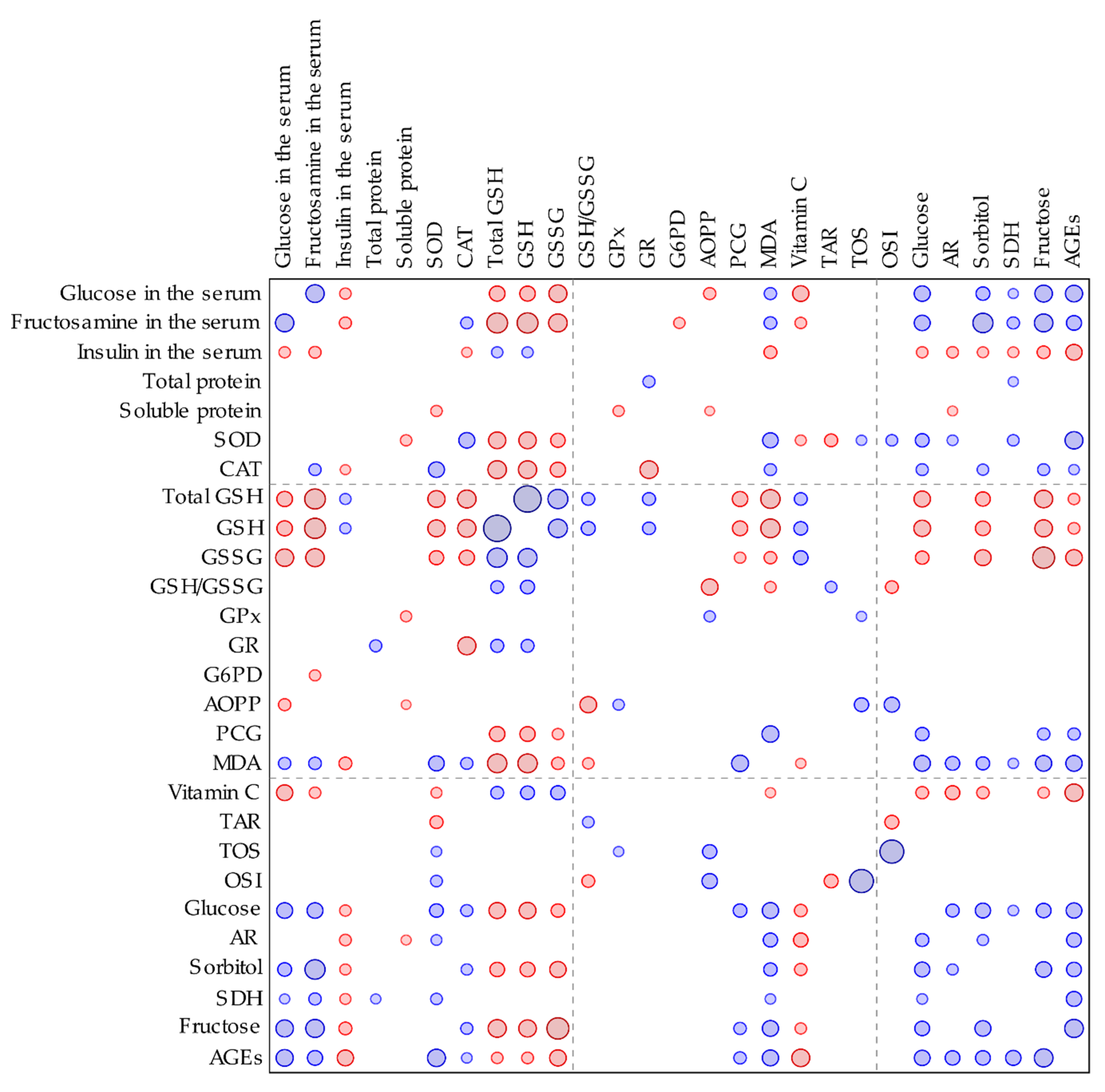
60]. In our study, the total and reduced GSH levels in the lenses of diabetic rats were significantly lower than in the lenses of the healthy animals. These parameters correlated negatively with the serological glucose level and the fructosamine level—a parameter representing long-term glycemia (
Appendix A,
Figure A1).
