Polyphenolic and Methylxanthine Bioaccessibility of Cocoa Bean Shell Functional Biscuits: Metabolomics Approach and Intestinal Permeability through Caco-2 Cell Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples and Formulation of the Model Food (Biscuits)
2.3. Preparation of Polyphenolic Extracts, In Vitro Digests, and Non-Digested Extracts
2.4. Analytical Determinations
2.4.1. Total Phenolic, Flavonoid, and Tannin Contents
2.4.2. Radical Scavenging Activity
2.4.3. In Vitro α-Glucosidase Inhibition Capacity
2.4.4. Liquid Chromatography–High-Resolution Mass Spectrometry (LC–HRMS) Analysis
2.5. Cell Culture and Assays
2.5.1. Cell Viability Assay
2.5.2. Permeability Study through Caco-2 Cell Monolayers
2.5.3. Study of the Caco-2 Cell Monolayer Integrity by Means of the Lucifer Yellow Assay
2.6. Statistical Analyses
3. Results
3.1. Total Phenolic, Tannin, and Flavonoid Contents
3.2. Biofunctional Characteristics—Radical Scavenging Activity and α-Glucosidase Inhibition Capacity
3.3. In Vitro Bioaccessibility of Bioactive Compounds and Functional Characteristics through Spectrophotometric Analysis Results
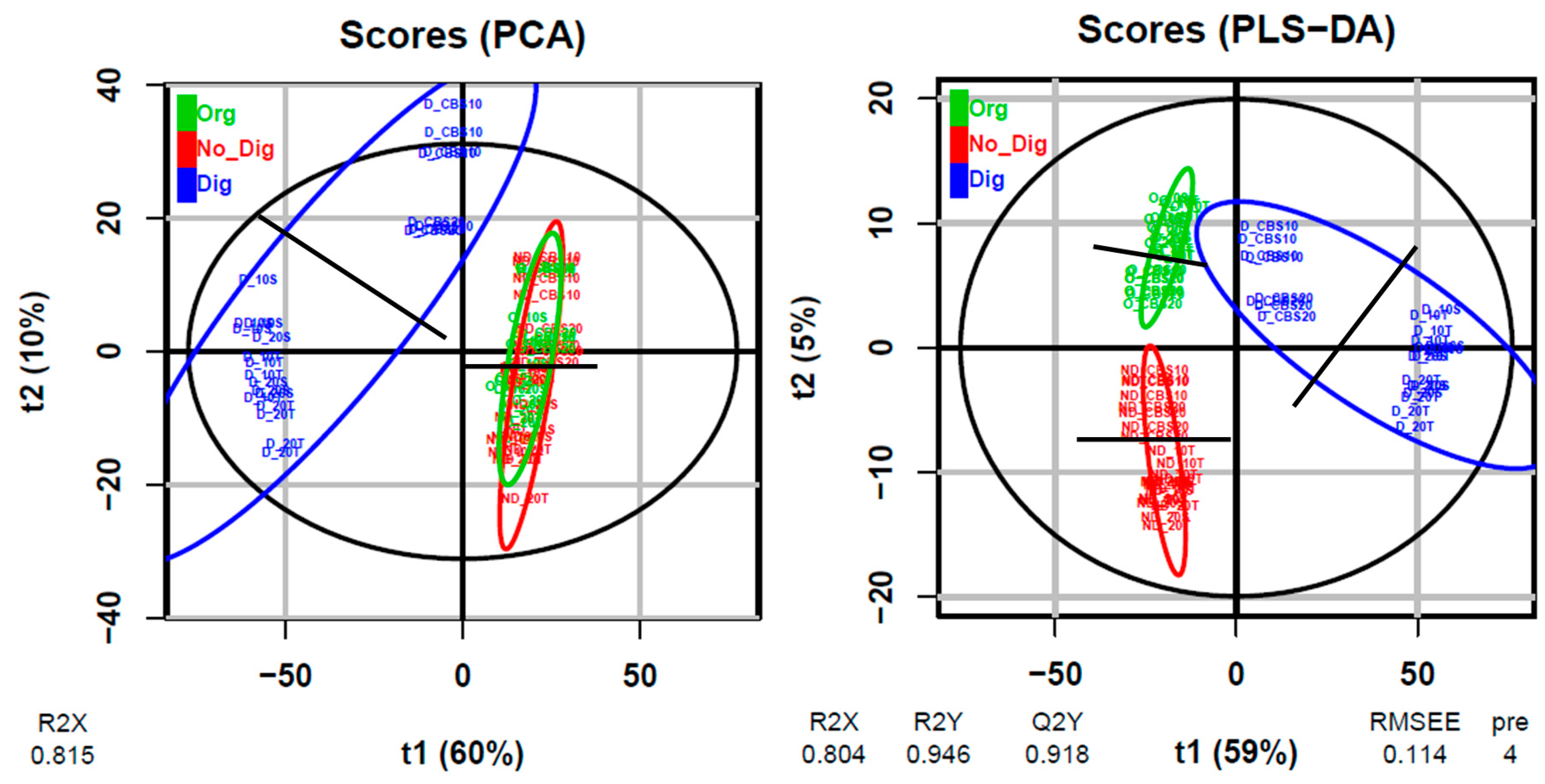
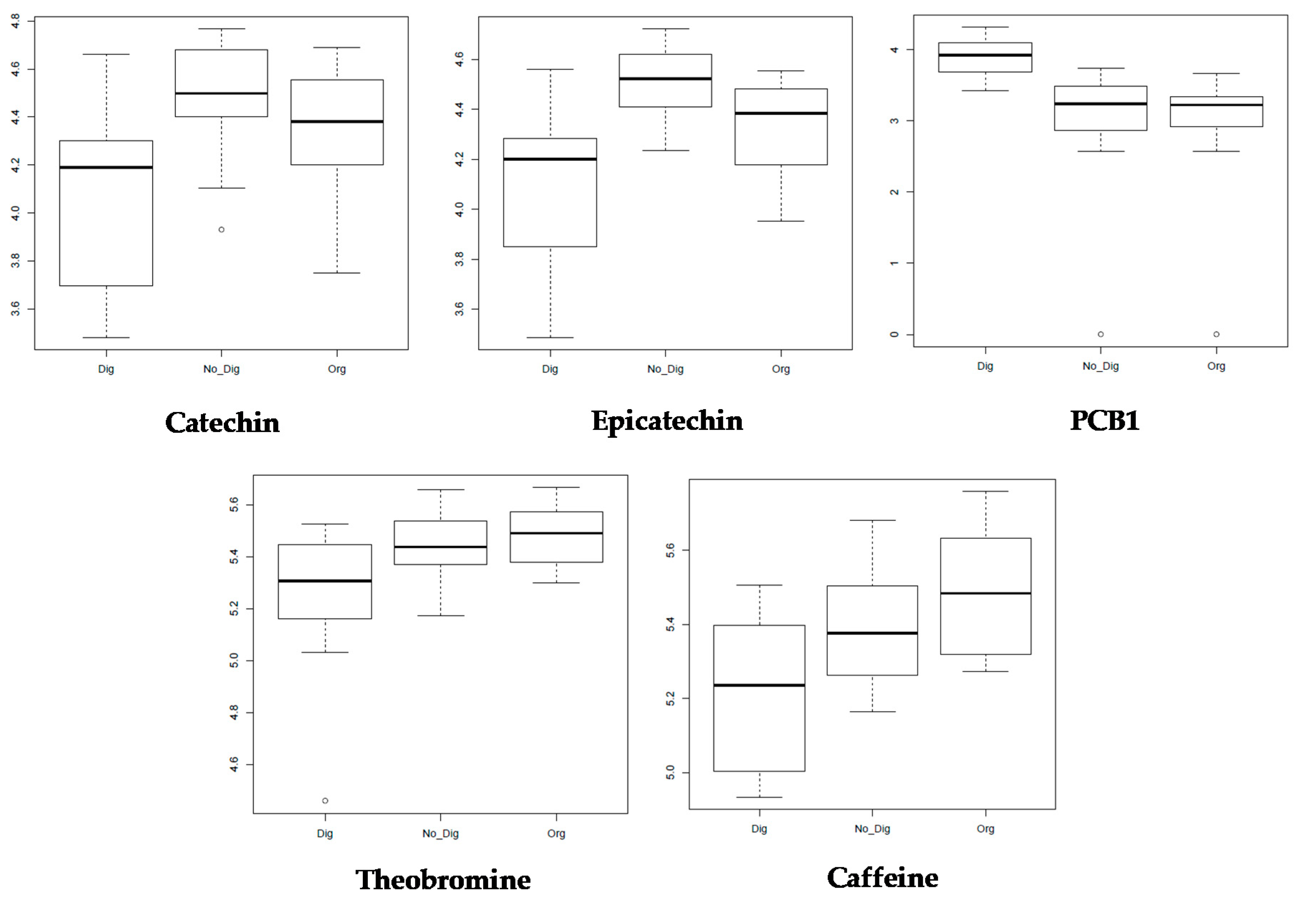
3.4. LC–HRMS and Metabolomics Analysis for Single Compound Bioaccessibility Evaluation
3.5. In Vitro Intestinal Permeability of Bioactive Compounds through Caco-2 Cell Monolayers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Galanakis, C.M.E. Food Waste Recovery: Processing Technologies and Industrial Techniques; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: London, UK, 2015. [Google Scholar]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Assessment of volatile fingerprint by HS-SPME/GC-qMS and E-nose for the classification of cocoa bean shells using chemometrics. Food Res. Int. 2019, 123, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Analytical dataset on volatile compounds of cocoa bean shells from different cultivars and geographical origins. Data Brief 2019, 25, 104268. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.A.; Ramos, S. Cocoa polyphenols in oxidative stress: Potential health implications. J. Funct. Foods 2016, 27, 570–588. [Google Scholar] [CrossRef]
- Martin, M.Á.; Goya, L.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Garti, N. Delivery and Controlled Release of Bioactives in Foods and Nutraceuticals; CRC Press: Boca Raton, FL, USA; Woodhead Publishing Limited: Cambridge, UK, 2008. [Google Scholar]
