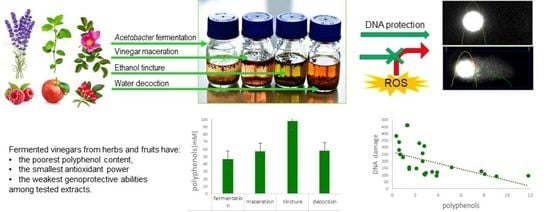
Fermented Vinegars from Apple Peels, Raspberries, Rosehips, Lavender, Mint, and Rose Petals: The Composition, Antioxidant Power, and Genoprotective Abilities in Comparison to Acetic Macerates, Decoctions, and Tinctures
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Extracts
2.2.1. Fermented Vinegars
2.2.2. Acetic Macerates
2.2.3. Decoctions
2.2.4. Tinctures
2.3. Total Polyphenol Content—Fast Blue BB Assay
2.4. Polyphenol Identification with HPLC
2.5. HPLC Analysis of Anthocyanins
2.6. Vitamin C Content with Tillman’s Method
2.7. Carotenoid Content
2.8. Metal Content
2.9. Ferric Reducing Antioxidant Power (FRAP) Assay
2.10. Cell Culture and Treatment
2.11. Evaluation of Cell Viability with MTT Assay
2.12. Evaluation of DNA Damage with Comet Assay
2.13. Statistical Analysis
3. Results
3.1. Composition
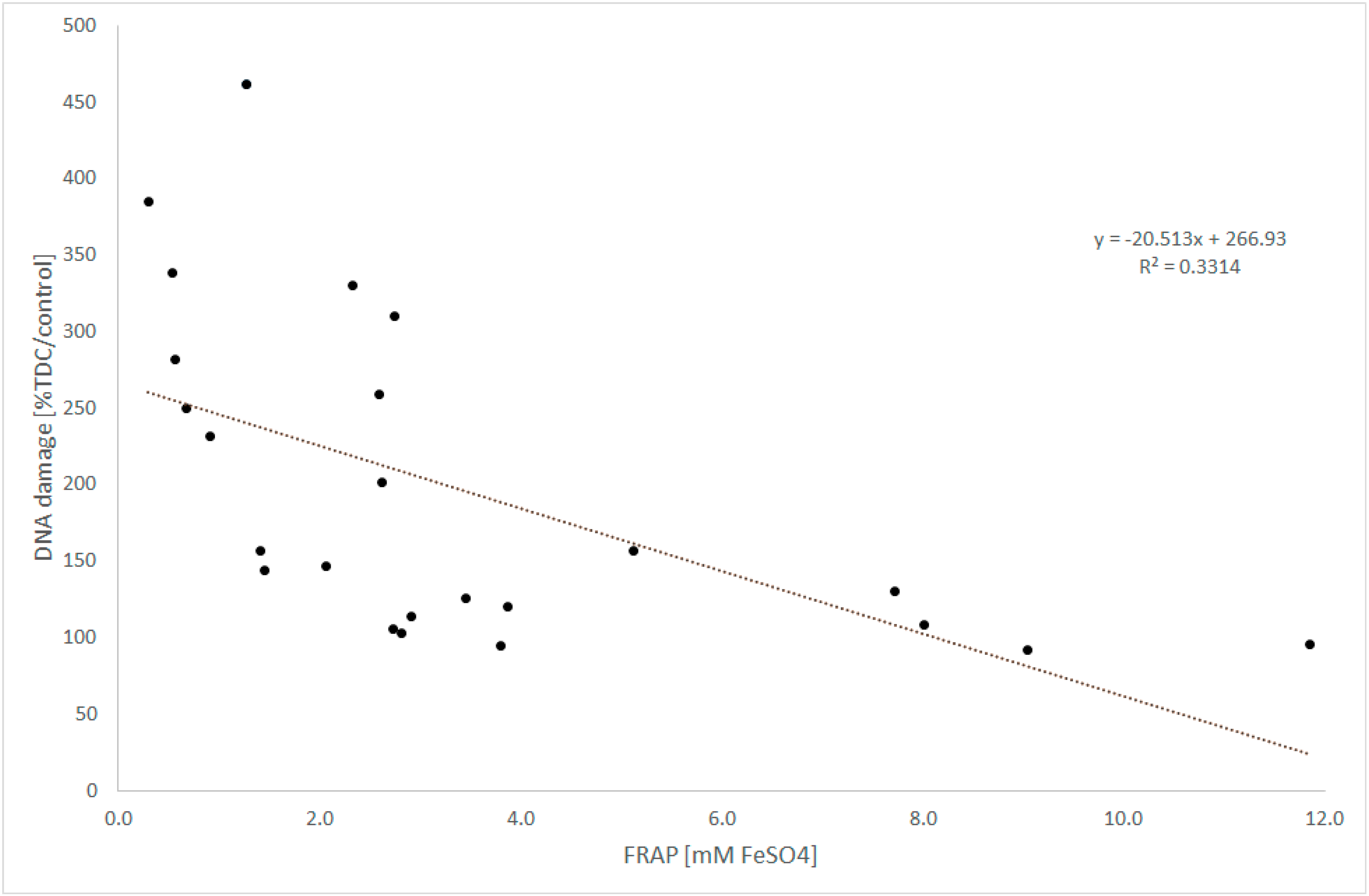
3.2. Antioxidant Power
3.3. Viability of Cells
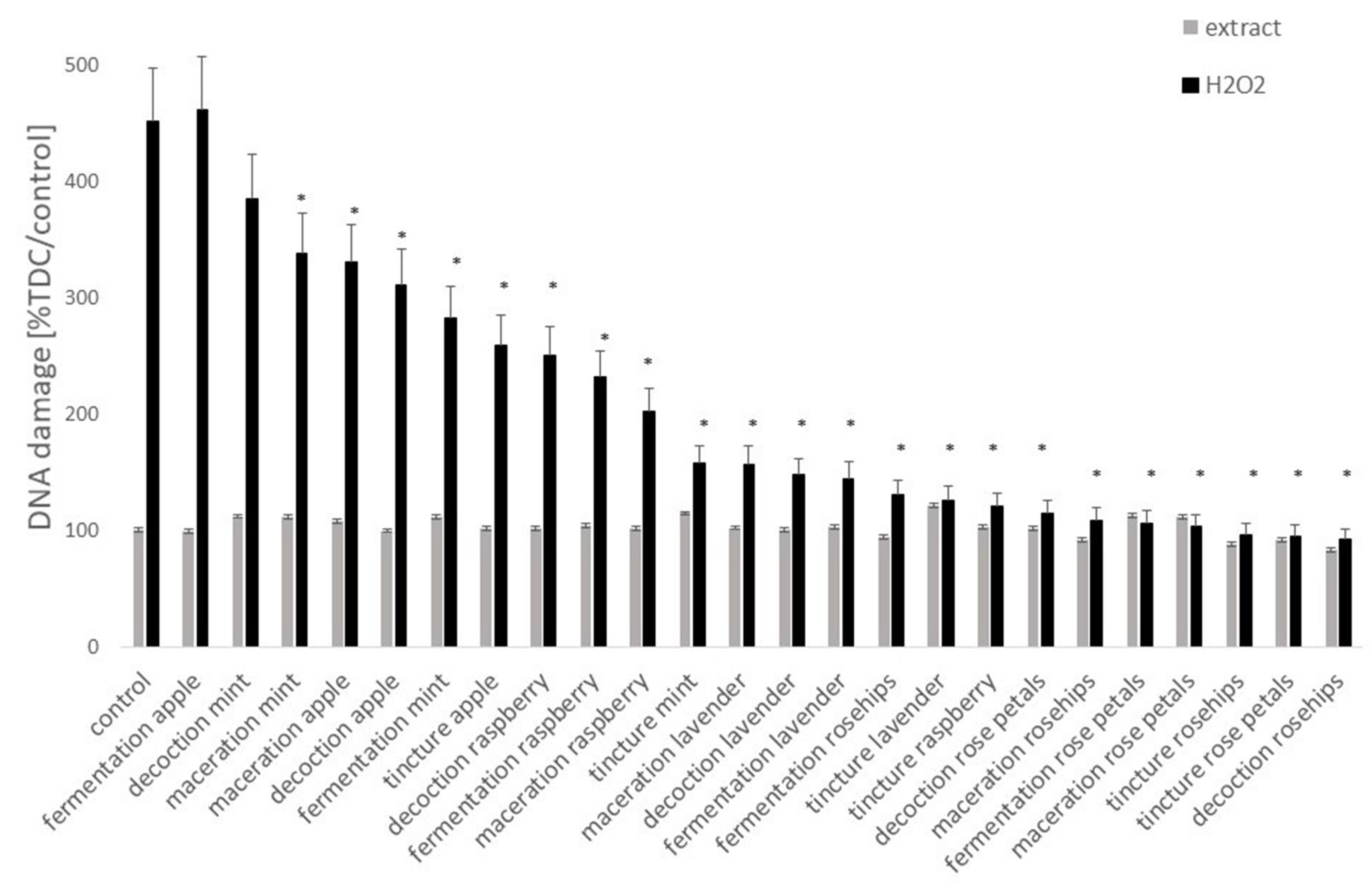
3.4. DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional properties of vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Štornik, A.; Skok, B.; Trček, J. Comparison of cultivable acetic acid bacterial microbiota in organic and conventional AppleCider vinegar. Food Technol. Biotechnol. 2016, 54, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Mitsonis, P.; Siorokos, I.; Dermishaj, E.; Samaras, Y. The physicochemical properties and antioxidant capacities of commercial and homemade Greek vinegars. Acta Sci. Pol. Technol. Aliment. 2019, 18, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; de Carvalho-Silva, S.; Trovatti Uetanabaro, A.P.; Villas-Boas, S.G. Vinegar metabolomics: An explorative study of commercial balsamic vinegars using gas chromatography-mass spectrometry. Metabolites 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, D.; Serafin, V.J.; Shah, A. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef]
- Gopal, J.; Anthonydhason, V.; Muthu, M.; Gansukh, E.; Jung, S.; Chul, S.; Iyyakkannu, S. Authenticating apple cider vinegar’s home remedy claims: Antibacterial, antifungal, antiviral properties and cytotoxicity aspect. Nat. Prod. Res. 2017, 11, 1–5. [Google Scholar] [CrossRef]