GSH decrease in the lenses of diabetic rats is widely described in scientific literature [
48,
49,
51,
61]. This effect is presumably a response to ROS generation by the elevated serological glucose level. In oxidative stress conditions, the GSH serves as a cofactor for antioxidative enzymes and is oxidized to GSSG. As a result, the GSSG level increases and the GSH/GSSG ratio consequently decreases [
62]. In our study, the GSSG level did not increase in the lenses of diabetic rats, but similarly to GSH level, was lowered instead. For this reason, the GSH/GSSG ratio did not change. This effect may be explained by a compensatory mechanism in the lens, in which the excess of GSSG is removed from the lens by the trabecular meshwork in order to maintain the GSH/GSSG ratio [
63]. A decrease in the total GSH level in the lenses of diabetic animals resulted from a decrease of both the reduced and oxidized forms of glutathione.
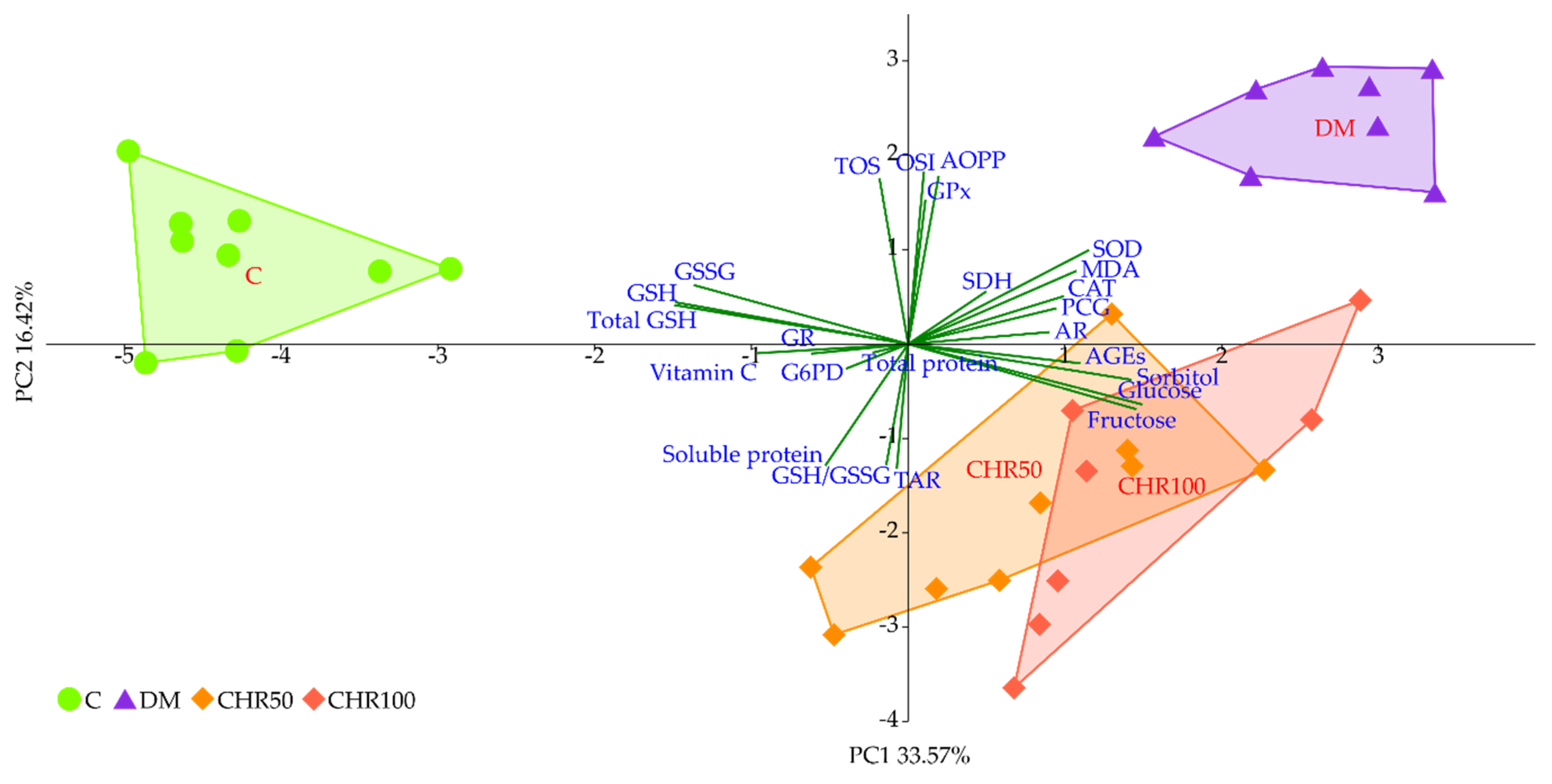
We observed that the GSH level in the lenses of diabetic rats treated with both the doses of chrysin was elevated in comparison to the non-treated rats. This increase, although statistically significant, was minor. Even though, from a mathematical point of view, the GSH/GSSG ratio in the lenses of chrysin-treated diabetic rats was restored to the values observed in the healthy control rats, it should be noted that the total GSH level remained unchanged after chrysin treatment. The main effect observed in the PCA shows a strong negative link between total GSH, GSH and GSSG levels and both chrysin-treated rat groups, suggesting low values in these groups and high values in the control rats. CHR50 and CHR100 rat groups clustered closer to the healthy C rats along PC1, but only ever so slightly. The observed statistically significant increase in reduced GSH level after chrysin administration is a probable result of a completely different mechanism. The PCA provides a clue supporting this reasoning as chrysin-treated rats separate from the remaining clusters also with regard to PC2. This statistically significant effect could not simply be explained by counteracting the diabetic state. Usually GSH is synthetized in the lens epithelial cells and outer cortex, but can also be transported from aqueous humor or be restored from GSSG [
