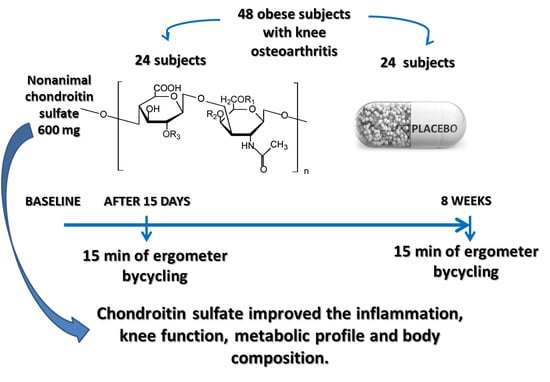
Short- and Long-Term Effectiveness of Supplementation with Non-Animal Chondroitin Sulphate on Inflammation, Oxidative Stress and Functional Status in Obese Subjects with Moderate Knee Osteoarthritis before and after Physical Stress: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Inclusion/Exclusion Criteria
2.3. Study Design
2.4. Description of the Intervention
2.5. Tolerance of the Test Product
2.6. Effectiveness of the Test Product
2.7. Pain and Knee Function
2.8. Biochemical Parameters
2.9. Body Composition
2.10. Ergometer Cycling
2.11. Adverse Events
2.12. Run-in, Randomization and Masking
2.13. Statistical Analysis
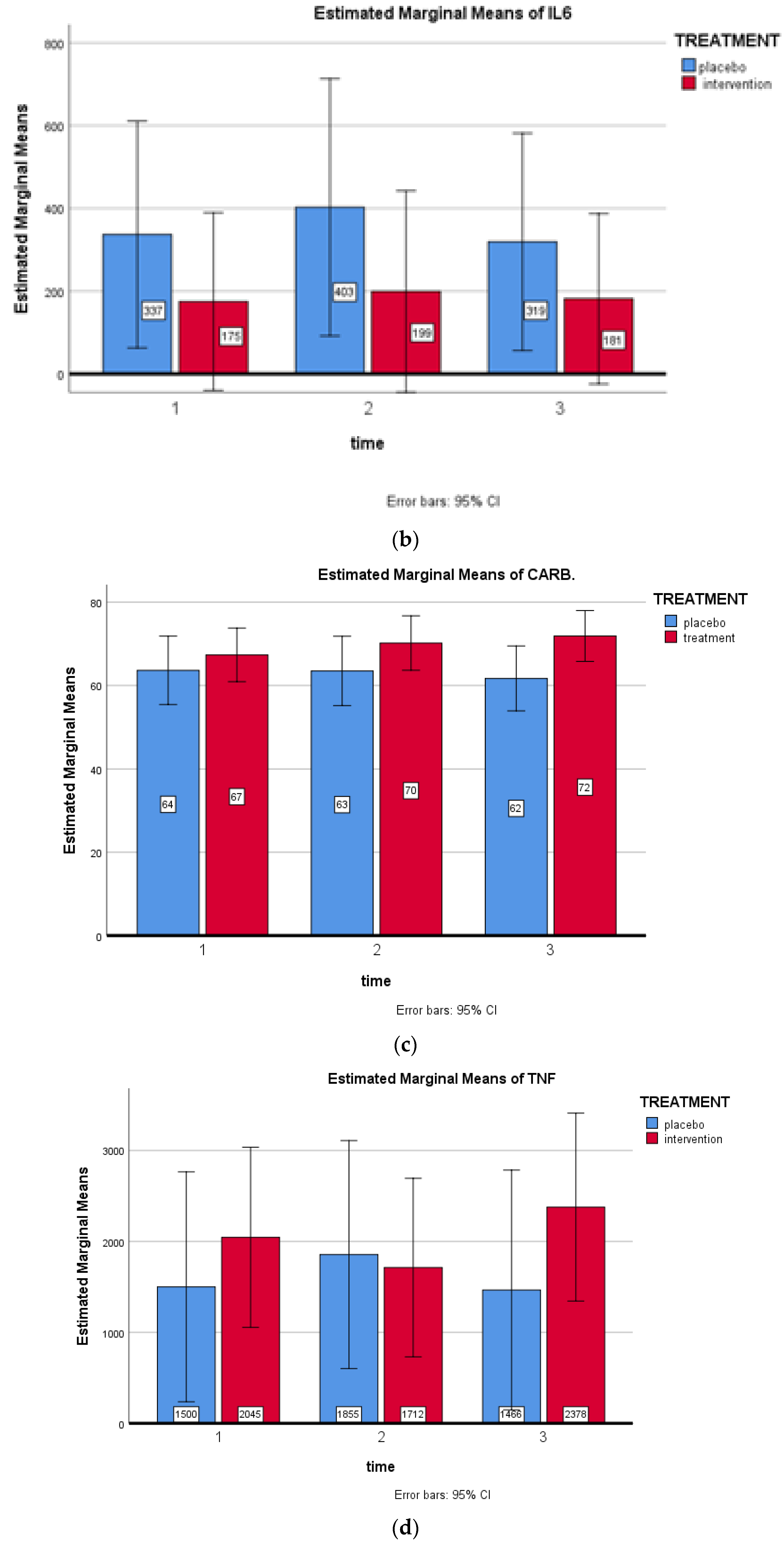
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reyes, C.; Leyland, K.M.; Peat, G.; Cooper, C.; Arden, N.K.; Prieto-Alhambra, D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016, 68, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, T.; Bruun, J.M.; Paulsen, S.K.; Ølholm, J.; Overgaard, K.; Pedersen, S.B.; Richelsen, B. Acute exercise increases circulating inflammatory markers in overweight and obese compared with lean subjects. Eur. J. Appl. Physiol. 2013, 113, 1635–1642. [Google Scholar] [CrossRef]
- Accattato, F.; Greco, M.; Pullano, S.A.; Caré, I.; Fiorillo, A.S.; Pujia, A.; Montalcini, T.; Foti, D.P.; Brunetti, A.; Gulletta, E. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS ONE 2017, 12, e0178900. [Google Scholar] [CrossRef]
- Henrotin, Y.; Marty, M.; Mobasheri, A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas 2014, 78, 184–187. [Google Scholar] [CrossRef] [PubMed]
- DiNubile, N. Glucosamine and chondroitin sulfate: What has been learned since the glucosamine/chondroitin arthritis intervention trial. Orthopedics 2018, 41, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, M.; Sánchez-García, A.; Vilchez-Cavazos, F.; Acosta-Olivo, C.A.; Peña-Martínez, V.M.; Simental-Mendía, L.E. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: A systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol. Int. 2018, 38, 1413–1428. [Google Scholar] [CrossRef]