- Halima, B.H.; Sonia, G.; Sarra, K.; Houda, B.J.; Fethi, B.S.; Abdallah, A. Apple cider vinegar attenuates oxidative stress and reduces the risk of obesity in high-fat-fed male wistar rats. J. Med. Food 2018, 21, 70–80. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Güler, M.; Özgül, C.; Saydam, G.; Küçükayaz, M.; Sözbir, E. Apple cider vinegar modulates serum lipid profile, erythrocyte, kidney, and liver membrane oxidative stress in ovariectomized mice fed high cholesterol. J. Membr. Biol. 2014, 247, 667–673. [Google Scholar] [CrossRef]
- Iizuka, M.; Tani, M.; Kishimoto, Y.; Saita, E.; Toyozaki, M.; Kondo, K. Inhibitory effects of balsamic vinegar on LDL oxidation and lipid accumulation in THP-1 macrophages. J. Nutr. Sci. Vitaminol. 2010, 56, 421–427. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; de Moraes, W.M.A.M.; da Silva, G.A.R.; Prestes, J.; Schoenfeld, B.J. Vinegar (acetic acid) intake on glucose metabolism: A narrative review. Clin. Nutr. ESPEN 2019, 32, 1–7. [Google Scholar] [CrossRef]
- Shishehbor, F.; Mansoori, A.; Shirani, F. Vinegar consumption can attenuate postprandial glucose and insulin responses; a systematic review and meta-analysis of clinical trials. Diabetes Res. Clin. Pract. 2017, 127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Petsiou, E.I.; Mitrou, P.I.; Raptis, S.A.; Dimitriadis, G.D. Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutr. Rev. 2014, 72, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.B. Simple and rapid method for the analysis of phenolic compounds in beverages and grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Maślanka, A.; Szewczyk, A.; Muszyńska, B. Physiologically active compounds in four species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef]
- Weber, N.; Veberic, R.; Miculic-Petkovsek, M.; Stampar, F.; Koron, D.; Munda, A.; Jakopic, J. Metabolite accumulation in strawberry (Fragaria x ananassa Duch.) fruits and runners in response to Colletotrichum nymphaeae infection. Physiol. Mol. Plant Pathol. 2015, 92, 119–129. [Google Scholar] [CrossRef]
- Kalemba-Drożdż, M.; Cierniak, A.; Cichoń, I. Berry fruit juices protect lymphocytes against DNA damage and ROS formation induced with heterocyclic aromatic amine PhIP. J. Berry Res. 2020, 10, 95–113. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Chu, W.K.; Benzie, I.F. Antioxidants in fruits and vegetables: A study of cellular availability and direct effects on human DNA. Biosci. Biotechnol. Biochem. 2006, 70, 2551–2555. [Google Scholar] [CrossRef]
