Biological Effects of Scattered Versus Scanned Proton Beams on Normal Tissues in Total Body Irradiated Mice: Survival, Genotoxicity, Oxidative Stress and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Ethics Considerations
2.3. Irradiation
2.4. Skin, Lung, Heart & Blood Sample Collection
2.5. Separation of Blood Components
2.6. Cytochalasin-Blocked Micronucleus Assay
2.7. Preparation of Erythrocytes Lysates
2.8. Preparation of Tissue Homogenates
2.9. Protein Quantification
2.10. Measurement of SOD activity
2.11. Measurement of CAT Activity
2.12. Measurement of Glutathione Peroxidase (GPx) Activity
2.13. Quantification of Oxidized Glutathione (GSSG)
2.14. Measurement of LPO
2.15. Measurement of Protein Carbonylation
2.16. Cytokine Quantification in Plasma
2.17. Statistical Analysis
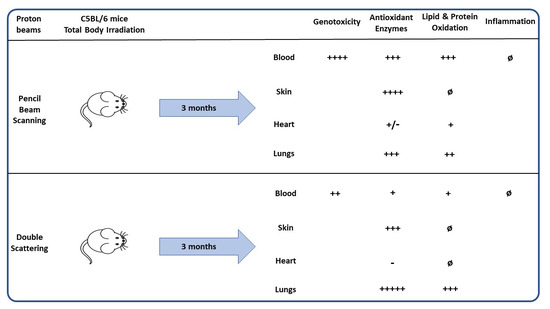
3. Results
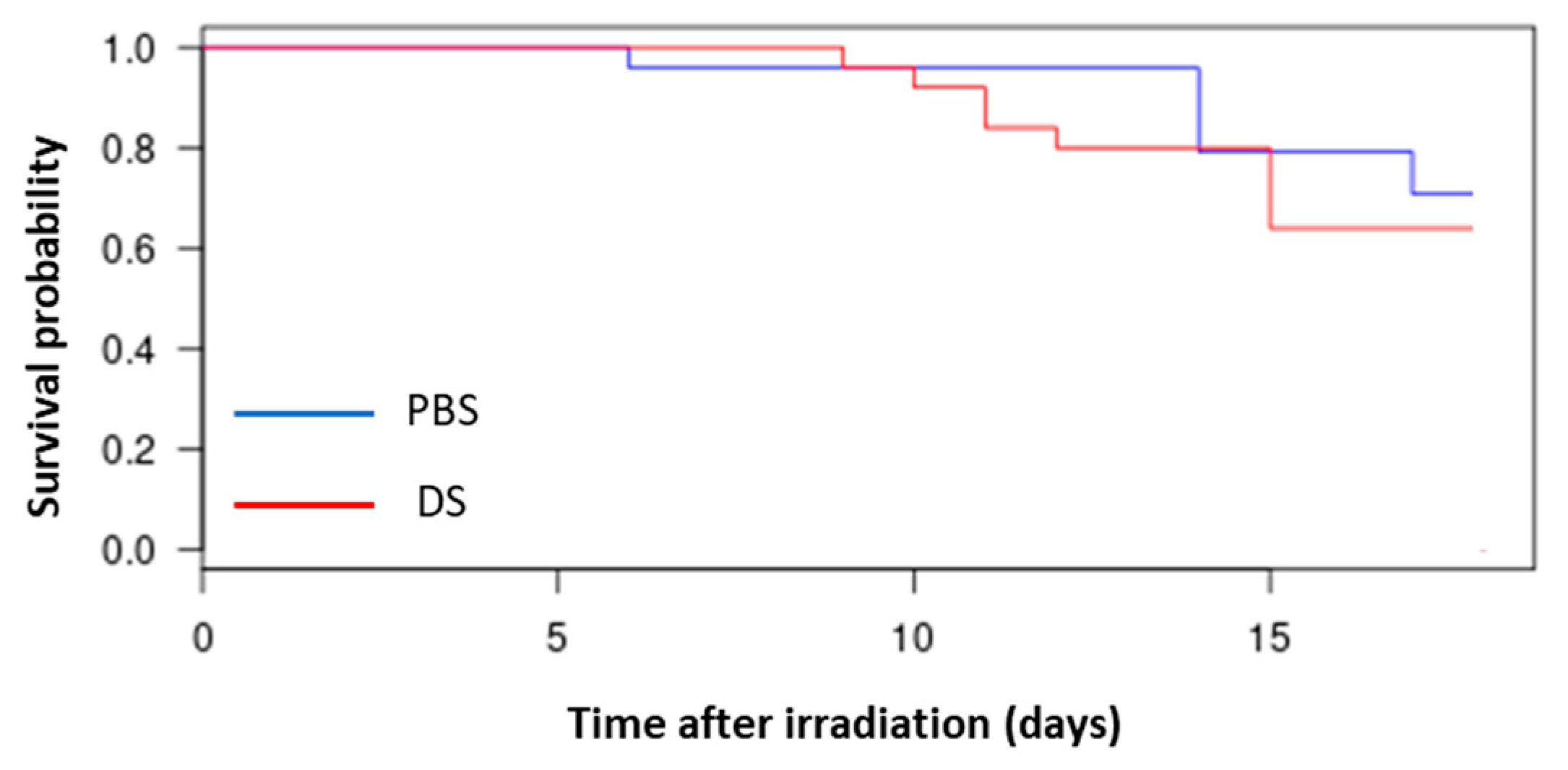
3.1. Evaluation of Survival of Mice
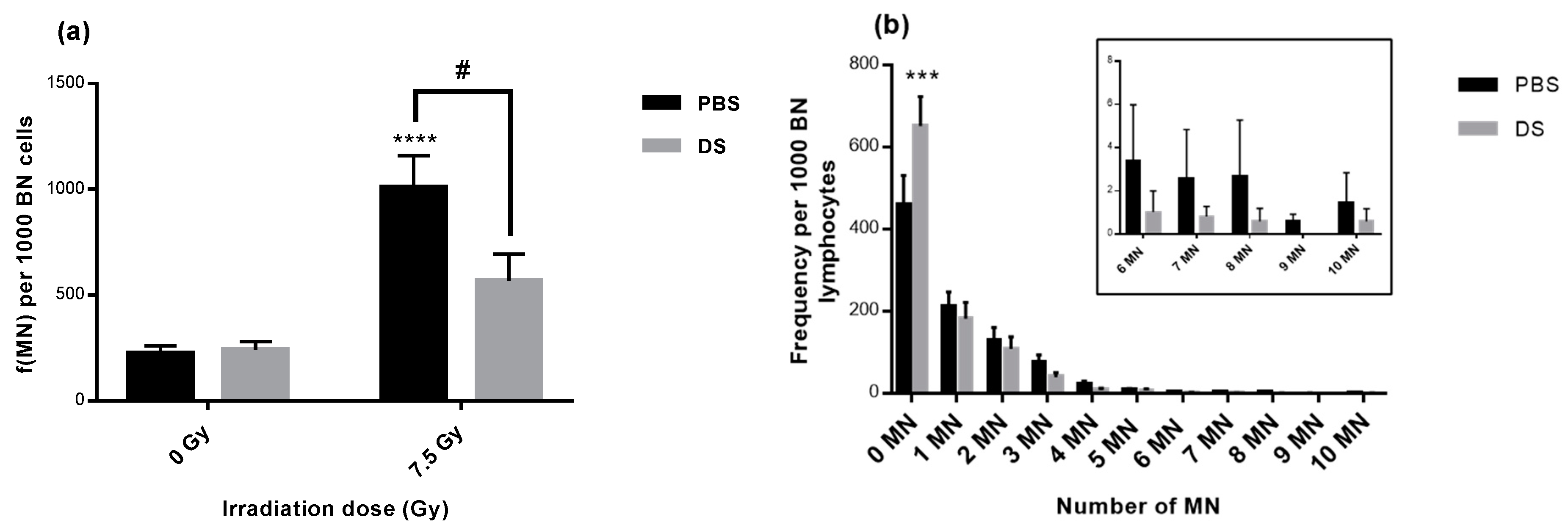
3.2. Lymphocyte Micronucleus Frequency and Distribution
3.3. Evaluation of Antioxidant Enzymes Activities and Oxidized Glutathione in Erythrocytes Lysates and Tissue Homogenates
3.4. LPO in Erythrocytes Lysates and Tissue Homogenates
3.5. Protein Carbonylation in Erythrocytes Lysates and Tissue Homogenates
3.6. Quantification of Plasma Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baskar, R.; Lee, K.; Yeo, R.; Yeoh, K.W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Loeffler, J.S.; Durante, M. Charged particle therapy--optimization, challenges and future directions. Nat. Rev. Clin. Oncol. 2013, 10, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C. Oxidative Stress in Hadrontherapy. In Novel Prospects in Oxidative and Nitrosative Stress; Atukeren, P., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Brown, J.M. Exploiting the hypoxic cancer cell: Mechanisms and therapeutic strategies. Mol. Med. Today 2000, 6, 157–162. [Google Scholar] [CrossRef]
- Emami, B.; Lyman, J.; Brown, A.; Coia, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Chaouni, S.; Lecomte, D.D.; Stefan, D.; Leduc, A.; Barraux, V.; Leconte, A.; Grellard, J.-M.; Habrand, J.-L.; Guillamin, M.; Sichel, F.; et al. The Possibility of Using Genotoxicity, Oxidative Stress and Inflammation Blood Biomarkers to Predict the Occurrence of Late Cutaneous Side Effects after Radiotherapy. Antioxidants 2020, 9, 220. [Google Scholar] [CrossRef]
