Biological Redox Impact of Tocopherol Isomers Is Mediated by Fast Cytosolic Calcium Increases in Living Caco-2 Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Ca2+ and Na+ Imaging
2.3. HyPer Biosensor Imaging
2.4. Statistical Analysis
3. Results
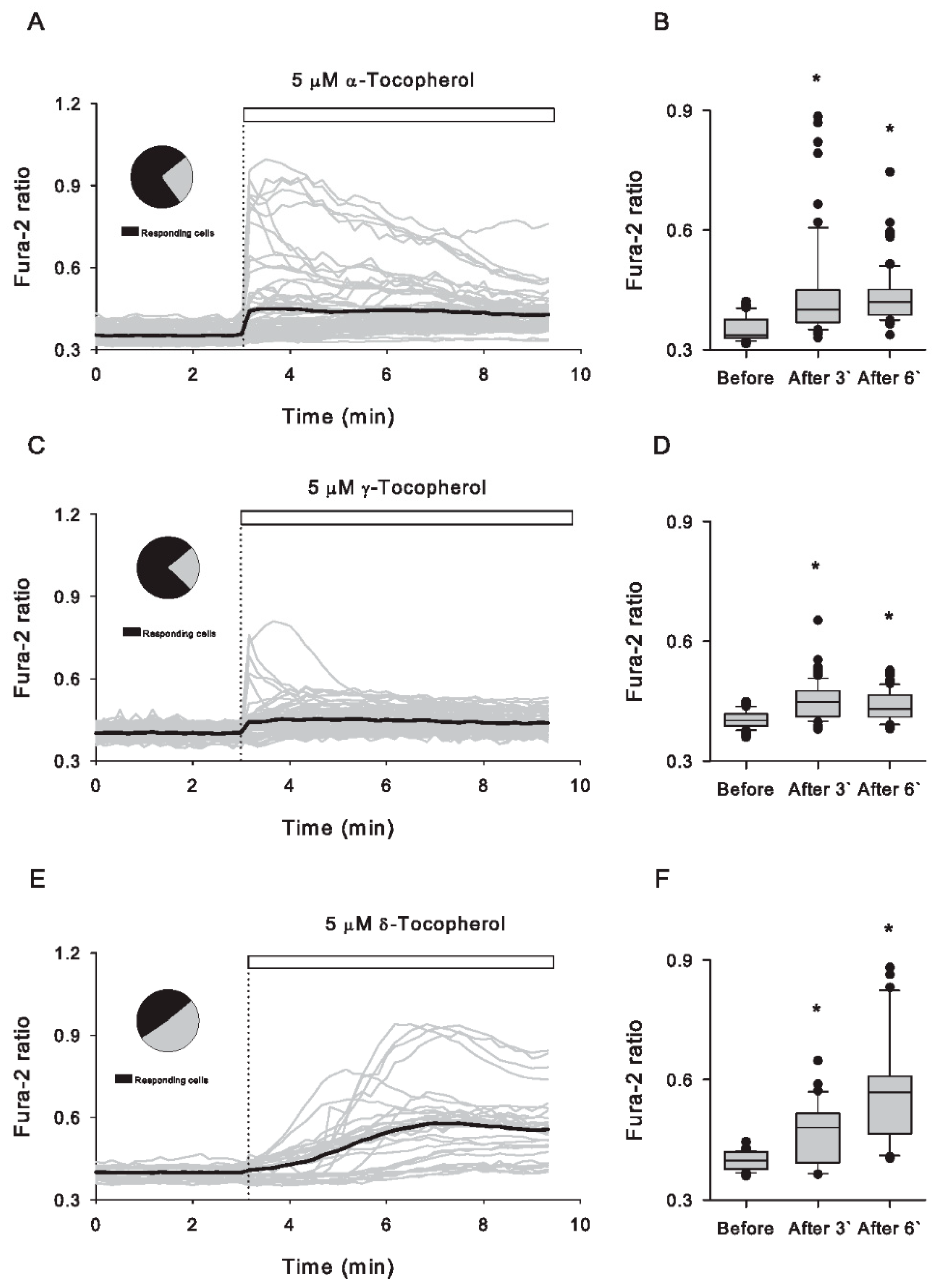
3.1. Tocopherol Isomers Evoked Rapid Increases in Cytosolic Ca2+ with Different Patterns in Caco-2 Cells
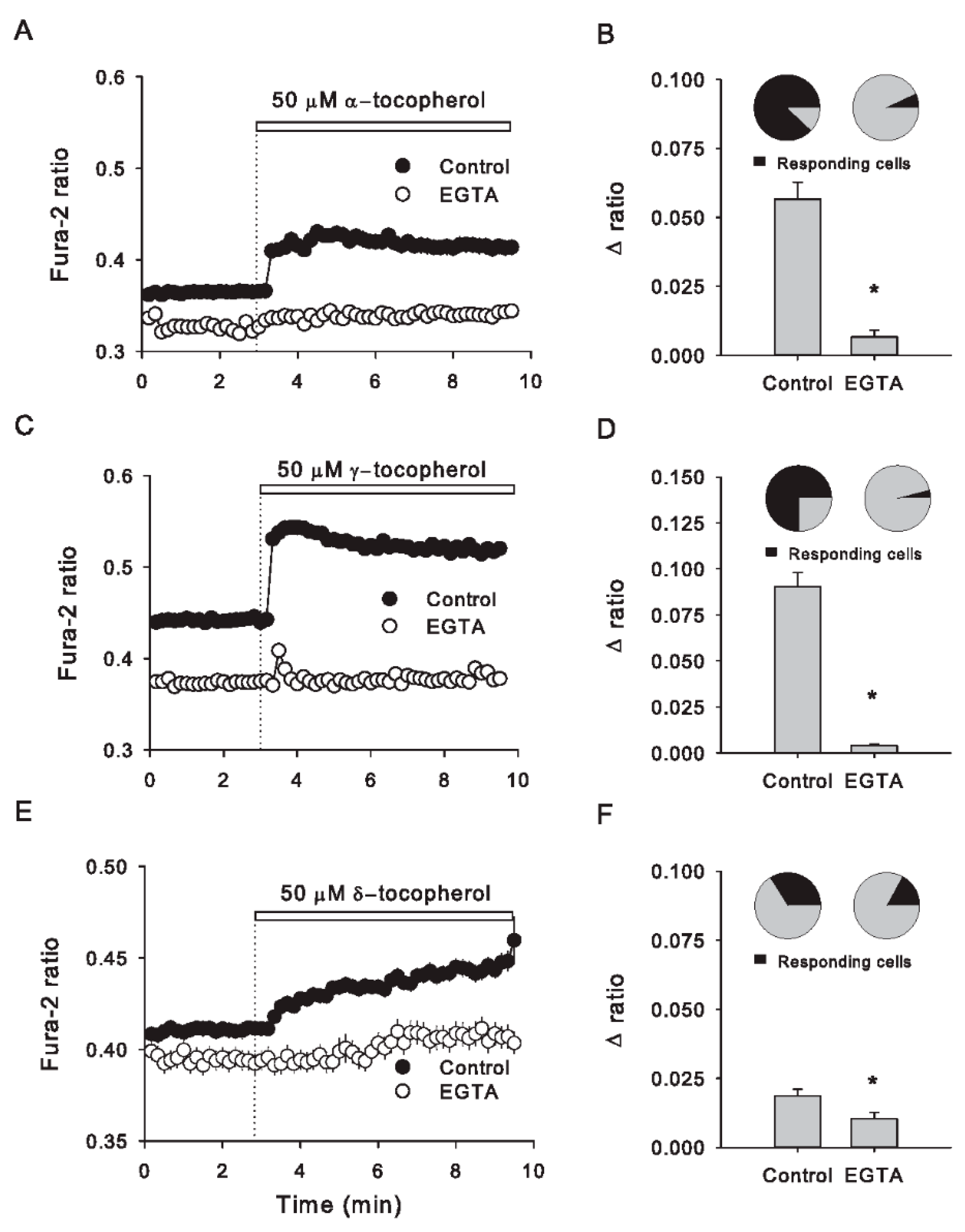
3.2. Removal of Extracellular Ca2+ Abolishes the Cytosolic Ca2+ Increases Induced by All Tocopherol Isomers
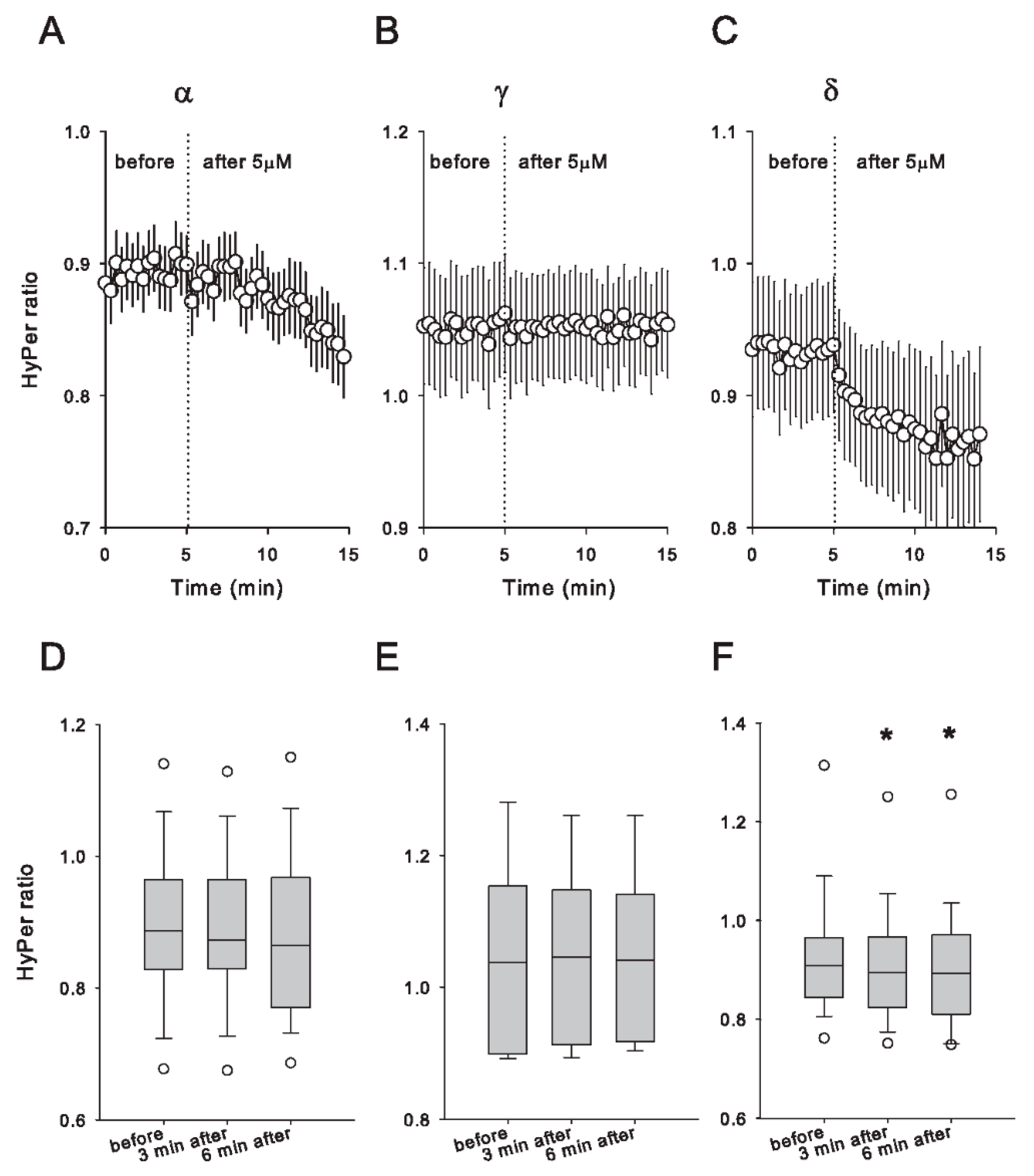
3.3. Only δ-Tocopherol Evokes an Acute Redox Effect on Caco-2 Cells at Low Concentration
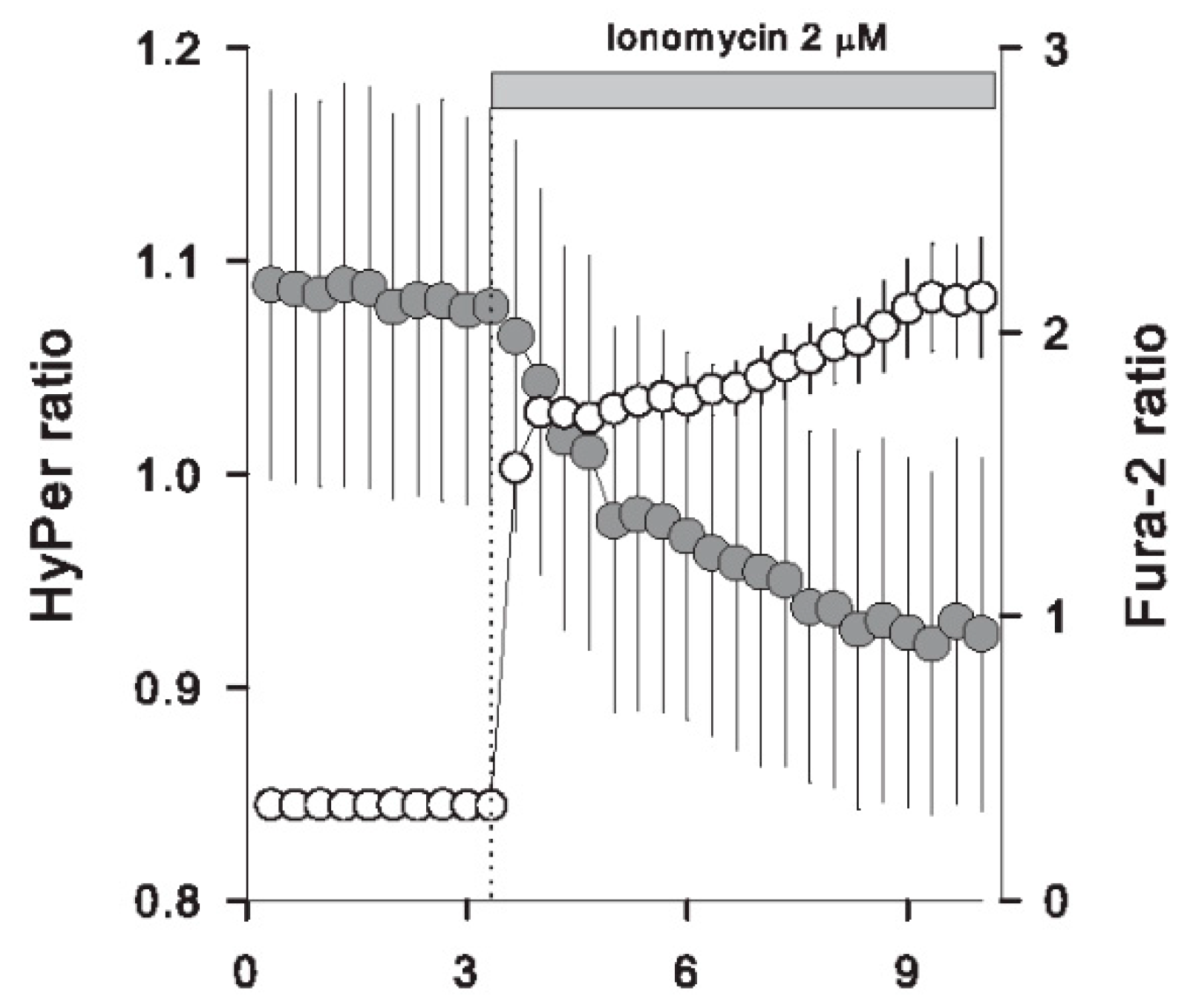
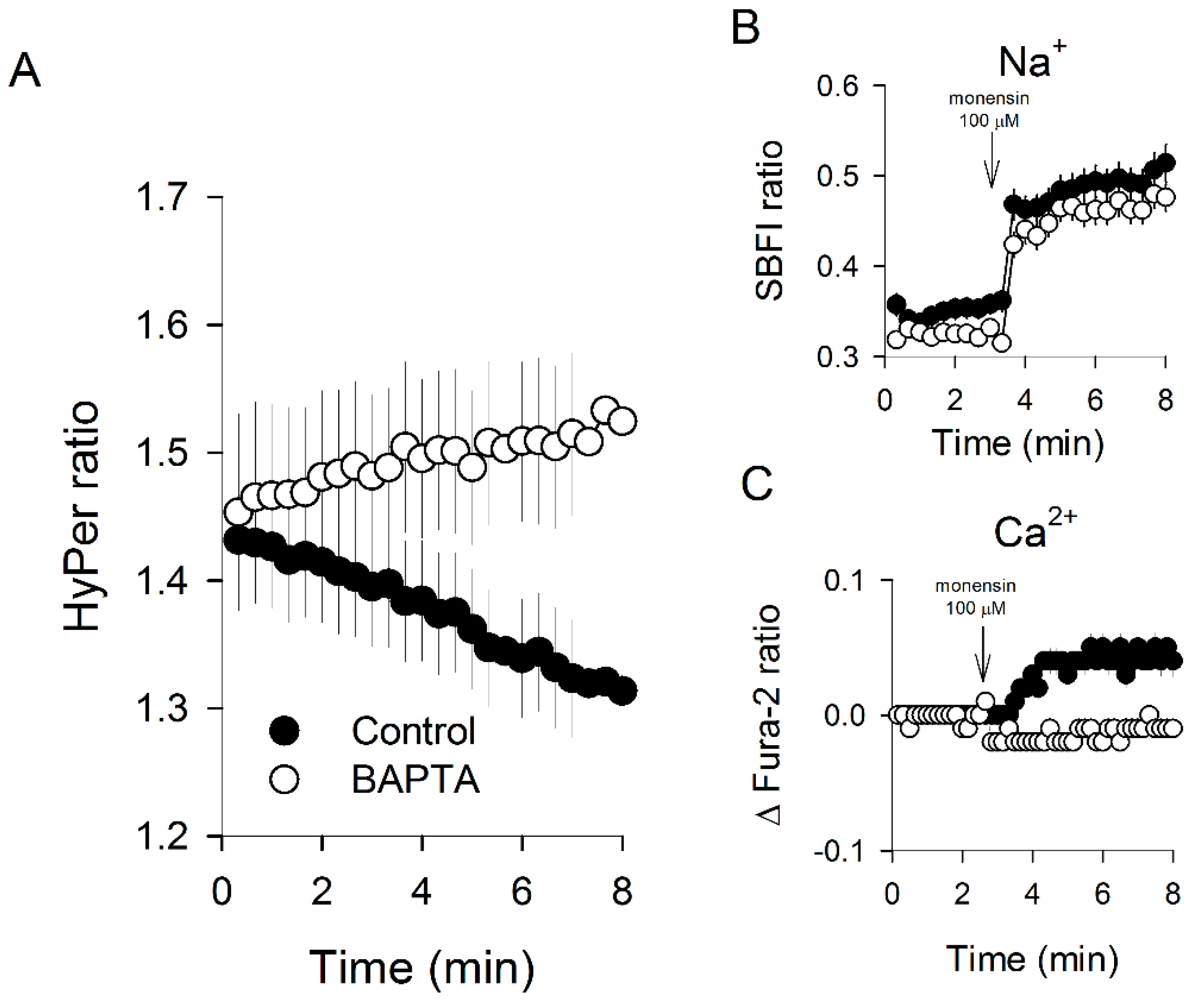
3.4. Massive Calcium Entry Using Ionophores Induced a Fast Redox Response
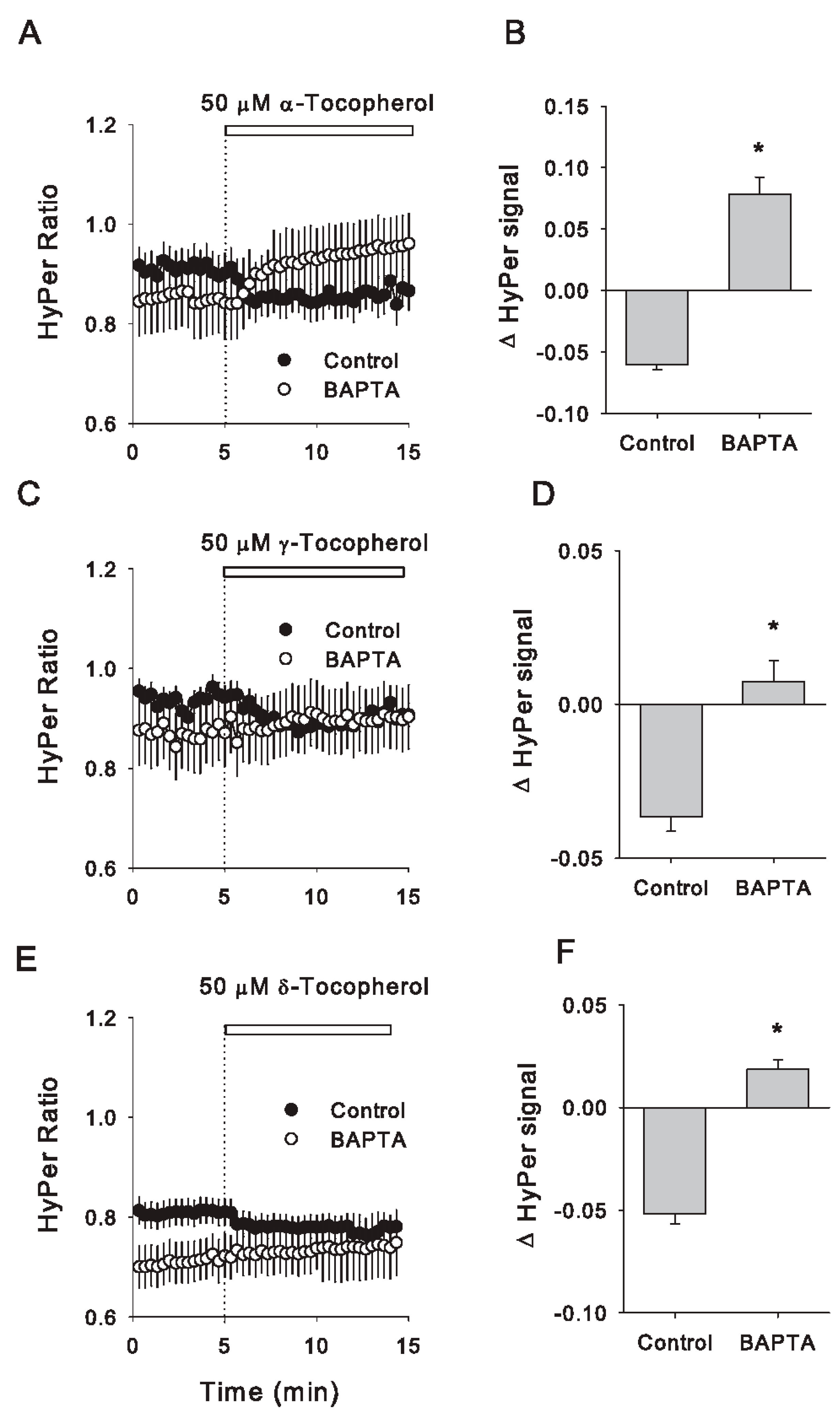
3.5. Acute Redox Changes Induced by Isomers of Tocopherol Are Dependent on Intracellular Calcium Increases
4. Discussion
5. Conclusions
- Tocopherol isomers evoked fast calcium responses in Caco-2 cells with different kinetics.
- δ-tocopherol was the only isomer capable of inducing a redox change in HyPer biosensor at 5 µM.
- An increase in cytosolic Ca2+, but not in Na+, was necessary and sufficient to induce a reduction in the HyPer biosensor for all the isomers evaluated.