- Kalemba-Drożdż, M.; Cierniak, A. Antioxidant and genoprotective properties of extracts from edible flowers. J. Food Nutr. Res. 2019, 58, 42–50. [Google Scholar] [CrossRef]
- Hollman, P.C.; van Trijp, J.M.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997, 114, 139–140. [Google Scholar] [CrossRef]
- Nguyen, V.; Tang, J.; Oroudjev, E.; Lee, C.J.; Marasigan, C.; Wilson, L.; Ayoub, G. Cytotoxic effects of bilberry extract on MCF7-GFP-tubulin breast cancer cells. J. Med. Food 2010, 13, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 6. [Google Scholar] [CrossRef]
- Bhave, A.; Schulzova, V.; Chmelarova, H.; Mrnka, L.; Hajslova, J. Assessment of rosehips based on the content of their biologically active compounds. J. Food Drug Anal. 2017, 25, 681–690. [Google Scholar] [CrossRef]
- Abacı, Z.T.; Zarıfıkhosroshahı, M.; Kafkas, E.; Sevindik, E. Characterization of bioactive compounds in rosehip species from East Anatolia region of Turkey. Ital. J. Food Sci. 2016, 28, 314–325. [Google Scholar]
- Cunja, V.; Mikulic-Petkovsek, M.; Weber, N.; Jakopic, J.; Zupan, A.; Veberic, R.; Stampar, F.; Schmitzer, V. Fresh from the ornamental garden: Hips of selected rose cultivars rich in phytonutrients. J. Food Sci. 2016, 81, C369–C379. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Pecio, Ł.; Łoś, R.; Malm, A.; Rzymowska, J.; Oleszek, W. Multidirectional characterisation of chemical composition and health-promoting potential of Rosa rugosa hips. Nat. Prod. Res. 2017, 31, 667–671. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from polana raspberry (Rubus idaeus L.) fruit and juice—In vitro study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E. The effects of organic and conventional farm management and harvest time on the polyphenol content in different raspberry cultivars. Food Chem. 2019, 301, 125295. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- USDA. United States Department of Agriculture Food Composition Databases. Available online: https://www.nal.usda.gov/fnic (accessed on 21 December 2019).