- Doyen, J.; Bondiau, P.-Y.; Benezery, K.; Thariat, J.; Vidal, M.; Gérard, A.; Hérault, J.; Carrie, C.; Hannoun-Lévi, J.-M. Indications et résultats de la protonthérapie dans le traitement des cancers. Cancer/Radiothérapie 2016, 20, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Ilicic, K.; Combs, S.E.; Schmid, T.E. New insights in the relative radiobiological effectiveness of proton irradiation. Radiat. Oncol. Lond. Engl. 2018, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Girdhani, S.; Sachs, R.; Hlatky, L. Biological effects of proton radiation: What we know and don’t know. Radiat. Res. 2013, 179, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, F.; Durante, M. Proton Radiobiology. Cancers 2015, 7, 353–381. [Google Scholar] [CrossRef]
- Patyal, B. Dosimetry aspects of proton therapy. Technol. Cancer Res. Treat. 2007, 6, 17–23. [Google Scholar]
- Lundkvist, J.; Ekman, M.; Ericsson, S.R.; Jönsson, B.; Glimelius, B. Cost-effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer 2005, 103, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Rutz, H.P.; Weber, D.C.; Goitein, G.; Ares, C.; Bolsi, A.; Lomax, A.J.; Pedroni, E.; Coray, A.; Hug, E.B.; Timmermann, B. Postoperative spot-scanning proton radiation therapy for chordoma and chondrosarcoma in children and adolescents: Initial experience at Paul Scherrer institute. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Kadkhoda, Z.; Björk-Eriksson, T.; Nill, S.; Wilkens, J.J.; Oelfke, U.; Johansson, K.-A.; Huber, P.E.; Münter, M.W. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: A comparative treatment planning study of photons and protons. Radiat. Oncol. 2008, 3, 4. [Google Scholar] [CrossRef]
- Miyawaki, D.; Murakami, M.; Demizu, Y.; Sasaki, R.; Niwa, Y.; Terashima, K.; Nishimura, H.; Hishikawa, Y.; Sugimura, K. Brain injury after proton therapy or carbon ion therapy for head-and-neck cancer and skull base tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 378–384. [Google Scholar] [CrossRef]
- Björk-Eriksson, T.; Glimelius, B. The potential of proton beam radiation therapy in breast cancer. Acta Oncol. Stockh. Swed. 2005, 44, 884–889. [Google Scholar] [CrossRef]