- Roy, S.; Chikkerur, J.; Roy, S.C.; Dhali, A.; Kolte, A.P.; Sridhar, M.; Samanta, A.K. Tagatose as a potential nutraceutical: Production, properties, biological roles, and applications. J. Food Sci. 2018, 83, 2699–2709. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Orden, D.; Stévigny, C.; Zeppa, G.; Bertolino, M. Physical Properties and Consumer Evaluation of Cocoa Bean Shell-Functionalized Biscuits Adapted for Diabetic Consumers by the Replacement of Sucrose with Tagatose. Foods 2020, 9, 814. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Botta, C.; Ferrocino, I.; Giordano, M.; Bertolino, M.; Dolci, P.; Cannoni, M.; Cocolin, L. Dynamics and biodiversity of bacterial and yeast communities during fermentation of cocoa beans. Appl. Environ. Microbiol. 2018, 84, e01118–e01164. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In vitro bioaccessibility and functional properties of phenolic pompounds from enriched beverages based on cocoa bean shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed electric field assisted extraction of bioactive compounds from cocoa bean shell and coffee silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Coïsson, J.D.; Arlorio, M. Cocoa hulls polyphenols stabilized by microencapsulation as functional ingredient for bakery applications. Food Res. Int. 2019, 115, 511–518. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G. Effects of particle size and extraction methods on cocoa bean shell functional beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Giacomoni, F.; Le Corguillé, G.; Monsoor, M.; Landi, M.; Pericard, P.; Pétéra, M.; Duperier, C.; Tremblay-Franco, M.; Martin, J.-F.; Jacob, D. Workflow4Metabolomics: A collaborative research infrastructure for computational metabolomics. Bioinformatics 2015, 31, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Souard, F.; Delporte, C.; Stoffelen, P.; Thévenot, E.A.; Noret, N.; Dauvergne, B.; Kauffmann, J.-M.; Van Antwerpen, P.; Stevigny, C. Metabolomics fingerprint of coffee species determined by untargeted-profiling study using LC-HRMS. Food Chem. 2018, 245, 603–612. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111. [Google Scholar] [CrossRef]
- Kosińska, A.; Andlauer, W. Cocoa polyphenols are absorbed in Caco-2 cell model of intestinal epithelium. Food Chem. 2012, 135, 999–1005. [Google Scholar] [CrossRef]