- EU Commission Regulation. No 420/2011 of 29 April 2011 Amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2011, 111/3, 3–6. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Z.; Hao, W.; Zhu, H.; Liang, N.; Liu, J.; Zhang, C.; Ma, K.Y.; He, W.; Yang, Y.; et al. Vinegars but not acetic acid are effective in reducing plasma cholesterol in hamsters fed a high-cholesterol diet. Food Funct. 2020, 11, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J. Food systems approach to cancer prevention. Crit. Rev. Food Sci. Nutr. 2015, 57, 2573–2588. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Kausarb, H.; Munagalaa, R.; Singhd, I.P.; Gupta, R. Lung cancer inhibitory activity of dietary berries and berry polyphenolics. J. Berry Res. 2017, 6, 105–114. [Google Scholar] [CrossRef]
- López-Romero, D.; Izquierdo-Vega, J.A.; Morales-González, J.A.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M.; Betanzos-Cabrera, G.; Alvarez-Gonzalez, I.; Morales-González, Á.; Madrigal-Santillán, E. Evidence of some natural products with antigenotoxic effects. Part 2: Plants, vegetables, and natural resin. Nutrients 2018, 10, 1954. [Google Scholar] [CrossRef]
- Wang, F.; Miao, M.; Xia, H.; Yang, L.G.; Wang, S.K.; Sun, G.J. Antioxidant activities of aqueous extracts from 12 Chinese edible flowers in vitro and in vivo. Food Nutr. Res. 2017, 61, 1265324. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Yang, Y.; Bakri, M.; Chen, Q.; Xin, X.; Aisa, H.A. A LC/QTOF-MS/MS application to investigate chemical compositions in a fraction with protein tyrosine phosphatase 1B inhibitory activity from Rosa rugosa flowers. Phytochem. Anal. 2013, 24, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J. Preventive therapy for cancer. Lancet Oncol. 2017, 18, e472–e482. [Google Scholar] [CrossRef]
- Lansky, E.; Harrison, G.; Froom, P.; Jiang, W. Pomegranate (Punica granatum) pure chemical show possible synergistic inhibition of human PC-3 prostate cancer cell invasion across MatrigelTM. Investig. New Drugs 2018, 23, 121–122. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Akbulut, M.B.; Guneser, M.B.; Eldeniz, A.U. Effects of fruit vinegars on root dentin microhardness and roughness. J. Conserv. Dent. 2019, 22, 97–101. [Google Scholar] [CrossRef]
- Lachat, C. Dietary species richness as a measure of food biodiversity and nutritional quality of diets. Proc. Natl. Acad. Sci. USA 2018, 115, 127–132. [Google Scholar] [CrossRef]
- Rodriguez-Casado, A. The health potential of fruits and vegetables phytochemicals: Notable examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2019. [Google Scholar] [CrossRef]


| Plant | Extract | pH ± S.D. | Polyphenols [mg/100 g] ± S.D. | Vitamin C [mg/100 g] ± S.D. | Carotenoids [µg/100 g] ± S.D. |
|---|---|---|---|---|---|
| food vinegar | 2.51 ± 0.13 | 0.56 ± 0.34 | ND | 0.01 ± 0.01 | |
| Apple peels | Fermented | 2.93 ± 0.17 | 22.07 ± 1.84 | ND | 0.13 ± 0.08 |
| Macerate | 2.51 ± 0.08 | 38.87 ± 0.98 | ND | 0.06 ± 0.03 | |
| Tincture | 4.72 ± 0.14 | 59.74 ± 2.67 | ND | 0.19 ± 0.06 | |
| Decoction | 4.11 ± 0.17 | 47.24 ± 1.54 | ND | 0.12 ± 0.08 | |
| Raspberries | Fermented | 2.82 ± 0.19 | 46.44 ± 2.54 | 1.67 ± 1.16 | 0.23 ± 0.07 |