- Fernandes, L.; Hagen, K.B.; Bijlsma, J.W.J.; Andreassen, O.; Christensen, P.; Conaghan, P.G.; Doherty, M.; Geenen, R.; Hammond, A.; Kjeken, I.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013, 72, 1125–1135. [Google Scholar] [CrossRef]
- Jordan, K.M.; Arden, N.K.; Doherty, M.; Bannwarth, B.; Bijlsma, J.W.J.; Dieppe, P.; Gunther, K.; Hauselmann, H.; Herrero-Beaumont, G.; Kaklamanis, P.; et al. EULAR Recommendations 2003: An evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 2003, 62, 1145–1155. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Leeb, B.F.; Alekseeva, L.; Arden, N.K.; Bijlsma, J.W.; Dinçer, F.; Dziedzic, K.; Häuselmann, H.J.; Herrero-Beaumont, G.; et al. EULAR evidence based recommendations for the management of hand osteoarthritis: Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 2007, 66, 377–388. [Google Scholar] [CrossRef]
- Rondanelli, M.; Braschi, V.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; Peroni, G.; Naso, M.; Iannello, G.; Spadaccini, D.; Miraglia, N.; et al. Effectiveness of Non-Animal Chondroitin Sulfate Supplementation in the Treatment of Moderate Knee Osteoarthritis in a Group of Overweight Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2019, 11, 2027. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Ferlazzo, A.M.; Samà, D.; Calatroni, A. Purified human chondroitin-4-sulfate reduced MMP/TIMP imbalance induced by iron plus ascorbate in human fibroblast cultures. Cell Biol. Int. 2006, 30, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H. Oxidative stress in ovariectomy menopause and role of chondroitin sulfate. Arch. Pharmacal. Res. 2004, 27, 867–872. [Google Scholar] [CrossRef]
- Suttkus, A.; Morawski, M.; Arendt, T. Protective Properties of Neural Extracellular Matrix. Mol. Neurobiol. 2016, 53, 73–82. [Google Scholar] [CrossRef]