- Widesott, L.; Amichetti, M.; Schwarz, M. Proton therapy in lung cancer: Clinical outcomes and technical issues. A systematic review. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2008, 86, 154–164. [Google Scholar] [CrossRef]
- Johansson, B.; Ridderheim, M.; Glimelius, B. The potential of proton beam radiation therapy in prostate cancer, other urological cancers and gynaecological cancers. Acta Oncol. Stockh. Swed. 2005, 44, 890–895. [Google Scholar] [CrossRef]
- Girdhani, S.; Lamont, C.; Hahnfeldt, P.; Abdollahi, A.; Hlatky, L. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat. Res. 2012, 178, 33–45. [Google Scholar] [CrossRef]
- Gridley, D.S.; Freeman, T.L.; Makinde, A.Y.; Wroe, A.J.; Luo-Owen, X.; Tian, J.; Mao, X.W.; Rightnar, S.; Kennedy, A.R.; Slater, J.M.; et al. Comparison of proton and electron radiation effects on biological responses in liver, spleen and blood. Int. J. Radiat. Biol. 2011, 87, 1173–1181. [Google Scholar] [CrossRef]
- Grutters, J.P.C.; Kessels, A.G.H.; Pijls-Johannesma, M.; De Ruysscher, D.; Joore, M.A.; Lambin, P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: A meta-analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Lee, C.; Shin, S.-W.; Kim, S.-Y.; Hong, S.N.; Park, H.C. Comparison of Proton and Photon Beam Irradiation in Radiation-Induced Intestinal Injury Using a Mouse Model. Int. J. Mol. Sci. 2019, 20, 1894. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Nuclear interactions in proton therapy: Dose and relative biological effect distributions originating from primary and secondary particles. Phys. Med. Biol. 2002, 47, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419. [Google Scholar] [CrossRef] [PubMed]
- James, S.S.; Grassberger, C.; Lu, H.-M. Considerations when treating lung cancer with passive scatter or active scanning proton therapy. Transl. Lung Cancer Res. 2018, 7, 210–215. [Google Scholar] [CrossRef]
- Mishra, M.V.; Khairnar, R.; Bentzen, S.M.; Larson, G.; Tsai, H.; Sinesi, C.; Vargas, C.; Laramore, G.; Rossi, C.; Rosen, L.; et al. Proton beam therapy delivered using pencil beam scanning vs. passive scattering/uniform scanning for localized prostate cancer: Comparative toxicity analysis of PCG 001–09. Clin. Transl. Radiat. Oncol. 2019, 19, 80–86. [Google Scholar] [CrossRef]
- Wang, D. A critical appraisal of the clinical utility of proton therapy in oncology. Med. Devices Auckl. NZ 2015, 8, 439–446. [Google Scholar] [CrossRef][Green Version]