| Macerate | 2.7 ± 0.13 | 56.98 ± 3.00 | 5.00 ± 1.67 | 0.54 ± 0.02 | |
| Tincture | 3.85 ± 0.01 | 97.57 ± 2.06 | 1.67 ± 1.00 | 0.35 ± 0.04 | |
| Decoction | 3.98 ± 0.14 | 57.48 ± 1.87 | ND | 0.57 ± 0.67 | |
| Rosehips | Fermented | 2.85 ± 0.13 | 231.32 ± 8.09 | 88.33 ± 2.33 | 0.27 ± 0.02 |
| Macerate | 2.67 ± 0.10 | 303.38 ± 6.67 | 118.33 ± 1.00 | 0.27 ± 0.03 | |
| Tincture | 4.23 ± 0.09 | 227.42 ± 6.11 | 141.67 ± 3.67 | 0.29 ± 0.11 | |
| Decoction | 4.91 ± 1.00 | 449.68 ± 9.33 | 256.67 ± 5.43 | 1.17 ± 0.12 | |
| Mint | Fermented | 2.79 ± 0.13 | 8.94 ± 1.00 | ND | 0.04 ± 0.01 |
| Macerate | 2.59 ± 0.05 | 10.68 ± 0.67 | ND | 0.18 ± 0.00 | |
| Tincture | 6.96 ± 0.11 | 8.90 ± 1.22 | ND | 0.03 ± 0.00 | |
| Decoction | 6.98 ± 1.06 | 4.76 ± 0.89 | ND | 0.31 ± 0.02 | |
| Lavender | Fermented | 2.86 ± 0.30 | 20.84 ± 1.50 | ND | 0.13 ± 0.04 |
| Macerate | 2.61 ± 0.18 | 20.29 ± 1.12 | ND | 0.06 ± 0.05 | |
| Tincture | 6.99 ± 0.02 | 46.63 ± 1.89 | ND | ND | |
| Decoction | 6.59 ± 0.16 | 30.10 ± 1.51 | ND | 0.17 ± 0.01 | |
| Rose petals | Fermented | 2.93 ± 0.07 | 97.17 ± 1.45 | ND | 0.09 ± 0.01 |
| Macerate | 2.78 ± 0.13 | 138.51 ± 3.99 | ND | 0.09 ± 0.00 | |
| Tincture | 6.87 ± 0.20 | 199.81 ± 3.33 | ND | 0.29 ± 0.01 | |
| Decoction | 6.77 ± 0.09 | 109.82 ± 4.67 | ND | 0.11 ± 0.01 |
| Plant | Apple Peels | Raspberries | Rosehips Flesh | Mint Leaves | Lavender Flowers | Rose Petals | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extract | Fermented | Macerate | Tincture | Decoction | Fermented | Macerate | Tincture | Decoction | Fermented | Vinegar | Tincture | Decoction | Fermented | Macerate | Tincture | Decoction | Fermented | Macerate | Tincture | Decoction | Fermented | Macerate | Tincture | Decoction |
| PHENOLIC ACIDS | [mg/100mL] | |||||||||||||||||||||||
| caffeic acid | 0.07 ± 0.04 | 0.38 ± 0.02 | 0.18 ± 0.00 | 0.06 ± 0.00 | 5.08 ± 0.22 | 2.98 ± 0.06 | 4.47 ± 0.14 | 3.71 ± 0.07 | ||||||||||||||||
| chlorogenic acid | 0.63 ± 0.00 | 0.77 ± 0.29 | 2.08 ± 0.13 | 0.82 ± 0.01 | 0.38 ± 0.02 | 0.43 ± 0.02 | 2.32 ± 0.19 | 1.65 ± 0.10 | 0.13 ± 0.04 | 0.17 ± 0.02 | 0.04 ± 0.00 | 7.23 ± 0.59 | 3.86 ± 0.16 | |||||||||||
| cinnamic acid | 0.39 ± 0.02 | 0.59 ± 0.02 | 0.92 ± 0.01 | |||||||||||||||||||||
| elagic acid | 2.11 ± 0.07 | 3.76 ± 0.51 | 12.45 ± 1.05 | 4.31 ± 0.15 | 0.62 ± 0.07 | 2.21 ± 0.32 | 1.28 ± 0.05 | |||||||||||||||||
| ferulic acid | 2.63 ± 0.12 | 3.13 ± 0.43 | 3.26 ± 0.12 | 3.48 ± 0.07 | ||||||||||||||||||||
| isoferulic acid | 1.85 ± 0.01 | 2.03 ± 0.14 | 2.99 ± 0.22 | 2.73 ± 0.05 | ||||||||||||||||||||
| gallic acid | 0.24 ± 0.02 | 0.26 ± 0.03 | 0.10 ± 0.01 | 0.34 ± 0.03 | 3.09 ± 0.02 | 1.30 ± 0.02 | 3.71 ± 0.41 | 10.60 ± 0.32 | 0.12 ± 0.04 | 0.20 ± 0.01 | 0.04 ± 0.01 | 0.13 ± 0.01 | 0.41 ± 0.02 | 1.26 ± 1.16 | 4.53 ± 0.10 | |||||||||
| p-coumaric acid | 1.27 ± 0.02 | 0.59 ± 0.02 | 3.04 ± 0.29 | 1.31 ± 0.10 | ||||||||||||||||||||
| p-hydroxybenzoic acid | 1.08 ± 0.01 | 1.09 ± 0.01 | ||||||||||||||||||||||
| protocatechuic acid | 0.34 ± 0.01 | 0.37 ± 0.00 | 0.13 ± 0.00 | 0.39 ± 0.01 | 0.48 ± 0.07 | 0.95 ± 0.03 | 0.91 ± 0.04 | 1.04 ± 0.03 | ||||||||||||||||