63,
64]. However, GSH synthesis may also be promoted by flavonoid supplementation. Moskaug et al. provided evidence on the de novo GSH synthesis—the cellular GSH level is then increased via activating gamma-glutamylcysteine synthetase induced by polyphenols [
65]. Since in our study the activity of glutathione reductase was not altered by chrysin supplementation, we presume that chrysin promotes de novo GSH synthesis rather than the restoration of GSH from GSSG. Chrysin is reported to increase the GSH level in various organs of diabetic animals [
27,
66], which is consistent with our report.
MDA formed during lipid peroxidation can be metabolized by enzymes using GSH as a cofactor to various metabolites which are removed with urine [
46]. GSH is also a crucial molecule in the prevention of protein oxidation. GSH may form a reversible disulfide bond with proteins, which protects these molecules (including crystallins) from misfolding and aggregation [
52,
53,
67]. Therefore, the increased GSH level in the lenses of the diabetic rats treated with chrysin (especially the 50 mg/kg dose) may be connected with increased soluble protein level and decreased levels of protein and lipid oxidation markers. Similarly to our in vivo experiment, in the ex vivo study conducted on the goat lenses incubated in the medium with a high concentration of glucose, chrysin elevated the soluble protein level and decreased the PCG level [
20]. In our previous study, we observed that administration of another flavone—diosmin—resulted in the AOPP level decrease in the lenses of diabetic rats [
29]. Treatment with other natural-derived compounds or plant extracts resulted in the MDA and PCG levels’ decrease in the lenses of diabetic rats, in comparison to the untreated diabetic animals [
49,
51,
59,
68], which overlaps with our results. In the study conducted ex vivo on the rat lenses, chrysin at a concentration of 200 µM added to the medium: (1) simultaneously with sodium selenite, (2) as a pretreatment before sodium selenite, or (3) after a 30-minute long preincubation with this salt, reduced the MDA level and elevated the GSH level [
21].