- Ju, C.; Hou, L.; Sun, F.; Zhang, L.; Zhang, Z.; Gao, H.; Wang, L.; Wang, D.; Lv, Y.; Zhao, X. Anti-oxidation and Antiapoptotic Effects of Chondroitin Sulfate on 6-Hydroxydopamine-Induced Injury Through the Up-Regulation of Nrf2 and Inhibition of Mitochondria-Mediated Pathway. Neurochem. Res. 2015, 40, 1509–1519. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef]
- Volpi, N. Oral absorption and bioavailability of ichthyic origin chondroitin sulfate in healthy male volunteers. Osteoarthr. Cartil. 2003, 11, 433–441. [Google Scholar] [CrossRef]
- Volpi, N. The pathobiology of osteoarthritis and the rationale for using the chondroitin sulfate for its treatment. Curr. Drug Targets Immune. Endocr. Metabol. Disord. 2004, 4, 119–127. [Google Scholar] [CrossRef]
- Volpi, N. Analytical aspects of pharmaceutical grade chondroitin sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef]
- Singh, J.A.; Noorbaloochi, S.; Macdonald, R.; Maxwell, L.J. Chondroitin for osteoarthritis. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Salaffi, F.; Leardini, G.; Canesi, B.; Mannoni, A.; Fioravanti, A.; Caporali, R.; Lapadula, G.; Punzi, L.; Bucci, R.; Cimmino, M.A.; et al. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index in Italian patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2003, 11, 551–560. [Google Scholar] [CrossRef]
- Haffner, S.M.; Kennedy, E.; Gonzalez, C.; Stern, M.P.; Miettinen, H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care 1996, 19, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Scott, J.M. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997, 281, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; De Lucia Rolfe, E.; Sleigh, A.; Kivisild, T.; Behbehani, K.; Wareham, N.J.; Brage, S.; Mohammad, T. Validity of visceral adiposity estimates from DXA against MRI in Kuwaiti men and women. Nutr. Diabetes 2017, 7, e238. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kobayashi, T.; Moroi, S.; Kotake, H.; Ikoma, T.; Saeki, H.; Ura, K.; Takagi, Y. Anti-obesity effects of chondroitin sulfate oligosaccharides from the skate Raja pulchra. Carbohydr. Polym. 2019, 214, 303–310. [Google Scholar] [CrossRef]