- De Marzi, L. Effets Physiques et Biologiques des Faisceaux de Protons Balayés: Mesures et Modélisation pour des Balayages Séquentiels à Haut Débit. Ph.D. Thesis, Université Paris Saclay (COmUE), Paris, France, 2016. [Google Scholar]
- MacDonald, S.M.; Jimenez, R.; Paetzold, P.; Adams, J.; Beatty, J.; DeLaney, T.F.; Kooy, H.; Taghian, A.G.; Lu, H.-M. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat. Oncol. Lond. Engl. 2013, 8, 71. [Google Scholar] [CrossRef]
- Hall, E.J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1–7. [Google Scholar] [CrossRef]
- Gridley, D.S.; Pecaut, M.J.; Mao, X.W.; Wroe, A.J.; Luo-Owen, X. Biological effects of passive versus active scanning proton beams on human lung epithelial cells. Technol. Cancer Res. Treat. 2015, 14, 81–98. [Google Scholar] [CrossRef]
- Matsubara, H.; Karasawa, K.; Furuichi, W.; Wakaisami, M.; Shiba, S.; Wakatsuki, M.; Omatsu, T.; Inaniwa, T.; Fukuda, S.; Kamada, T. Comparison of passive and scanning irradiation methods for carbon-ion radiotherapy for breast cancer. J. Radiat. Res. 2018, 59, 625–631. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Baluchamy, S.; Zhang, Y.; Ravichandran, P.; Ramesh, V.; Sodipe, A.; Hall, J.C.; Jejelowo, O.; Gridley, D.S.; Wu, H.; Ramesh, G.T. Differential oxidative stress gene expression profile in mouse brain after proton exposure. In Vitro Cell. Dev. Biol. Anim. 2010, 46, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Suman, S.; Kallakury, B.V.S.; Fornace, A.J. Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS ONE 2012, 7, e42224. [Google Scholar] [CrossRef]
- Suman, S.; Kumar, S.; Fornace, A.; Datta, K. Persistent oxidative stress in mouse intestinal and colonic epithelial cells after exposure to 12C-ion radiation. Am. Assoc. Cancer Res. 2017, 77. [Google Scholar] [CrossRef]
- Chi, C.; Tanaka, R.; Okuda, Y.; Ikota, N.; Yamamoto, H.; Urano, S.; Ozawa, T.; Anzai, K. Quantitative measurements of oxidative stress in mouse skin induced by X-ray irradiation. Chem. Pharm. Bull. 2005, 53, 1411–1415. [Google Scholar] [CrossRef][Green Version]
- Barshishat-Kupper, M.; Tipton, A.J.; McCart, E.A.; McCue, J.; Mueller, G.P.; Day, R.M. Effect of ionizing radiation on liver protein oxidation and metabolic function in C57BL/6J mice. Int. J. Radiat. Biol. 2014, 90, 1169–1178. [Google Scholar] [CrossRef]