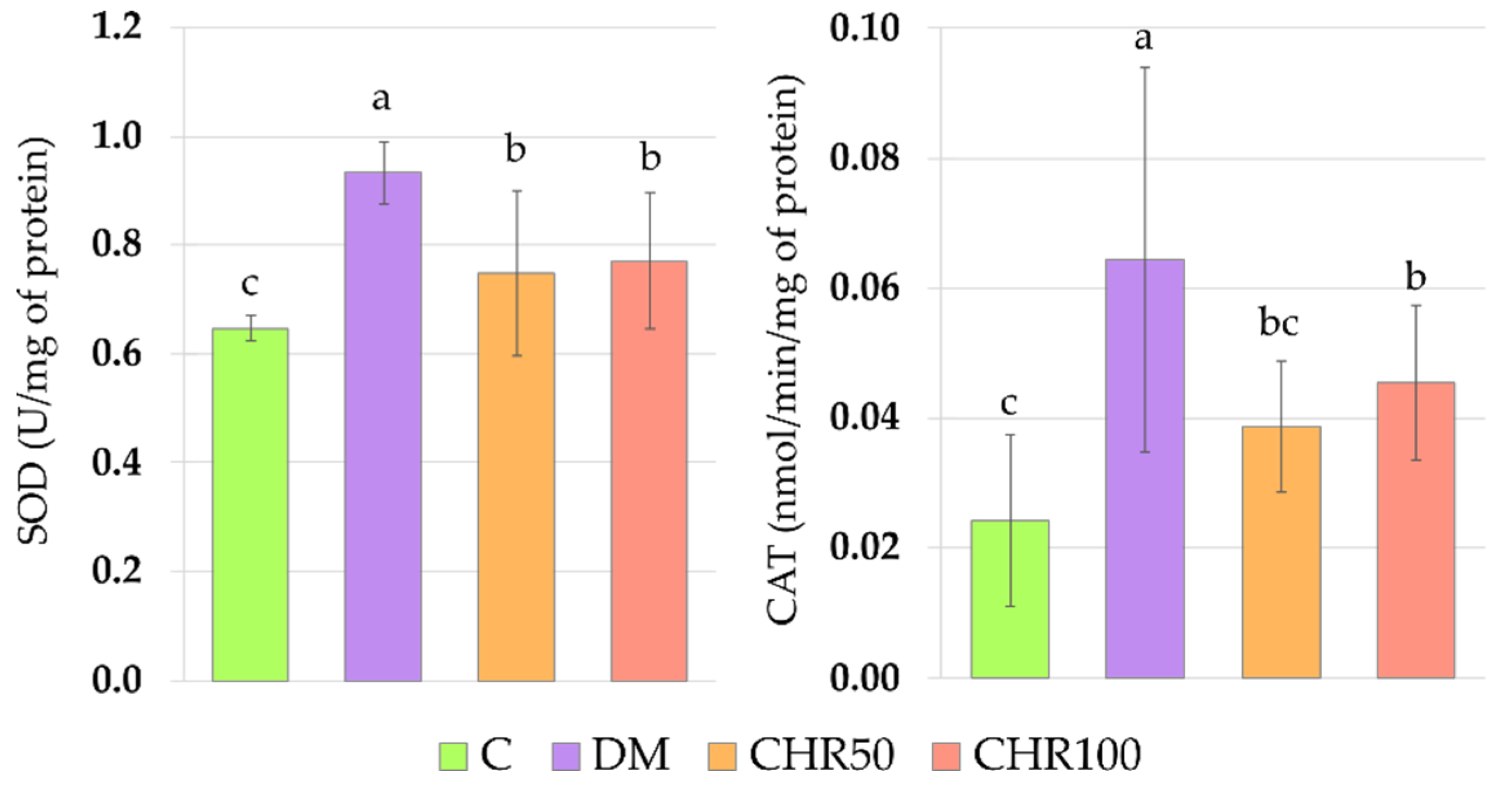
Antioxidative enzymes, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), are involved in the antioxidative defense of the organism [
69]. Their activity is regulated via nuclear factor erythroid 2–related factor 2 (Nrf2) dependent pathway. In normal non-stressed conditions, the Nrf2 is connected to its cytoplasmatic inhibitor—Kelch-like ECH-associated protein-1 (Keap1). When oxidative stress increases, Nrf2 is released from Keap1, then translocated to the nucleus where it connects with antioxidant response element (ARE), inducing the expression of Nrf2-ARE dependent genes, including the genes coding antioxidative enzymes [
70]. We observed that SOD and CAT activity was significantly increased in the lenses of diabetic rats. This elevated activity may be connected to the fact that oxidative stress in these lenses was observed, based on the OSI value. We did not note an increase in the GPx activity. GPx possesses a very high affinity to H
2O
2 and eliminates it even when the latter is in low concentration. However, this process requires electron donors such as GSH, therefore is disadvantageous for the cell. CAT has a lower affinity to hydrogen peroxide but a very high Michaelis constant (K
M) to the substrate, thus may eliminate H
2O
2 even at very high concentrations [
71]. We hypothesize that GPx activity in the lenses was not altered by diabetes, since GSH was utilized to form S-conjugates with proteins in order to protect them against oxidative stress, and its resources had depleted. After chrysin administration at both the doses, the activity of SOD and CAT decreased. This may be a result of the overall reduction of oxidative stress in the lenses. The OSI parameter was significantly reduced in the lenses of diabetic rats treated with 50 and 100 mg/kg of chrysin. As OSI correlates with SOD, its decrease would result in the SOD activity reduction. When SOD does not dismutate the superoxide radical, there is no need to activate CAT which is also positively correlated with SOD (
Appendix A,
Figure A1). This may suggest that chrysin, being an exogenous antioxidant, administered to the diabetic rats, scavenged ROS, and thus reduced oxidative stress in the lenses (lowered OSI), which led to the decrease of Nrf2-ARE dependent antioxidative gene expression.
Glucose is transported to the lens in an insulin-independent process. In normal physiological conditions, glucose in the lenses is metabolized mainly by the hexokinase-involving pathway, but in a hyperglycemic state the hexokinases become saturated and the polyol pathway takes the leading role in the lenticular glucose metabolism [