- Han, L.K.; Sumiyoshi, M.; Takeda, T.; Chihara, H.; Nishikiori, T.; Tsujita, T.; Kimura, Y.; Okuda, H. Inhibitory effects of chondroitin sulfate prepared from salmon nasal cartilage on fat storage in mice fed a high-fat diet. Int. J. Obes. 2000, 24, 1131–1138. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Campo, S.; Ferlazzo, A.M.; Calatroni, A. Antioxidant Activity of Chondroitin Sulfate. Adv. Pharmacol. 2006, 53, 417–431. [Google Scholar]
- Nakasone, Y.; Watabe, K.; Watanabe, K.; Tomonaga, A.; Nagaoka, I.; Yamamoto, T.; Yamaguchi, H. Effect of a glucosamine-based combination supplement containing chondroitin sulfate and antioxidant micronutrients in subjects with symptomatic knee osteoarthritis: A pilot study. Exp. Ther. Med. 2011, 2, 893–899. [Google Scholar] [CrossRef]
- Grover, A.K.; Samson, S.E. Benefits of antioxidant supplements for knee osteoarthritis: Rationale and reality. Nutr. J. 2016, 15, 1. [Google Scholar]
- Lippi, G.; Chiozza, L.; Mattiuzzi, C.; Plebani, M. Patient and Sample Identification. out of the Maze? J. Med. Biochem. 2017, 36, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D.; Lippi, G. Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clin. Biochem. 2017, 50, 568–573. [Google Scholar] [CrossRef] [PubMed]


| Variable | Control (24, 11 M, 13 F) Mean; SD | Intervention (24, 11 M, 13 F) Mean; SD | Total Sample (48) | p-Value between Groups |
|---|---|---|---|---|
| Age (years) | 43.36 ± 8.67 | 48.73 ± 16.33 | 46.64 ± 13.97 | 0.267 |
| Height (m) | 1.68 ± 0.11 | 1.65 ± 0.14 | 1.66 ± 0.13 | 0.481 |
| Weight (kg) | 95.38 ± 16.15 | 88.482 ± 18.84 | 91.16 ± 17.93 | 0.267 |
| BMI (kg/m2) | 33.47 ± 1.85 | 32.09 ± 1.77 | 32.63 ± 1.91 | 0.031 |
| FFM (kg) | 51.43 ± 9.43 | 49.61 ± 10.88 | 50.32 ± 10.24 | 0.611 |
| FM (kg) | 41.27 ± 10.12 | 36.13 ± 10.72 | 38.13 ± 10.65 | 0.162 |