- Van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Hochkogler, C.M.; Somoza, V.; Del Castillo, M.D. Biscuits with no added sugar containing stevia, coffee fibre and fructooligosaccharides modifies α-glucosidase activity and the release of GLP-1 from HuTu-80 cells and serotonin from Caco-2 cells after in vitro digestion. Nutrients 2017, 9, 694. [Google Scholar] [CrossRef]
- Altin, G.; Gültekin-Özgüven, M.; Ozcelik, B. Liposomal dispersion and powder systems for delivery of cocoa hull waste phenolics via Ayran (drinking yoghurt): Comparative studies on in-vitro bioaccessibility and antioxidant capacity. Food Hydrocoll. 2018, 81, 364–370. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Hilary, S.; Tomás-Barberán, F.A.; Martinez-Blazquez, J.A.; Kizhakkayil, J.; Souka, U.; Al-Hammadi, S.; Habib, H.; Ibrahim, W.; Platat, C. Polyphenol characterisation of Phoenix dactylifera L.(date) seeds using HPLC-mass spectrometry and its bioaccessibility using simulated in-vitro digestion/Caco-2 culture model. Food Chem. 2020, 311, 125969. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Ova, G. Evaluation of Cocoa Bean Hulls as a Fat Replacer On Functional Cake Production. Turk. J. Agric. Food Sci. Technol. 2018, 6, 1043–1050. [Google Scholar] [CrossRef]
- Mertes, G. Safety and efficacy of acarbose in the treatment of type 2 diabetes: Data from a 5-year surveillance study. Diabetes Res. Clin. Pract. 2001, 52, 193–204. [Google Scholar] [CrossRef]
- Philippe, J.; Raccah, D. Treating type 2 diabetes: How safe are current therapeutic agents? Int. J. Clin. Pract. 2009, 63, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Borotto Dalla Vecchia, S.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Bertolino, M.; Ghirardello, D.; Zeppa, G. Polyphenols Bioaccessibility and Antioxidant Activity in Yogurt Enriched with Cocoa Bean Shell. In Proceedings of the 16th ISANH World Congress on Antioxidants, Porto, Portugal, 29 June–1 July 2016; Volume 3, p. 75. [Google Scholar]
- Kan, L.; Oliviero, T.; Verkerk, R.; Fogliano, V.; Capuano, E. Interaction of bread and berry polyphenols affects starch digestibility and polyphenols bio-accessibility. J. Funct. Foods 2020, 68, 103924. [Google Scholar] [CrossRef]