11,
72]. As we observed in our study, the non-fasting glucose level in the serum as well as the fructosamine level were considerably elevated in comparison with the healthy, non-diabetic rats. These elevated glycemia-related markers correlated with the increased markers connected to the polyol pathway in the lenses (
Appendix A,
Figure A1). In other studies concerning the lenses of the diabetic animals, the polyol pathway was also intensified and markers describing the sugars and enzymes connected to this pathway were elevated [
51,
61,
73]. Administration of chrysin did not reduce the non-fasting glucose level in the serum of the tested rats. This was confirmed by a marker representing a long-term glycemia state—fructosamine, which was not altered after 28 days of chrysin treatment. Lack of changes in the glycemia-related markers in the serum of the rats treated with chrysin may explain why the polyol pathway was not inhibited in the lenses. Some studies indicate that chrysin has no effect on hyperglycemia in the rats [
74,
75], which is consistent with our study. An increase in the advanced glycation-end products (AGEs) level is a result of an intensified polyol pathway and fructose accumulation in the lenses [
76]. We observed that in the lenses of the diabetic animals, the AGEs level was significantly higher than in the non-diabetic rats. The AGEs level correlates positively with the polyol pathway, serological glucose and fructosamine levels (
Appendix A,
Figure A1). Since the polyol pathway in the lenses and serological glucose level were not altered by chrysin administration, the AGEs level in the lenses also remained unchanged. AGEs interact with the specific receptors—RAGE (receptor of AGE). As a result of this interaction, ROS are generated through the activation of NADPH oxidase. Therefore, the excess of AGEs results in oxidative stress progression [
77,
78]. We noted that the AGEs level in the lenses positively correlates with the SOD activity in this organ (
Appendix A,
Figure A1), indicating that increased SOD activity is a response to overly produced ROS, generated by AGEs. Moreover, AGEs level correlates negatively with GSH and vitamin C levels in the lenses (
Appendix A,