| FM (%) | 44.29 ± 6.47 | 41.82 ± 6.36 | 42.78 ± 6.43 | 0.268 |
| VAT (kg) | 1.77 ± 0.92 | 1.40 ± 1.17 | 1.55 ± 1.08 | 0.327 |
| WOMAC | 23.61 ± 16.71 | 22.19 ± 16.16 | 22.74 ± 16.15 | 0.802 |
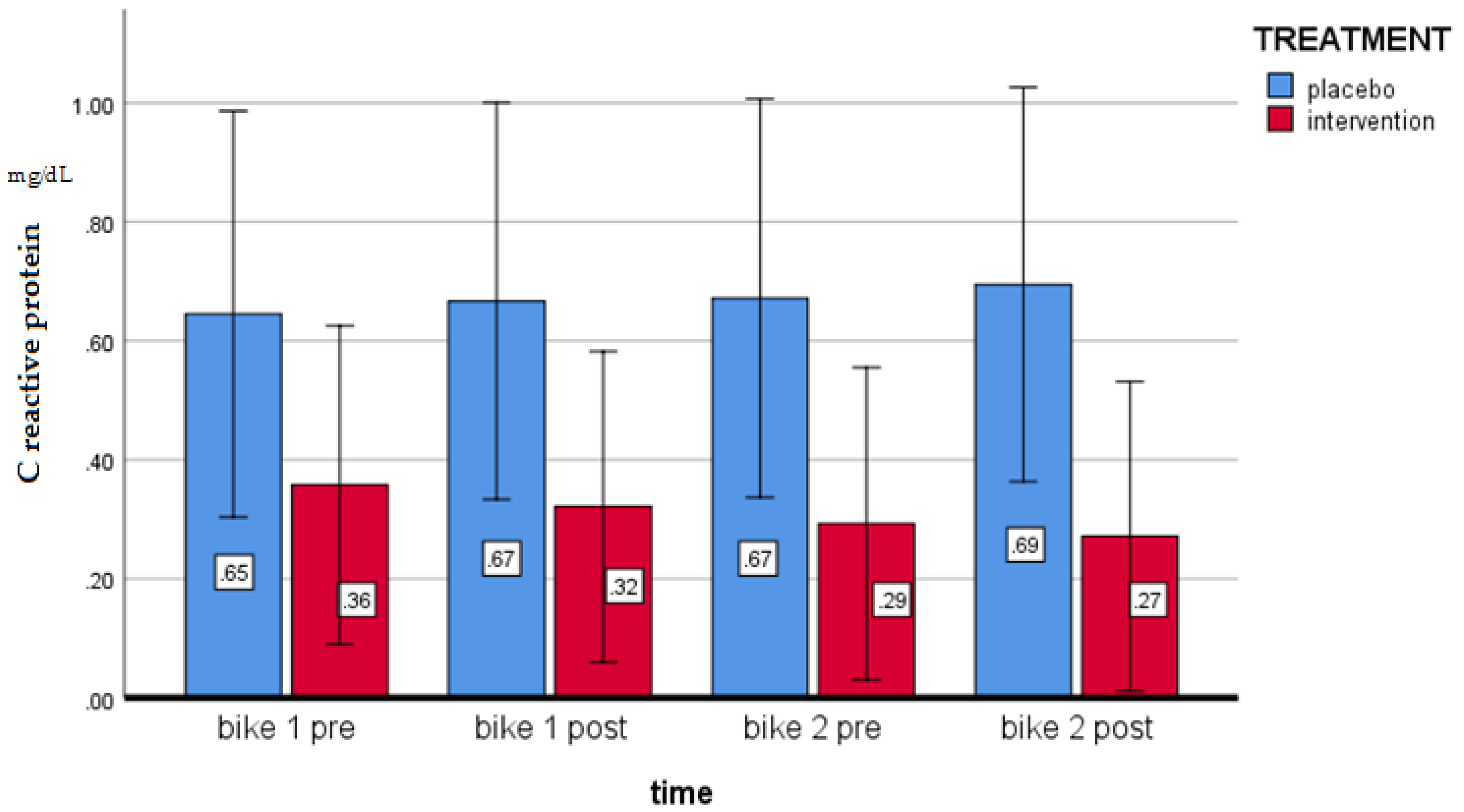
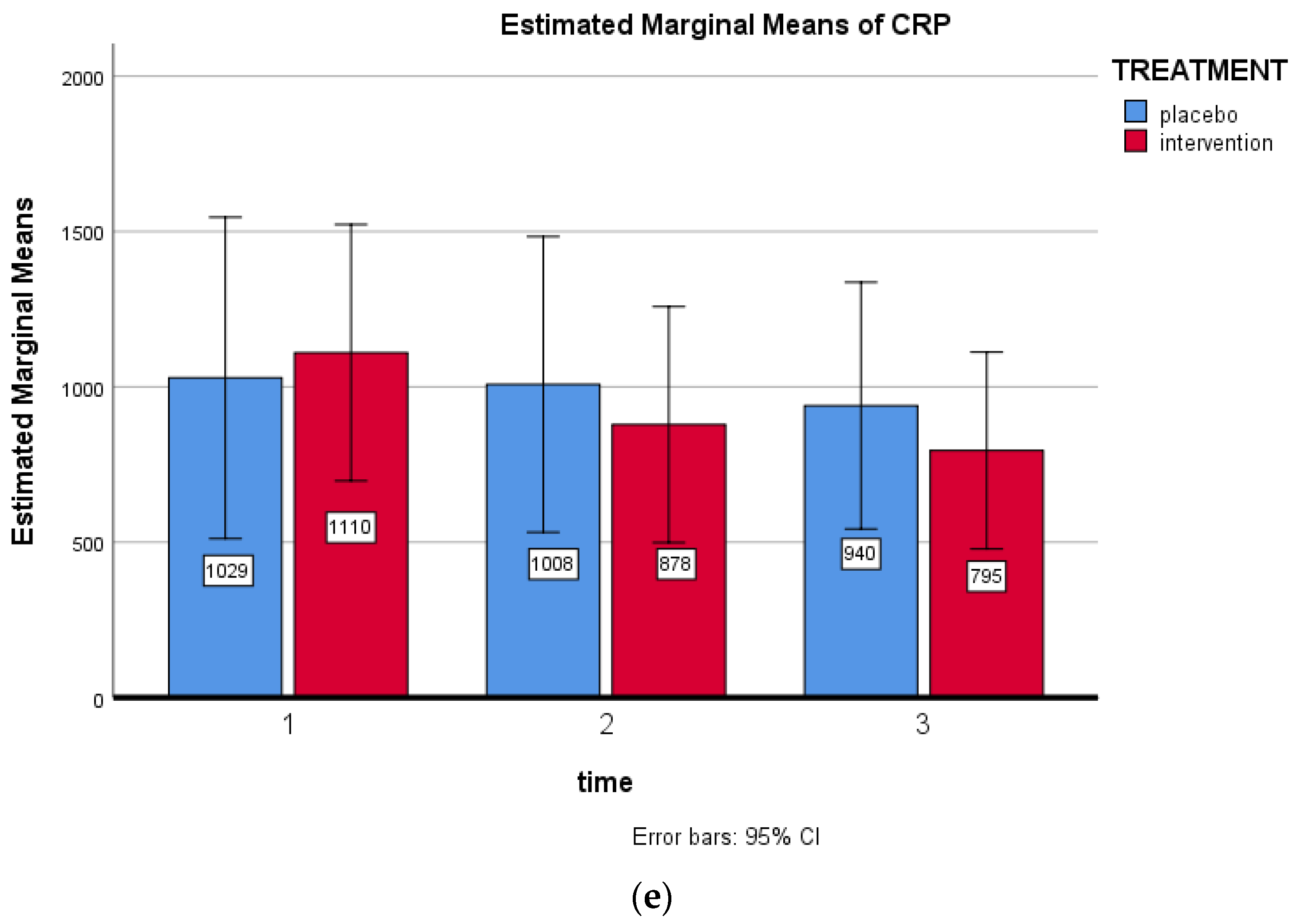
| CRP (mg/dL) | 0.68 ± 0.88 | 0.65 ± 0.77 | 0.66 ± 0.80 | 0.918 |
| Total cholesterol (mg/dL) | 192.14 ± 32.39 | 182.59 ± 40.31 | 186.31 ± 37.24 | 0.461 |
| Triglycerides (mg/dL) | 117.93 ± 57.91 | 104.00 ± 51.78 | 109.42 ± 53.87 | 0.457 |
| Glucose (mg/dL) | 86.71 ± 9.14 | 86.73 ± 9.96 | 86.72 ± 9.52 | 0.997 |
| HDL (mg/dL) | 54.36 ± 17.85 | 52.46 ± 15.93 | 53.19 ± 16.48 | 0.741 |
| LDL (mg/dL) | 132.29 ± 27.10 | 121.36 ± 40.01 | 125.61 ± 35.53 | 0.376 |
| VLDL (mg/dL) | 23.59 ± 11.58 | 20.80 ± 10.36 | 21.88 ± 10.77 | 0.457 |
| Insulin (IU/mL) | 12.66 ± 6.78 | 13.30 ± 9.67 | 13.05 ± 8.56 | 0.829 |
| HOMA-IR | 2.73 ± 1.50 | 2.93 ± 2.21 | 2.85 ± 1.94 | 0.770 |
| AST (U/l) | 18.71 ± 7.75 | 17.64 ± 4.73 | 18.06 ± 6.0 | 0.606 |
| ALT (U/l) | 24.07 ± 16.10 | 19.46 ± 9.28 | 21.25 ± 12.38 | 0.282 |
| GGT (U/l) | 25.79 ± 16.36 | 19.86 ± 14.61 | 22.17 ± 15.36 | 0.265 |
| Creatinine (mg/dL) | 0.79 ± 0.16 | 0.79 ± 0.16 | 0.79 ± 0.16 | 0.921 |
| Vitamin B12 (pg/mL) | 362.64 ± 131.25 | 419.05 ± 205.86 | 397.11 ± 180.56 | 0.368 |
| Folic acid (ng/mL) | 6.89 ± 6.80 | 8.06 ± 8.36 | 7.61 ± 7.71 | 0.664 |
| Homocysteine (µmol/L) | 14.15 ± 3.69 | 15.30 ± 8.11 | 14.85 ± 6.70 | 0.624 |
| WBC (×103/µL) | 6.35 ± 1.64 | 6.37 ± 1.58 | 6.36 ± 1.58 | 0.971 |
| Lymphocytes (×103/µL) | 1.87 ± 0.41 | 2.05 ± 0.66 | 1.98 ± 0.57 | 0.346 |
| Lymphocytes (%) | 30.64 ± 7.90 | 33.04 ± 8.13 | 32.11 ± 8.02 | 0.388 |
| RBC (×106/µL) | 4.96 ± 0.38 | 4.77 ± 0.36 | 4.84 ± 0.38 | 0.135 |
| Hemoglobin (g/dL) | 14.12 ± 1.98 | 13.64 ± 1.11 | 13.83 ± 1.50 | 0.353 |
| Hematocrit (%) | 41.67 ± 3.06 | 40.81 ± 3.04 | 41.14 ± 3.03 | 0.411 |
| MCV (fl) | 84.38 ± 7.82 | 85.95 ± 7.44 | 85.34 ± 7.52 | 0.549 |