| rosmarinic acid | 0.38 ± 0.01 | 0.70 ± 0.01 | 0.23 ± 0.01 | 1.73 ± 0.04 | 4.61 ± 0.33 | 1.33 ± 0.14 | ||||||||||||||||||
| vanillic acid | 0.07 ± 0.04 | 0.08 ± 0.00 | 3.66 ± 0.12 | 2.70 ± 0.06 | 4.53 ± 0.05 | 3.63 ± 0.11 | 0.22 ± 0.01 | 0.89 ± 0.03 | ||||||||||||||||
| PHENOLIC ACIDS SUM | 1.21 ± 0.11 | 1.40 ± 0.03 | 2.32 ± 0.11 | 1.55 ± 0.03 | 2.59 ± 0.08 | 4.71 ± 0.11 | 13.36 ± 1.01 | 5.35 ± 0.16 | 3.47 ± 0.02 | 2.34 ± 0.08 | 8.24 ± 0.53 | 13.53 ± 0.33 | 1.46 ± 0.40 | 2.30 ± 0.07 | 0.93 ± 0.01 | 0.46 ± 0.01 | 13.22 ± 0.23 | 12.95 ± 0.46 | 20.44 ± 0.26 | 15.79 ± 0.21 | 1.27 ± 0.02 | 1.21 ± 0.02 | 12.41 ± 0.58 | 9.70 ± 0.15 |
| FLAVONOIDS | [mg/100mL] | |||||||||||||||||||||||
| apigenin | 2.03 ± 0.01 | 5.17 ± 0.18 | 2.45 ± 0.12 | 3.92 ± 0.22 | ||||||||||||||||||||
| catechin | 8.32 ± 0.13 | 12.04 ± 1.04 | 58.90 ± 0.69 | 47.20 ± 0.21 | 2.28 ± 0.40 | 3.46 ± 0.07 | 0.60 ± 0.03 | 1.98 ± 0.20 | 8.36 ± 0.52 | 2.76 ± 0.05 | ||||||||||||||
| epicatechin | 7.80 ± 0.55 | 8.99 ± 0.28 | 13.28 ± 0.45 | 9.49 ± 0.52 | 7.03 ± 0.05 | 1.12 ± 0.04 | ||||||||||||||||||
| kaempferol | 5.70 ± 0.05 | 6.64 ± 0.05 | 5.54 ± 0.08 | 3.06 ± 0.02 | 6.39 ± 0.06 | 6.23 ± 0.14 | ||||||||||||||||||
| kaempferol 3-O-galactoside | 0.25 ± 0.01 | 1.57 ± 0.04 | 6.37 ± 0.21 | 0.89 ± 0.06 | 20.11 ± 1.69 | 11.63 ± 0.86 | 4.79 ± 0.03 | |||||||||||||||||
| kaempferol 3-O-glucoside | 1.52 ± 0.08 | 2.33 ± 0.17 | 1.39 ± 0.08 | 8.46 ± 1.02 | 12.87 ± 0.86 | 5.88 ± 0.83 | ||||||||||||||||||
| kaempferol 7-ramnoside | 1.33 ± 0.02 | 1.29 ± 0.05 | 1.20 ± 0.02 | 2.99 ± 0.09 | 4.60 ± 0.22 | 9.49 ± 1.16 | 3.84 ± 0.15 | |||||||||||||||||
| luteolin | 0.63 ± 0.02 | |||||||||||||||||||||||
| isoquercetin | 1.51 ± 0.12 | 0.54 ± 0.01 | 1.69 ± 0.04 | 0.71 ± 0.02 | 0.29 ± 0.08 | 0.57 ± 0.02 | 1.79 ± 0.04 | 0.92 ± 0.01 | ||||||||||||||||
| quercetin | 1.74 ± 0.09 | 1.95 ± 0.11 | 1.66 ± 0.02 | 1.83 ± 0.09 | 1.57 ± 0.04 | 2.54 ± 0.08 | 1.79 ± 0.00 | 1.29 ± 0.01 | 1.46 ± 0.05 | 1.36 ± 0.01 | 5.69 ± 0.04 | 7.72 ± 0.08 | 16.41 ± 0.36 | 6.28 ± 0.19 | ||||||||||
| quercetin 3-O-arabinofuranoside | 2.21 ± 0.22 | 3.69 ± 0.31 | 7.57 ± 0.02 | 4.06 ± 0.10 | ||||||||||||||||||||
| quercetin 3-O-glucuronide | 7.63 ± 0.14 | 8.51 ± 0.15 | 19.85 ± 0.65 | 10.92 ± 0.32 | ||||||||||||||||||||
| quercetin 3-O-ramnoside | 10.94 ± 0.69 | 12.86 ± 0.55 | 21.28 ± 2.48 | 11.02 ± 0.13 | ||||||||||||||||||||
| quercetin 7-O-glucoside | 1.15 ± 0.02 | 1.14 ± 0.02 | 1.17 ± 0.04 | 1.13 ± 0.00 | 5.14 ± 0.33 | |||||||||||||||||||
| quercitrin | 2.03 ± 0.02 | 4.37 ± 0.02 | 3.52 ± 0.06 | 2.97 ± 0.02 | 1.73 ± 0.04 | |||||||||||||||||||
| rutoside | 1.82 ± 0.17 | 2.91 ± 0.14 | 5.70 ± 0.28 | 2.87 ± 0.04 | 0.77 ± 0.04 | 5.83 ± 0.08 | 3.40 ± 0.02 | 0.27 ± 0.02 | 0.23 ± 0.01 | 5.92 ± 0.25 | 1.56 ± 0.03 | 2.28 ± 0.11 | 4.55 ± 0.36 | 3.15 ± 0.04 | ||||||||||
| FLAVONOIDS SUM | 33.65 ± 0.95 | 39.46 ± 0.73 | 71.02 ± 2.62 | 40.91 ± 0.65 | 1.40 ± 0.02 | 12.83 ± 0.54 | 20.34 ± 1.10 | 11.92 ± 0.21 | 10.35 ± 0.21 | 18.47 ± 1.04 | 69.71 ± 0.90 | 54.93 ± 0.46 | 2.28 ± 0.40 | 5.75 ± 0.07 | 5.99 ± 0.20 | 4.43 ± 0.20 | 0.29 ± 0.08 | 0.57 ± 0.02 | 19.68 ± 0.26 | 2.49 ± 0.03 | 18.22 ± 0.36 | 59.05 ± 2.06 | 61.18 ± 1.89 | 26.69 ± 0.90 |
| ANTHOCYANINS | [mg/100mL] | |||||||||||||||||||||||