Figure A1), which also suggests a compensative mechanism for ROS removal from the lens by small-molecule antioxidants. These non-enzymatic antioxidants (GSH and vitamin C) are restored from their oxidized forms by enzymes requiring NADPH—glutathione reductase and thioredoxin reductases, respectively. Moreover, synthesis of vitamin C requires NADPH as a driving force for the enzymes involved in this process [
79,
80]. Aldose reductase (AR), a first enzyme in the polyol pathway catalyzing the conversion of glucose to sorbitol, competes for NADPH with GR [
76]. It is possible, that in hyperglycemic condition, AR utilizes most of the NADPH supplies in the cell, thus GSH cannot be restored from GSSG. AR activity in the lenses negatively correlates with the vitamin C level in this organ (
Appendix A,
Figure A1), which was also confirmed by the PCA. This suggests that due to the lack of NADPH, vitamin C cannot be reduced from the oxidized forms or be synthetized de novo in the rat lenses. In our study, AR activity was not affected by chrysin administration. On the contrary, in the ex vivo study, chrysin revealed a strong AR inhibitory effect [
20]. However, it should be noted that in the ex vivo experiment, chrysin was added to the medium in which lenses were incubated, thus the lenses were exposed directly to this flavonoid action. In the in vivo experiments, when substances are given orally or by intragastric tube, they may be metabolized or bound to plasma proteins and, in consequence, they do not interact directly with the organ [
81,
82]. Therefore, the discrepancies between our investigation and the ex vivo study are a result of experimental design and the complexity of the living organism.
In order to evaluate the significance of the dose-dependent effect of chrysin on the lenses of diabetic rats, the PCA was performed. According to this analysis, no differences were observed between 50 and 100 mg/kg doses. Due to the composite nature of PCA, only the most prominent differences regarding individual parameters are exposed. This reveals itself in different weights represented by variable correlation strengths with regard to the PC1 and PC2 axes, along which the experimental group clusters separate from each other, thus providing insight into the significance of each parameter to the total observed variance [
83]. This phenomenon explains the inconsistencies observed between measurements of the individual parameters, providing only a quantitative aspect when compared to the effect revealed by the PCA, where all parameters are considered simultaneously in both quantitative and qualitative manner.