| Variable | Control Intra-Group Δ Change (CI 95%) | Intervention Intra-Group Δ Change (CI 95%) | Intervention Effect between Groups p-Value |
|---|---|---|---|
| Weight (kg) | −1.12 (−2.83; 0.59) | −2.23 (−3.57; −0.89) ** | −1.10 (−3.36; 1.15) (p = 0.325) |
| −0.40 (−1.02; 0.21) | −0.73 (−1.21; −0.25) ** | −0.33 (−1.14; 0.48) (p = 0.416) | |
| FFM (kg) | −0.16 (−1.13; 0.81) | −0.32 (−1.09; 0.44) | −0.16 (−1.44; 1.12) (p = 0.797) |
| FM (kg) | −1.13 (−2.57; 0.32) | −1.44 (−2.58; −0.31) | −0.32 (−2.22; 1.59) (p = 0.739) |
| FM (%) | −0.56 (−1.94; 0.83) | −0.63 (−1.72; 0.46) | −0.07 (−1.90; 1.76) (p = 0.937) |
| VAT (kg) | −0.05 (−0.34; 0.24) | −0.16 (−0.39; 0.07) | −0.11 (−0.49; 0.27) (p = 0.552) |
| WOMAC | 13.23 (5.85; 20.62) | −8.83 (−14.62; −3.04) * | −22.06 (−31.79; −12.33) (p = 0.000) |
| CRP (mg/dL) | 0.28 (−0.23; 0.29) | −0.38 (−0.58; −0.17) * | −0.40 (−0.75; −0.06) (p = 0.022) |
| Total cholesterol (mg/dL) | −0.92 (−9.28; 11.11) | −9.77 (−17.75; −1.78) ** | −10.68 (−24.11; 2.75) (p = 0.115) |
| Triglycerides (mg/dL) | −1.44 (−22.09; 19.20) | −5.40 (−21.57; 10.78) | −3.95 (−31.16; 23.25) (p = 0.769) |
| Glucose (mg/dL) | 3.42 (−3.04; 9.87) | −3.13 (−8.18; 1.93) | −6.54 (−15.05; 1.96) (p = 0.127) |
| HDL (mg/dL) | 2.84 (−3.67; 9.35) | 2.05 (−3.05; 7.15) | −0.79 (−9.37: 7.79) (p = 0.852) |
| LDL (mg/dL) | −0.11 (−10.07; 9.85) | −5.93 (−13.74; 1.87) | −5.83 (−18.95; 7.30) (p = 0.373) |
| VLDL (mg/dL) | −0.29 (−4.42; 3.84) | −1.08 (−4.32; 2.16) | −0.79 (−6.23; 4.65) (p = 0.769) |
| Insulin (IU/mL) | −0.37 (−4.08; 3.35) | −2.87 (−5.78; 0.04) | −2.50 (−7.40; 2.39) (p = 0.306) |
| HOMA–IR | −0.01 (−0.91; 0.89) | −0.77 (−1.47; −0.07) ** | −0.76 (−1.94; 0.42) (p = 0.200) |
| AST (U/L) | −0.41 (−3.95; 3.13) | −0.03 (−2.74; 2.81) | 0.44 (−4.23; 5.10) (p = 0.850) |
| ALT (U/L) | 0.54 (−4.59; 5.66) | −2.30 (−6.31; 1.72) | −2.83 (−9.58; 3.92) (p = 0.399) |
| GGT (U/l) | −1.08 (−5.66; 3.51) | −3.91 (−7.50; −0.31) ** | −2.83 (−8.88; 3.21) (p = 0.347) |