- Paz-Yépez, C.; Peinado, I.; Heredia, A.; Andrés, A. Lipids digestibility and polyphenols release under in vitro digestion of dark, milk and white chocolate. J. Funct. Foods 2019, 52, 196–203. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A. Influence of Food Matrix on the Bioaccessibility of Fruit Polyphenolic Compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A. In vitro digestion of meat-and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Reguant, J.; Romero, M.-P.; Macia, A.; Motilva, M.-J. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.P.; George, J.C.; Janle, E.M.; Mattes, R.D.; Rudolph, R.; Matusheski, N.V.; Ferruzzi, M.G. Influence of chocolate matrix composition on cocoa flavan-3-ol bioaccessibility in vitro and bioavailability in humans. J. Agric. Food Chem. 2009, 57, 9418–9426. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Campiglia, P.; Giannetti, D.; Novellino, E. Simulated gastrointestinal digestion, intestinal permeation and plasma protein interaction of white, green, and black tea polyphenols. Food Chem. 2015, 169, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Mendes, T.M.N.; Murayama, Y.; Yamaguchi, N.; Sampaio, G.R.; Fontes, L.C.B.; da Silva Torres, E.A.F.; Tamura, H.; Yonekura, L. Guaraná (Paullinia cupana) catechins and procyanidins: Gastrointestinal/colonic bioaccessibility, Caco-2 cell permeability and the impact of macronutrients. J. Funct. Foods 2019, 55, 352–361. [Google Scholar] [CrossRef]
- Shimizu, M. Modulation of intestinal functions by food substances. Food Nahr. 1999, 43, 154–158. [Google Scholar] [CrossRef]
- Deprez, S.; Mila, I.; Huneau, J.-F.; Tome, D.; Scalbert, A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid. Redox Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, W.; Xia, M.; Pan, S.; Xu, X. Study of structure and permeability relationship of flavonoids in Caco-2 cells. Nutrients 2017, 9, 1301. [Google Scholar] [CrossRef]
- Xie, Y.; Kosińska, A.; Xu, H.; Andlauer, W. Milk enhances intestinal absorption of green tea catechins in in vitro digestion/Caco-2 cells model. Food Res. Int. 2013, 53, 793–800. [Google Scholar] [CrossRef]
- Alvarez, A.I.; Real, R.; Pérez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Sarria, B.; Goya, L.; Bravo-Clemente, L.; Mateos, R. Experimental confounding factors affecting stability, transport and metabolism of flavanols and hydroxycinnamic acids in Caco-2 cells. Food Res. Int. 2020, 129, 108797. [Google Scholar] [CrossRef] [PubMed]