| kuromanin | 1.57 ± 0.00 | 2.03 ± 0.01 | 1.90 ± 0.00 | |||||||||||||||||||||
| idaein | 2.23 ± 0.02 | 2.88 ± 0.01 | 7.14 ± 0.03 | 3.24 ± 0.00 | ||||||||||||||||||||
| keracyanin | 0.92 ± 0.04 | 1.44 ± 0.02 | 5.35 ± 0.03 | 2.42 ± 0.05 | ||||||||||||||||||||
| ANTHOCYANINS SUM | 1.57 ± 0.00 | 2.03 ± 0.01 | 1.90 ± 0.00 | 3.15 ± 0.04 | 4.32 ± 0.02 | 12.49 ± 0.03 | 5.66 ± 0.05 | |||||||||||||||||
| TOTAL POLYPHENOLS | 34.86 ± 0.96 | 42.43 ± 0.73 | 75.36 ± 2.62 | 44.35 ± 0.65 | 7.14 ± 0.08 | 21.86 ± 0.08 | 46.19 ± 0.55 | 22.93 ± 1.49 | 13.82 ± 0.26 | 20.81 ± 0.21 | 77.95 ± 1.04 | 68.46 ± 1.04 | 3.74 ± 0.57 | 8.05 ± 0.57 | 6.92 ± 0.10 | 4.89 ± 0.02 | 13.51 ± 0.20 | 13.52 ± 0.24 | 40.12 ± 0.46 | 18.28 ± 0.37 | 19.49 ± 0.21 | 60.26 ± 2.06 | 73.59 ± 1.98 | 36.38 ± 0.91 |
| Plant | Extract | Ca [mg/100 mL] ± S.D. | Fe [mg/100 mL] ± S.D. | Mg [mg/100 mL] ± S.D. | Zn [mg/100 mL] ± S.D. | Al [μg/L] ± S.D. | Ni [μg/L] ± S.D. | Cd [μg/L] ± S.D. | Cr [μg/L] ± S.D. |
|---|---|---|---|---|---|---|---|---|---|
| Apple peels | Fermented | 1.50 ± 0.01 | 0.02 ± 0.00 | 2.45 ± 0.02 | ND | 68.1 ± 1.4 | 13.9 ± 1.1 | 0.3 ± 0.0 | 202.2 ± 12.2 |
| Macerate | 5.10 ± 0.00 | 0.06 ± 0.00 | 2.31 ± 0.01 | 0.01 ± 0.00 | 80.2 ± 2.3 | 13.3 ± 1.0 | 0.3 ± 0.0 | 192.9 ± 10.9 | |
| Tincture | 0.35 ± 0.01 | ND | 1.55 ± 0.00 | ND | 34.2 ± 1.9 | 17.2 ± 1.5 | ND | 167.9 ± 11.6 | |
| Decoction | 1.02 ± 0.01 | ND | 1.77 ± 0.01 | 0.01 ± 0.00 | 33.1 ± 2.6 | 10.9 ± 1.0 | 0.1 ± 0.0 | 66.9 ± 6.6 | |
| Raspberries | Fermented | 4.27 ± 0.01 | 0.04 ± 0.00 | 5.42 ± 0.00 | 0.03 ± 0.00 | 40.6 ± 2.1 | 62.7 ± 2.5 | 4.5 ± 0.0 | 68.1 ± 5.9 |
| Macerate | 6.66 ± 0.02 | 0.04 ± 0.00 | 5.75 ± 0.00 | 0.06 ± 0.00 | 64.8 ± 3.4 | 63.2 ± 2.3 | 7.8 ± 0.1 | 133.1 ± 4.3 | |
| Tincture | 2.47 ± 0.00 | 0.01 ± 0.00 | 5.38 ± 0.00 | 0.07 ± 0.00 | 62.4 ± 2.8 | 103.2 ± 5.6 | 2.8 ± 0.0 | 184.8 ± 15.0 | |
| Decoction | 3.78 ± 0.00 | 0.01 ± 0.00 | 5.17 ± 0.02 | 0.07 ± 0.00 | 49.8 ± 5.8 | 73.9 ± 7.6 | 11.1 ± 0.8 | 158.8 ± 11.3 | |
| Rosehips | Fermented | ND | ND | ND | ND | 1.0 ± 0.1 | 6.9 ± 1.1 | ND | 38.3 ± 4.0 |
| Macerate | ND | ND | ND | ND | 1.1 ± 0.0 | 7.1 ± 0.6 | ND | 57.0 ± 1.9 | |
| Tincture | 0.01 ± 0.00 | ND | 0.02 ± 0.00 | ND | 5.4 ± 0.1 | 6.8 ± 0.4 | ND | 25.4 ± 1.0 | |
| Decoction | 20.43 ± 0.09 | 0.21 ± 0.00 | 16.52 ± 0.10 | 0.07 ± 0.00 | 61.0 ± 2.7 | 72.0 ± 3.2 | 0.2 ± 0.0 | 39.2 ± 1.1 | |
| Mint | Fermented | 5.39 ± 0.01 | 0.04 ± 0.00 | 1.34 ± 0.00 | 0.02 ± 0.00 | 64.6 ± 4.6 | 11.9 ± 0.9 | 0.5 ± 0.0 | 138.5 ± 15.9 |
| Macerate | 9.70 ± 0.04 | 0.21 ± 0.00 | 1.89 ± 0.01 | 0.04 ± 0.00 | 175.0 ± 7.8 | 12.9 ± 0.8 | 3.1 ± 0.1 | 157.1 ± 13.0 | |
| Tincture | 0.18 ± 0.00 | ND | 0.61 ± 0.00 | 0.01 ± 0.00 | 40.8 ± 3.9 | 10.0 ± 0.9 | ND | 167.5 ± 12.6 | |
| Decoction | 3.00 ± 0.00 | ND | 0.89 ± 0.00 | 0.01 ± 0.00 | 6.7 ± 2.4 | 6.6 ± 0.1 | 0.2 ± 0.0 | 93.5 ± 5.6 | |
| Lavender | Fermented | 6.04 ± 0.01 | 0.08 ± 0.00 | 4.66 ± 0.01 | 0.05 ± 0.00 | 297.5 ± 11.9 | 18.3 ± 2.0 | 0.5 ± 0.0 | 133.0 ± 3.3 |
| Macerate | 6.71 ± 0.00 | 0.09 ± 0.00 | 6.42 ± 0.02 | 0.06 ± 0.00 | 304.3 ± 9.8 | 20.8 ± 1.9 | 0.4 ± 0.0 | 162.9 ± 7.8 | |
| Tincture | 0.18 ± 0.00 | ND | 0.95 ± 0.00 | 0.01 ± 0.00 | 43.2 ± 3.6 | 23.2 ± 1.0 | ND | 190.7 ± 8.9 | |