- A massive cytosolic Ca2+ increase induced by both ionophores, ionomycin and monensin, was enough to generate a redox change in HyPer.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BAPTA | 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid |
| CCD | Charge-Coupled Device |
| DMSO | Dimethyl Sulfoxide |
| DTT | Dithiothreitol |
| EGTA | Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid |
| KRH | Krebs Ringer HEPES buffer |
| ROS | Reactive Oxygen Species |
| SBFI | Sodium-binding benzofuran isophthalate |
| TRP | Transient Receptor Potential |
References
- Chow, C.K. Distribution of tocopherols in human plasma and red blood cells. Am. J. Clin. Nutr. 1975, 28, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Khan, D.A. Evaluation of Pharmacokinetics, and Bioavailability of Higher Doses of Tocotrienols in Healthy Fed Humans. Clin. Exp. Cardiol. 2016, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wallmon, A.; Olsson-Mortlock, C.; Wallin, R.; Saldeen, T. Mixed tocopherols inhibit platelet aggregation in humans: Potential mechanisms. Am. J. Clin. Nutr. 2003, 77, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Helbig, D.; Wagner, A.; Schubert, R.; Jahreis, G. Tocopherol isomer pattern in serum and stool of human following consumption of black currant seed press residue administered in whole grain bread. Clin. Nutr. 2009, 28, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.W.; Ward, N.C.; Wu, J.H.Y.; Hodgson, J.M.; Puddey, I.B.; Croft, K.D. Supplementation with mixed tocopherols increases serum and blood cell γ-tocopherol but does not alter biomarkers of platelet activation in subjects with type 2 diabetes. Am. J. Clin. Nutr. 2006, 83, 95–102. [Google Scholar] [CrossRef]
- Robledo, S.N.; Zachetti, V.G.L.; Zon, M.A.; Fernández, H. Quantitative determination of tocopherols in edible vegetable oils using electrochemical ultra-microsensors combined with chemometric tools. Talanta 2013, 116, 964–971. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Hanson, C.; Lyden, E.; Furtado, J.; van Ormer, M.; Anderson-Berry, A. A Comparison of Nutritional Antioxidant Content in Breast Milk, Donor Milk, and Infant Formulas. Nutrients 2016, 8, 681. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Different tocopherol isoforms vary in capacity to scavenge free radicals, prevent inflammatory response, and induce apoptosis in both adult- and fetal-derived intestinal epithelial cells. BioFactors 2013, 39, 663–671. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Differences in vitamin E and C profile between infant formula and human milk and relative susceptibility to lipid oxidation. Int. J. Vitam. Nutr. Res. 2013. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, A.D.; Tsuchida, M.A.; Amodaj, N.; Pinkard, H.; Vale, R.D.; Stuurman, N. Advanced methods of microscope control using μManager software. J. Biol. Methods 2014. [Google Scholar] [CrossRef] [PubMed]
- Hernández, H.; Parra, A.; Tobar, N.; Molina, J.; Kallens, V.; Hidalgo, M.; Varela, D.; Martínez, J.; Porras, O. Insights into the HyPer biosensor as molecular tool for monitoring cellular antioxidant capacity. Redox Biol. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Klein, A.; Bietrix, F.; Gleize, B.; Malezet-Desmoulins, C.; Schneider, M.; Margotat, A.; Lagrost, L.; Collet, X.; Borel, P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J. Biol. Chem. 2006. [Google Scholar] [CrossRef] [PubMed]
- Narushima, K.; Takada, T.; Yamanashi, Y.; Suzuki, H. Niemann-Pick C1-Like 1 Mediates -Tocopherol Transport. Mol. Pharmacol. 2008. [Google Scholar] [CrossRef]
- Lee, M.-H.; Appleton, K.M.; El-Shewy, H.M.; Sorci-Thomas, M.G.; Thomas, M.J.; Lopes-Virella, M.F.; Luttrell, L.M.; Hammad, S.M.; Klein, R.L. S1P in HDL promotes interaction between SR-BI and S1PR1 and activates S1PR1-mediated biological functions: Calcium flux and S1PR1 internalization. J. Lipid Res. 2017. [Google Scholar] [CrossRef]