- Bonvehı, J.S.; Coll, F.V. Protein quality assessment in cocoa husk. Food Res. Int. 1999, 32, 201–208. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, Y.; Deng, S.; Zhu, F.; Liu, Q.; Wang, R.; Li, T.; Cai, H.; Wan, X.; Xie, Z. Using caffeine and free amino acids to enhance the transepithelial transport of catechins in Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5477–5485. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takahashi, H.; Suzuki, K.; Hirano, A.; Kamei, M.; Goto, T.; Takahashi, N.; Kawada, T. Theobromine enhances absorption of cacao polyphenol in rats. Biosci. Biotechnol. Biochem. 2014, 78, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Juaristi, M.; Sarria, B.; Martínez-López, S.; Bravo Clemente, L.; Mateos, R. Flavanol Bioavailability in Two Cocoa Products with Different Phenolic Content. A Comparative Study in Humans. Nutrients 2019, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Yasuda, A.; Natsume, M.; Takizawa, T.; Nakamura, T.; Terao, J. Bioavailability of (-)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic. Res. 2000, 33, 635–641. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Testa, G.; Sottero, B.; Poli, G.; Zeppa, G.; Biasi, F. A Dietary Mixture of Oxysterols Induces In Vitro Intestinal Inflammation through TLR2/4 Activation: The Protective Effect of Cocoa Bean Shells. Antioxidants 2019, 8, 151. [Google Scholar] [CrossRef]
- Smetanova, L.; Stetinova, V.; Kholova, D.; Kvetina, J.; Smetana, J.; Svoboda, Z. Caco-2 cells and Biopharmaceutics Classification System (BCS) for prediction of transepithelial transport of xenobiotics (model drug: Caffeine). Neuroendocrinol. Lett. 2009, 30, 101. [Google Scholar]
- Monente, C.; Ludwig, I.A.; Stalmach, A.; de Peña, M.P.; Cid, C.; Crozier, A. In vitro studies on the stability in the proximal gastrointestinal tract and bioaccessibility in Caco-2 cells of chlorogenic acids from spent coffee grounds. Int. J. Food Sci. Nutr. 2015, 66, 657–664. [Google Scholar] [CrossRef]
- Martínez-López, S.; Sarriá, B.; Gómez-Juaristi, M.; Goya, L.; Mateos, R.; Bravo-Clemente, L. Theobromine, caffeine, and theophylline metabolites in human plasma and urine after consumption of soluble cocoa products with different methylxanthine contents. Food Res. Int. 2014, 63, 446–455. [Google Scholar] [CrossRef]
- Mumford, G.; Benowitz, N.; Evans, S.; Kaminski, B.; Preston, K.; Sannerud, C.; Silverman, K.; Griffiths, R. Absorption rate of methylxanthines following capsules, cola and chocolate. Eur. J. Clin. Pharmacol. 1996, 51, 319–325. [Google Scholar] [CrossRef] [PubMed]