| Decoction | 1.43 ± 0.00 | 0.01 ± 0.00 | 1.97 ± 0.00 | 0.01 ± 0.00 | 7.3 ± 3.8 | 8.3 ± 0.3 | ND | 119.1 ± 10.0 | |
| Rose petals | Fermented | ND | ND | ND | 0.05 ± 0.00 | ND | ND | 0.3 ± 0.0 | 146.5 ± 7.9 |
| Macerate | 3.17 ± 0.01 | 0.03 ± 0.00 | 1.72 ± 0.00 | 0.01 ± 0.00 | 25.6 ± 2.7 | 20.0 ± 0.4 | ND | 156.0 ± 8.0 | |
| Tincture | 0.27 ± 0.00 | ND | 1.01 ± 0.00 | 0.01 ± 0.00 | 23.3 ± 2.7 | 17.7 ± 0.2 | ND | 113.9 ± 4.6 | |
| Decoction | 0.27 ± 0.01 | ND | 1.02 ± 0.00 | ND | 24.9 ± 2.3 | 16.4 ± 1.1 | ND | ND |
| FRAP [mM FeSO4] ± S.D. | ||||
|---|---|---|---|---|
| Extract Type | ||||
| Plant | Fermented Vinegar | Acetic Macerate | Tincture | Decoction |
| Apple peels | 1.9 ± 0.9 | 2.3 ± 0.2 | 2.6 ± 0.3 | 2.7 ± 0.3 |
| Raspberries | 0.9 ± 0.9 | 2.6 ± 0.3 | 3.9 ± 0.3 | 0.7 ± 0.1 |
| Rosehips flesh | 7.7 ± 2.6 | 8.0 ± 0.9 | 11.8 ± 0.9 | 9.0 ± 0.8 |
| Mint leaves | 0.6 ± 0.3 | 0.5 ± 0.2 | 5.1 ± 0.5 | 0.3 ± 0.1 |
| Lavender flower | 1.5 ± 0.6 | 1.4 ± 0.1 | 3.4 ± 0.3 | 2.1 ± 0.3 |
| Rose petals | 2.7 ± 0.9 | 2.8 ± 0.3 | 3.8 ± 0.4 | 2.9 ± 0.4 |
| Cell Viability [%Control] ± S.D. | ||||||
|---|---|---|---|---|---|---|
| Plant Material | ||||||
| Extract | Apple | Raspberry | Rosehips | Mint | Lavender | Rose Petals |
| Fermentation | 82 ± 3 | 91 ± 8 | 86 ± 3 | 79 ± 0 | 80 ± 0 | 73 ± 1 |
| Maceration | 88 ± 2 | 90 ± 4 | 106 ± 9 | 85 ± 1 | 79 ± 4 | 93 ± 2 |
| Tinctures | 103 ± 0 | 105 ± 2 | 107 ± 0 | 89 ± 0 | 91 ± 3 | 103 ± 0 |
| Decoctions | 99 ± 2 | 93 ± 1 | 98 ± 0 | 89 ± 0 | 93 ± 0 | 100 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalemba-Drożdż, M.; Kwiecień, I.; Szewczyk, A.; Cierniak, A.; Grzywacz-Kisielewska, A. Fermented Vinegars from Apple Peels, Raspberries, Rosehips, Lavender, Mint, and Rose Petals: The Composition, Antioxidant Power, and Genoprotective Abilities in Comparison to Acetic Macerates, Decoctions, and Tinctures. Antioxidants 2020, 9, 1121. https://doi.org/10.3390/antiox9111121
Kalemba-Drożdż M, Kwiecień I, Szewczyk A, Cierniak A, Grzywacz-Kisielewska A. Fermented Vinegars from Apple Peels, Raspberries, Rosehips, Lavender, Mint, and Rose Petals: The Composition, Antioxidant Power, and Genoprotective Abilities in Comparison to Acetic Macerates, Decoctions, and Tinctures. Antioxidants. 2020; 9(11):1121. https://doi.org/10.3390/antiox9111121
Chicago/Turabian StyleKalemba-Drożdż, Małgorzata, Inga Kwiecień, Agnieszka Szewczyk, Agnieszka Cierniak, and Agata Grzywacz-Kisielewska. 2020. "Fermented Vinegars from Apple Peels, Raspberries, Rosehips, Lavender, Mint, and Rose Petals: The Composition, Antioxidant Power, and Genoprotective Abilities in Comparison to Acetic Macerates, Decoctions, and Tinctures" Antioxidants 9, no. 11: 1121. https://doi.org/10.3390/antiox9111121
APA StyleKalemba-Drożdż, M., Kwiecień, I., Szewczyk, A., Cierniak, A., & Grzywacz-Kisielewska, A. (2020). Fermented Vinegars from Apple Peels, Raspberries, Rosehips, Lavender, Mint, and Rose Petals: The Composition, Antioxidant Power, and Genoprotective Abilities in Comparison to Acetic Macerates, Decoctions, and Tinctures. Antioxidants, 9(11), 1121. https://doi.org/10.3390/antiox9111121