- Barshishat-Kupper, M.; McCart, E.A.; Freedy, J.G.; Tipton, A.J.; Nagy, V.; Kim, S.-Y.; Landauer, M.R.; Mueller, G.P.; Day, R.M. Protein Oxidation in the Lungs of C57BL/6J Mice Following X-Irradiation. Proteomes 2015, 3, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Barshishat-Kupper, M.; McCart, E.A.; Mueller, G.P.; Day, R.M. Bone Marrow Protein Oxidation in Response to Ionizing Radiation in C57BL/6J Mice. Proteomes 2014, 2, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Otting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [PubMed]
- Chang, J.; Feng, W.; Wang, Y.; Luo, Y.; Allen, A.R.; Koturbash, I.; Turner, J.; Stewart, B.; Raber, J.; Hauer-Jensen, M.; et al. Whole-Body Proton Irradiation Causes Long-Term Damage to Hematopoietic Stem Cells in Mice. Radiat. Res. 2015, 183, 240–248. [Google Scholar] [CrossRef]
- Xue, X.-L.; Han, X.-D.; Li, Y.; Chu, X.-F.; Miao, W.-M.; Zhang, J.-L.; Fan, S.-J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7. [Google Scholar] [CrossRef]
- Chang, S.; Hu, L.; Xu, Y.; Li, X.; Ma, L.; Feng, X.; Wang, J.; Zhang, C.; Wang, S. Inorganic Nitrate Alleviates Total Body Irradiation-Induced Systemic Damage by Decreasing Reactive Oxygen Species Levels. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 945–957. [Google Scholar] [CrossRef]
- Michaelidesová, A.; Konířová, J.; Vachelová, J.; Vondráček, V.; Davídková, M. Nowadays proton therapy: Double scattering versus pencil beam scanning mode. Phys. Med. 2016, 32, 309–310. [Google Scholar] [CrossRef]
- Michaelidesová, A.; Vachelová, J.; Klementová, J.; Urban, T.; Pachnerová Brabcová, K.; Kaczor, S.; Falk, M.; Falková, I.; Depeš, D.; Vondráček, V.; et al. In Vitro Comparison of Passive and Active Clinical Proton Beams. Int. J. Mol. Sci. 2020, 21, 5650. [Google Scholar] [CrossRef]
- Liao, A.C.; Craver, B.M.; Tseng, B.P.; Tran, K.K.; Parihar, V.K.; Acharya, M.M.; Limoli, C.L. Mitochondrial-targeted human catalase affords neuroprotection from proton irradiation. Radiat. Res. 2013, 180, 1–6. [Google Scholar] [CrossRef]
- Pan, J.; Su, Y.; Hou, X.; He, H.; Liu, S.; Wu, J.; Rao, P. Protective effect of recombinant protein SOD-TAT on radiation-induced lung injury in mice. Life Sci. 2012, 91, 89–93. [Google Scholar] [CrossRef]