| Creatinine (mg/dL) | −0.03 (−0.07; 0.01) | 0.02 (−0.02; 0.05) | 0.05 (−0.01; 0.10) (p = 0.099) |
| Vitamin B12 (pg/mL) | 14.67 (−42.50; 71.84) | 8.26 (−36.54; 53.06) | −6.41 (−81.75; 68.93) (p = 0.864) |
| Folic acid (ng/mL) | −0.15 (−4.42; 4.12) | 1.31 (−2.03; 4.66) | 1.46 (−4.16: 7.09) (p = 0.600) |
| Homocysteine (µmol/L) | 1.97 (−11.47; 15.42) | 3.66 (−6.88; 14.19) | 1.69 (−16.03; 19.40) (p = 0.848) |
| WBC (×103/µL) | −0.37 (−1.27; 0.52) | 0.01 (−0.69; 0.71) | 0.39 (−0.80; 1.57) (p = 0.511) |
| Lymphocytes (×103/µL) | 0.03 (−0.17; 0.24) | −0.02 (−0.18; 0.15) | −0.05 (−0.32; 0.22) (p = 0.719) |
| Lymphocytes (%) | 2.31 (−2.28; 6.90) | −0.28 (−3.87; 3.32) | −2.59 (−8.63; 3.46) (p = 0.389) |
| RBC (×106/µL) | 0.02 (−0.09; 0.12) | 0.05 (−0.358; 0.13) | 0.03 (−0.11; 0.17) (p = 0.668) |
| Hemoglobin (g/dL) | −0.29 (−0.91; 0.33) | 0.19 (−0.30; 0.67) | 0.48 (−0.34; 1.29) (p = 0.243) |
| Hematocrit (%) | 4.70 (0.00; 9.40) ** | −0.09 (−3.77; 3.59) | −4.79 (−10.98; 1.40) (p = 0.125) |
| MCV (fl) | −4.96 (−10.75; 0.83) | 1.08 (−3.46; 5.61) | 6.04 (−1.59; 13.67) (p = 0.117) |
| VARIABLES | ΔWOMAC | ΔHOMA | ΔChol | ΔGlucose | ΔBMI | |
|---|---|---|---|---|---|---|
| ΔWOMAC | Correlation Coefficient | 1.000 | 0.027 | −0.128 | −0.147 | 0.010 |
| Sig. (2-tailed) | 0 | 0.906 | 0.570 | 0.513 | 0.966 | |
| ΔHOMA | Correlation Coefficient | 0.027 | 1.000 | −0.100 | −0.080 | 0.286 |
| Sig. (2-tailed) | 0.906 | 0 | 0.659 | 0.724 | 0.197 | |
| ΔChol | Correlation Coefficient | −0.128 | −0.100 | 1.000 | 0.149 | −0.023 |
| Sig. (2-tailed) | 0.570 | 0.659 | 0 | 0.507 | 0.919 | |
| ΔGlucose | Correlation Coefficient | −0.147 | −0.080 | 0.149 | 1.000 | 0.303 |
| Sig. (2-tailed) | 0.513 | 0.724 | 0.507 | . | 0.170 | |
| ΔBMI | Correlation Coefficient | 0.010 | 0.286 | −0.023 | 0.303 | 1.000 |