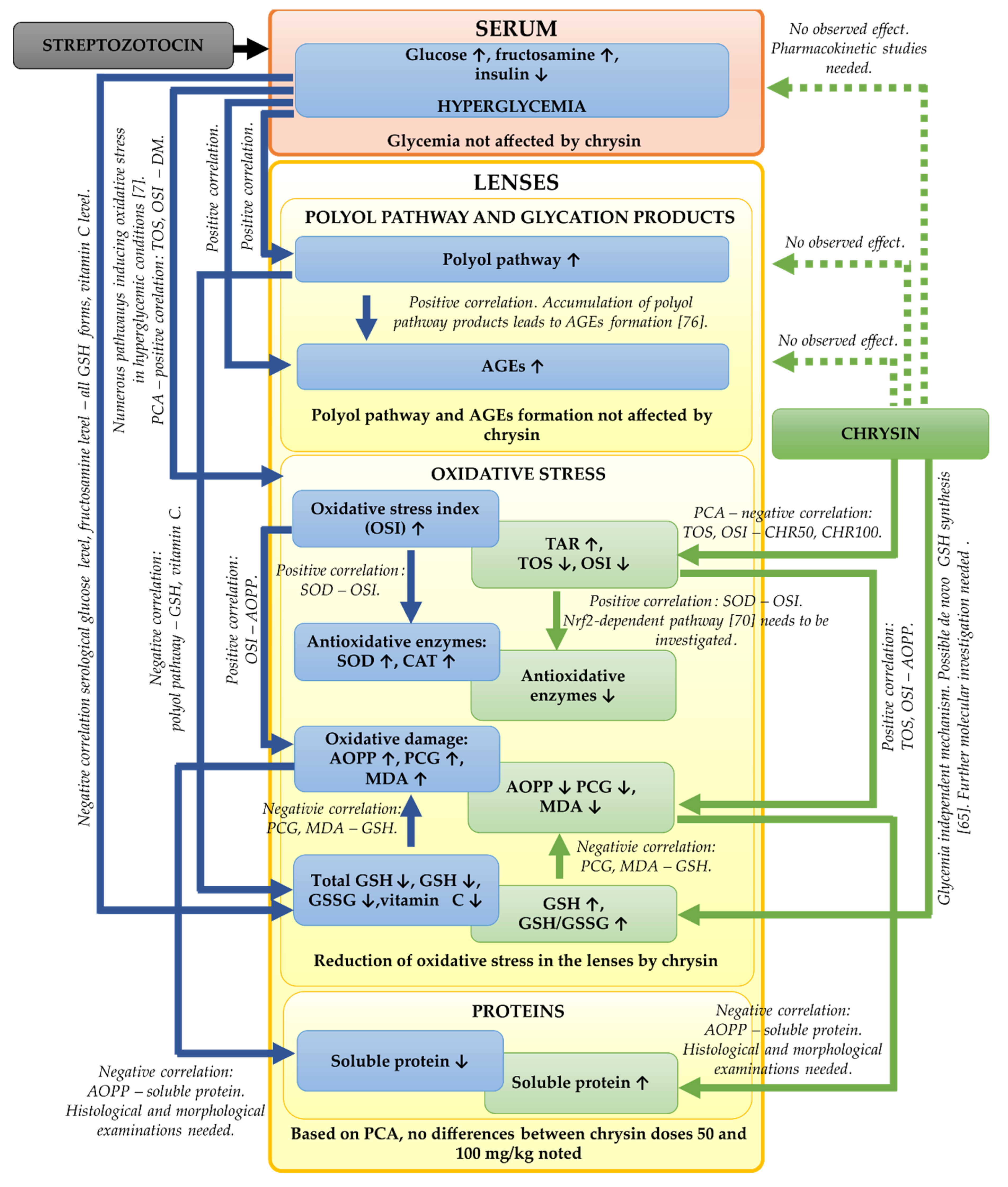
In the presented study, we focused mainly on the biochemical parameters related to the oxidative stress and polyol pathway in the lenses of the diabetic rats treated with chrysin. It should be noted that based on our results, further, more detailed examinations are needed. We did not examine the histological or morphological changes in these lenses. Such examinations should be assessed in the future in order to validate the biochemical results. Moreover, to explain molecular mechanisms behind the observed biochemical changes (e.g., the increase of GSH level in the lenses), genetic assays are crucial. The bioavailability of chrysin in the diabetic animals can be altered in comparison to the healthy rats, therefore pharmacokinetic studies regarding chrysin administered to the healthy and diabetic rats at doses 50 and 100 mg/kg could be informative. The effects observed during the study including further steps required in future experiments are presented in a combined form in
Figure 6.