- Focea, R.; Nadejde, C.; Creanga, D.; Luchian, T. Low dose X –ray effects on catalase activity in animal tissue. J. Phys. Conf. Ser. 2012, 398, 012032. [Google Scholar] [CrossRef]
- Park, E.M.; Ramnath, N.; Yang, G.Y.; Ahn, J.Y.; Park, Y.; Lee, T.; Shin, H.S.; Yu, J.; Ip, C.; Park, Y.M. High superoxide dismutase and low glutathione peroxidase activities in red blood cells predict susceptibility of lung cancer patients to radiation pneumonitis. Free Radic. Biol. Med. 2007, 42, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Baluchamy, S.; Ravichandran, P.; Ramesh, V.; He, Z.; Zhang, Y.; Hall, J.C.; Jejelowo, O.; Gridley, D.S.; Wu, H.; Ramesh, G.T. Reactive oxygen species mediated tissue damage in high energy proton irradiated mouse brain. Mol. Cell. Biochem. 2012, 360, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Iizawa, O.; Kato, T.; Tagami, H.; Akamatsu, H.; Niwa, Y. Long-term follow-up study of changes in lipid peroxide levels and the activity of superoxide dismutase, catalase and glutathione peroxidase in mouse skin after acute and chronic UV irradiation. Arch. Dermatol. Res. 1994, 286, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Alamil, H.; Galanti, L.; Heutte, N.; Van Der Schueren, M.; Dagher, Z.; Lechevrel, M. Genotoxicity of aldehyde mixtures: Profile of exocyclic DNA-adducts as a biomarker of exposure to tobacco smoke. Toxicol. Lett. 2020, 331, 57–64. [Google Scholar] [CrossRef]
- Okunieff, P.; Xu, J.; Hu, D.; Liu, W.; Zhang, L.; Morrow, G.; Pentland, A.; Ryan, J.L.; Ding, I. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 890–898. [Google Scholar] [CrossRef]
- Budagov, R.S.; Ul’ianova, L.P. Comparative analysis of proinflammatory cytokines in plasma of mice exposed to radiation or in combined radiation injury. Radiats. Biol. Radioecol. 2000, 40, 188–191. [Google Scholar]
- Warren, D.J.; Slørdal, L.; Moore, M.A. Tumor-necrosis factor induces cell cycle arrest in multipotential hematopoietic stem cells: A possible radioprotective mechanism. Eur. J. Haematol. 1990, 45, 158–163. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef]
- Kralik, S.F.; Ho, C.Y.; Finke, W.; Buchsbaum, J.C.; Haskins, C.P.; Shih, C.S. Radiation Necrosis in Pediatric Patients with Brain Tumors Treated with Proton Radiotherapy. AJNR Am. J. Neuroradiol. 2015, 36, 1572–1578. [Google Scholar] [CrossRef] [PubMed]