- Hong, C.; Kwak, M.; Myeong, J.; Ha, K.; Wie, J.; Jeon, J.H.; So, I. Extracellular disulfide bridges stabilize TRPC5 dimerization, trafficking, and activity. Pflugers Arch. Eur. J. Physiol. 2015, 467, 703–712. [Google Scholar] [CrossRef]
- Xu, S.Z.; Sukumar, P.; Zeng, F.; Li, J.; Jairaman, A.; English, A.; Naylor, J.; Ciurtin, C.; Majeed, Y.; Milligan, C.J.; et al. TRPC channel activation by extracellular thioredoxin. Nature 2008, 451, 69–72. [Google Scholar] [CrossRef]
- Rao, J.N.; Platoshyn, O.; Golovina, V.A.; Liu, L.; Zou, T.; Marasa, B.S.; Turner, D.J.; Yuan, J.X.-J.; Wang, J.-Y. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am. J. Physiol. Liver Physiol. 2005, 290, G782–G792. [Google Scholar] [CrossRef]
- Bertrand, C.A.; Laboisse, C.L.; Hopfer, U. Purinergic and cholinergic agonists induce exocytosis from the same granule pool in HT29-Cl.16E monolayers. Am. J. Physiol. Physiol. 2017. [Google Scholar] [CrossRef]
- Mitrovic, S.; Nogueira, C.; Cantero-Recasens, G.; Kiefer, K.; Fernández-Fernández, J.M.; Popoff, J.-F.; Casano, L.; Bard, F.A.; Gomez, R.; Valverde, M.A.; et al. TRPM5-mediated calcium uptake regulates mucin secretion from human colon goblet cells. eLife 2013. [Google Scholar] [CrossRef]