| Sig. (2-tailed) | 0.966 | 0.197 | 0.919 | 0.170 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondanelli, M.; Miraglia, N.; Putignano, P.; Peroni, G.; Faliva, M.A.; Naso, M.; Gasparri, C.; Infantino, V.; Nichetti, M.; Volpi, N.; et al. Short- and Long-Term Effectiveness of Supplementation with Non-Animal Chondroitin Sulphate on Inflammation, Oxidative Stress and Functional Status in Obese Subjects with Moderate Knee Osteoarthritis before and after Physical Stress: A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants 2020, 9, 1241. https://doi.org/10.3390/antiox9121241
Rondanelli M, Miraglia N, Putignano P, Peroni G, Faliva MA, Naso M, Gasparri C, Infantino V, Nichetti M, Volpi N, et al. Short- and Long-Term Effectiveness of Supplementation with Non-Animal Chondroitin Sulphate on Inflammation, Oxidative Stress and Functional Status in Obese Subjects with Moderate Knee Osteoarthritis before and after Physical Stress: A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants. 2020; 9(12):1241. https://doi.org/10.3390/antiox9121241
Chicago/Turabian StyleRondanelli, Mariangela, Niccolò Miraglia, Pietro Putignano, Gabriella Peroni, Milena Anna Faliva, Maurizio Naso, Clara Gasparri, Vittoria Infantino, Mara Nichetti, Nicola Volpi, and et al. 2020. "Short- and Long-Term Effectiveness of Supplementation with Non-Animal Chondroitin Sulphate on Inflammation, Oxidative Stress and Functional Status in Obese Subjects with Moderate Knee Osteoarthritis before and after Physical Stress: A Randomized, Double-Blind, Placebo-Controlled Trial" Antioxidants 9, no. 12: 1241. https://doi.org/10.3390/antiox9121241
APA StyleRondanelli, M., Miraglia, N., Putignano, P., Peroni, G., Faliva, M. A., Naso, M., Gasparri, C., Infantino, V., Nichetti, M., Volpi, N., Capitani, F., Mantovani, V., & Perna, S. (2020). Short- and Long-Term Effectiveness of Supplementation with Non-Animal Chondroitin Sulphate on Inflammation, Oxidative Stress and Functional Status in Obese Subjects with Moderate Knee Osteoarthritis before and after Physical Stress: A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants, 9(12), 1241. https://doi.org/10.3390/antiox9121241