| Irradiation Group | ||||||
|---|---|---|---|---|---|---|
| Organ | 0 Gy | 7.5 Gy PBS | % Increase ↑ % Decrease ↓ | 7.5 Gy DS | % Increase ↑ % Decrease ↓ | |
| SOD activity (U/mg of proteins) | Erythrocytes | 27.29 ± 2.20 | 33.35 ± 2.53 | 22.2% ↑ | 33.68 ± 5.14 | 23.41% ↑ |
| Lung | 58.51 ± 3.89 | 103.14 ± 14.81 | 76.27% ↑ * | 119.96 ± 23.00 | 105.02% ↑ ** | |
| Heart | 87.11 ± 8.07 | 96.59 ± 10.45 | 10.88% ↑ | 61.00 ± 8.51 | 29.97% ↓ | |
| Skin | 125.61 ± 13.61 | 192.46 ± 26.59 | 53.22% ↑ | 201.45 ± 27.46 | 60.37% ↑ | |
| Irradiation Group | ||||||
|---|---|---|---|---|---|---|
| Organ | 0 Gy | 7.5 Gy PBS | % Increase ↑ % Decrease ↓ | 7.5 Gy DS | % Increase ↑ % Decrease ↓ | |
| GPx activity (nmol of GSSG/min/mg proteins) | Erythrocytes | 178.46 ± 5.66 | 261.73 ± 13.49 | 46.66% ↑ *** ### | 204.06 ± 12.13 | 14.34% ↑ ### |
| Lung | 1404.86 ± 118.57 | 1755.60 ± 224.37 | 24.96% ↑ | 1574.60 ± 111.07 | 12.08% ↑ | |
| Heart | 699.81 ± 60.83 | 721.15 ± 56.02 | 3.04% ↑ | 878.41 ± 76.2 | 25.52% ↑ | |
| Skin | 120.41 ± 16.24 | 194.12 ± 23.85 | 61.21% ↑ * | 146.32 ± 4.16 | 21.51% ↑ | |
| Irradiation Group | ||||||
|---|---|---|---|---|---|---|
| Organ | 0 Gy | 7.5 Gy PBS | % Increase ↑ % Decrease ↓ | 7.5 Gy DS | % Increase ↑ % Decrease ↓ | |
| CAT activity (U/mg proteins) | Erythrocytes | 3.50 ± 0.15 | 4.44 ± 0.36 | 25.42% ↑ *** ### | 3.07 ± 0.13 | 12.28% ↑ ### |
| Skin | 2.79 ± 0.35 | 3.62 ± 0.40 | 29.74% ↑ | 3.63 ± 0.65 | 30.10% ↑ | |
| Heart | 0.12 ± 0.01 | 0.09 ± 0.002 | 25.00% ↓ | 0.12 ± 0.003 | No change | |
| Lung | 0.24 ± 0.04 | 0.25 ± 0.05 | 4.16% ↑ | 0.35 ± 0.06 | 66.66% ↑ | |
| Irradiation Group | ||||||
|---|---|---|---|---|---|---|
| Organ | 0 Gy | 7.5 Gy PBS | % Increase ↑ % Decrease ↓ | 7.5 Gy DS | % Increase ↑ % Decrease ↓ | |
| GSSG (µmol/mg of proteins) | Erythrocytes | 12.98 ± 0.42 | 12.45 ± 1.13 | 4.08% ↓ | 13.35 ± 1.33 | 2.85% ↑ |
| Lung | 9.18 ± 0.95 | 16.63 ± 2.95 | 81.15% ↑ ** | 13.28 ± 1.63 | 44.66% ↑ | |
| Heart | 9.80 ± 1.04 | 18.96 ± 1.84 | 93.46% ↑ *** # | 12.68 ± 1.90 | 29.38% ↑ # | |
| Skin | 8.64 ± 0.74 | 8.99 ± 0.92 | 4.05% ↑ # | 14.22 ± 2.73 | 7.03% ↑ *** # | |
| Irradiation Group | ||||||
|---|---|---|---|---|---|---|
| Organ | 0 Gy | 7.5 Gy PBS | % Increase ↑ % Decrease ↓ | 7.5 Gy DS | % Increase ↑ % Decrease ↓ | |