- Nyström, E.E.L.; Birchenough, G.M.H.; van der Post, S.; Arike, L.; Gruber, A.D.; Hansson, G.C.; Johansson, M.E.V. Calcium-activated Chloride Channel Regulator 1 (CLCA1) Controls Mucus Expansion in Colon by Proteolytic Activity. EBioMedicine 2018. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Williams, J.A.; Kučerka, N.; Atkinson, J.; Wassall, S.R.; Katsaras, J.; Harroun, T.A. Tocopherol activity correlates with its location in a membrane: A new perspective on the antioxidant vitamine. J. Am. Chem. Soc. 2013, 135, 7523–7533. [Google Scholar] [CrossRef] [PubMed]
- Gibhardt, C.S.; Zimmermann, K.M.; Zhang, X.; Belousov, V.V.; Bogeski, I. Imaging calcium and redox signals using genetically encoded fluorescent indicators. Cell Calcium. 2016. [Google Scholar] [CrossRef] [PubMed]
- Porras, O.H.; Stutzin, A. Glutamate-induced metabolic changes influence the cytoplasmic redox state of hippocampal neurons. Biochem. Biophys. Res. Commun. 2011, 411. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.W.; Regner, L.; Mett, J.; Stahlmann, C.P.; Schorr, P.; Nelke, C.; Streidenberger, O.; Stoetzel, H.; Winkler, J.; Zaidan, S.R.; et al. Tocotrienol affects oxidative stress, cholesterol homeostasis and the amyloidogenic pathway in neuroblastoma cells: Consequences for Alzheimer’s disease. Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef]