| Papp × 106 (cm/s) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catechin | Epicatechin | PCB1 | Caffeine | Theobromine | |||||||||
| CBS10 | 30.47 | ± | 3.64 a | 8.89 | ± | 0.39 c | n.d. | 42.22 | ± | 0.91 c | 41.89 | ± | 0.61 d |
| CBS20 | 22.83 | ± | 1.07 bc | 4.68 | ± | 0.13 d | n.d. | 45.00 | ± | 2.13 c | 49.14 | ± | 0.48 c |
| S10 | 25.35 | ± | 2.84 b | 15.92 | ± | 0.69 a | n.d. | 69.53 | ± | 0.42 a | 58.84 | ± | 0.58 b |
| S20 | 9.05 | ± | 0.60 d | 3.28 | ± | 0.15 e | n.d. | 62.46 | ± | 0.36 b | 61.02 | ± | 1.21 b |
| T10 | 19.73 | ± | 2.10 c | 11.33 | ± | 0.32 b | n.d. | 66.66 | ± | 4.47 a | 58.80 | ± | 2.35 b |
| T20 | 20.88 | ± | 3.10 bc | 4.44 | ± | 0.36 d | n.d. | 61.99 | ± | 2.67 b | 65.80 | ± | 1.80 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo-Poveda, O.; Barbosa-Pereira, L.; El Khattabi, C.; Youl, E.N.H.; Bertolino, M.; Delporte, C.; Pochet, S.; Stévigny, C. Polyphenolic and Methylxanthine Bioaccessibility of Cocoa Bean Shell Functional Biscuits: Metabolomics Approach and Intestinal Permeability through Caco-2 Cell Models. Antioxidants 2020, 9, 1164. https://doi.org/10.3390/antiox9111164
Rojo-Poveda O, Barbosa-Pereira L, El Khattabi C, Youl ENH, Bertolino M, Delporte C, Pochet S, Stévigny C. Polyphenolic and Methylxanthine Bioaccessibility of Cocoa Bean Shell Functional Biscuits: Metabolomics Approach and Intestinal Permeability through Caco-2 Cell Models. Antioxidants. 2020; 9(11):1164. https://doi.org/10.3390/antiox9111164
Chicago/Turabian StyleRojo-Poveda, Olga, Letricia Barbosa-Pereira, Charaf El Khattabi, Estelle N.H. Youl, Marta Bertolino, Cédric Delporte, Stéphanie Pochet, and Caroline Stévigny. 2020. "Polyphenolic and Methylxanthine Bioaccessibility of Cocoa Bean Shell Functional Biscuits: Metabolomics Approach and Intestinal Permeability through Caco-2 Cell Models" Antioxidants 9, no. 11: 1164. https://doi.org/10.3390/antiox9111164
APA StyleRojo-Poveda, O., Barbosa-Pereira, L., El Khattabi, C., Youl, E. N. H., Bertolino, M., Delporte, C., Pochet, S., & Stévigny, C. (2020). Polyphenolic and Methylxanthine Bioaccessibility of Cocoa Bean Shell Functional Biscuits: Metabolomics Approach and Intestinal Permeability through Caco-2 Cell Models. Antioxidants, 9(11), 1164. https://doi.org/10.3390/antiox9111164