| LPO (nmol/mg of proteins) | Erythrocytes | 57.12 ± 4.46 | 89.93 ± 9.63 | 57.44% ↑ *** | 68.76 ± 5.35 | 20.37% ↑ |
| Lung | 11.87 ± 1.43 | 16.12 ± 2.36 | 35.80% ↑ | 21.82 ± 3.77 | 83.82% ↑ ** | |
| Heart | 18.87 ± 1.26 | 22.74 ± 5.84 | 20.50% ↑ | 21.21 ± 0.91 | 12.40% ↑ | |
| Skin | below detection limit | |||||
| Irradiation Group | ||||||
|---|---|---|---|---|---|---|
| Organ | 0 Gy | 7.5 Gy PBS | % Increase ↑ % Decrease ↓ | 7.5 Gy DS | % Increase ↑ % Decrease ↓ | |
| Carbonyls (nmol/mg of proteins) | Erythrocytes | 202.62 ± 5.72 | 273.36 ± 8.58 | 34.91% ↑ *** | 234.85 ± 7.06 | 15.90% ↑ * |
| Lung | 11.92 ± 0.93 | 11.25 ± 1.46 | 5.62% ↑ | 10.73 ± 1.08 | 9.98% ↓ | |
| Heart | 7.04 ± 0.47 | 7.35 ± 1.04 | 4.40% ↑ | 6.53 ± 0.88 | 7.24% ↓ | |
| Skin | below detection limit | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaouni, S.; Leduc, A.; Pouzoulet, F.; De Marzi, L.; Megnin-Chanet, F.; Stefan, D.; Habrand, J.-L.; Sichel, F.; Laurent, C. Biological Effects of Scattered Versus Scanned Proton Beams on Normal Tissues in Total Body Irradiated Mice: Survival, Genotoxicity, Oxidative Stress and Inflammation. Antioxidants 2020, 9, 1170. https://doi.org/10.3390/antiox9121170
Chaouni S, Leduc A, Pouzoulet F, De Marzi L, Megnin-Chanet F, Stefan D, Habrand J-L, Sichel F, Laurent C. Biological Effects of Scattered Versus Scanned Proton Beams on Normal Tissues in Total Body Irradiated Mice: Survival, Genotoxicity, Oxidative Stress and Inflammation. Antioxidants. 2020; 9(12):1170. https://doi.org/10.3390/antiox9121170
Chicago/Turabian StyleChaouni, Samia, Alexandre Leduc, Frédéric Pouzoulet, Ludovic De Marzi, Frédérique Megnin-Chanet, Dinu Stefan, Jean-Louis Habrand, François Sichel, and Carine Laurent. 2020. "Biological Effects of Scattered Versus Scanned Proton Beams on Normal Tissues in Total Body Irradiated Mice: Survival, Genotoxicity, Oxidative Stress and Inflammation" Antioxidants 9, no. 12: 1170. https://doi.org/10.3390/antiox9121170
APA StyleChaouni, S., Leduc, A., Pouzoulet, F., De Marzi, L., Megnin-Chanet, F., Stefan, D., Habrand, J.-L., Sichel, F., & Laurent, C. (2020). Biological Effects of Scattered Versus Scanned Proton Beams on Normal Tissues in Total Body Irradiated Mice: Survival, Genotoxicity, Oxidative Stress and Inflammation. Antioxidants, 9(12), 1170. https://doi.org/10.3390/antiox9121170