- Casati, L.; Pagani, F.; Limonta, P.; Vanetti, C.; Stancari, G.; Sibilia, V. Beneficial effects of δ-tocotrienol against oxidative stress in osteoblastic cells: Studies on the mechanisms of action. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Starczak, M.; Zarakowska, E.; Modrzejewska, M.; Dziaman, T.; Szpila, A.; Linowiecka, K.; Guz, J.; Szpotan, J.; Gawronski, M.; Labejszo, A.; et al. In vivo evidence of ascorbate involvement in the generation of epigenetic DNA modifications in leukocytes from patients with colorectal carcinoma, benign adenoma and inflammatory bowel disease. J. Transl. Med. 2018. [Google Scholar] [CrossRef]
- Zhao, Y.; Monahan, F.J.; McNulty, B.A.; Brennan, L.; Gibney, M.J.; Gibney, E.R. α-Tocopherol Stereoisomers in Human Plasma Are Affected by the Level and Form of the Vitamin E Supplement Used. J. Nutr. 2015, 145, 2347–2354. [Google Scholar] [CrossRef]
- Fairus, S.; Nor, R.M.; Cheng, H.M.; Sundram, K. Alpha-tocotrienol is the most abundant tocotrienol isomer circulated in plasma and lipoproteins after postprandial tocotrienol-rich vitamin E supplementation. Nutr. J. 2012. [Google Scholar] [CrossRef]
- Stinco, C.M.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Hernanz, D.; Vicario, I.M. Simultaneous determination of dietary isoprenoids (carotenoids, chlorophylls and tocopherols) in human faeces by Rapid Resolution Liquid Chromatography. J. Chromatogr. A 2019. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Norkus, E.P.; Hertan, H.; Pitchumoni, C.S. Serum and colon mucosa micronutrient antioxidants: Differences between adenomatous polyp patients and controls. Am. J. Gastroenterol. 2001. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, M.; Rodríguez, V.; Kreindl, C.; Porras, O. Biological Redox Impact of Tocopherol Isomers Is Mediated by Fast Cytosolic Calcium Increases in Living Caco-2 Cells. Antioxidants 2020, 9, 155. https://doi.org/10.3390/antiox9020155
Hidalgo M, Rodríguez V, Kreindl C, Porras O. Biological Redox Impact of Tocopherol Isomers Is Mediated by Fast Cytosolic Calcium Increases in Living Caco-2 Cells. Antioxidants. 2020; 9(2):155. https://doi.org/10.3390/antiox9020155
Chicago/Turabian StyleHidalgo, Miltha, Vania Rodríguez, Christine Kreindl, and Omar Porras. 2020. "Biological Redox Impact of Tocopherol Isomers Is Mediated by Fast Cytosolic Calcium Increases in Living Caco-2 Cells" Antioxidants 9, no. 2: 155. https://doi.org/10.3390/antiox9020155
APA StyleHidalgo, M., Rodríguez, V., Kreindl, C., & Porras, O. (2020). Biological Redox Impact of Tocopherol Isomers Is Mediated by Fast Cytosolic Calcium Increases in Living Caco-2 Cells. Antioxidants, 9(2), 155. https://doi.org/10.3390/antiox9020155




