Plant Volatile, Phenylacetaldehyde Targets Breast Cancer Stem Cell by Induction of ROS and Regulation of Stat3 Signal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Mammnosphere Formation Assay
2.3. Cell Proliferation
2.4. Caspase-3/7 Assay
2.5. Annexin V/PI Assay and Analysis of Cell Apoptosis
2.6. Colony Formation and Migration Assay
2.7. CD44+/CD24−Expression Assay
2.8. Measurement of ROS Using DCFDA (2′, 7′-Dichlorofluorescein Diacetate)
2.9. Real-Time RT-qPCR
2.10. ALDEFLUOR Assay
2.11. Immunoblot Blotting
2.12. Electrophoretic Mobility Shift Assays (EMSA)
2.13. Small Interfering RNA (siRNA)
2.14. Tumor Xenografts
2.15. Statistical Analysis
3. Results
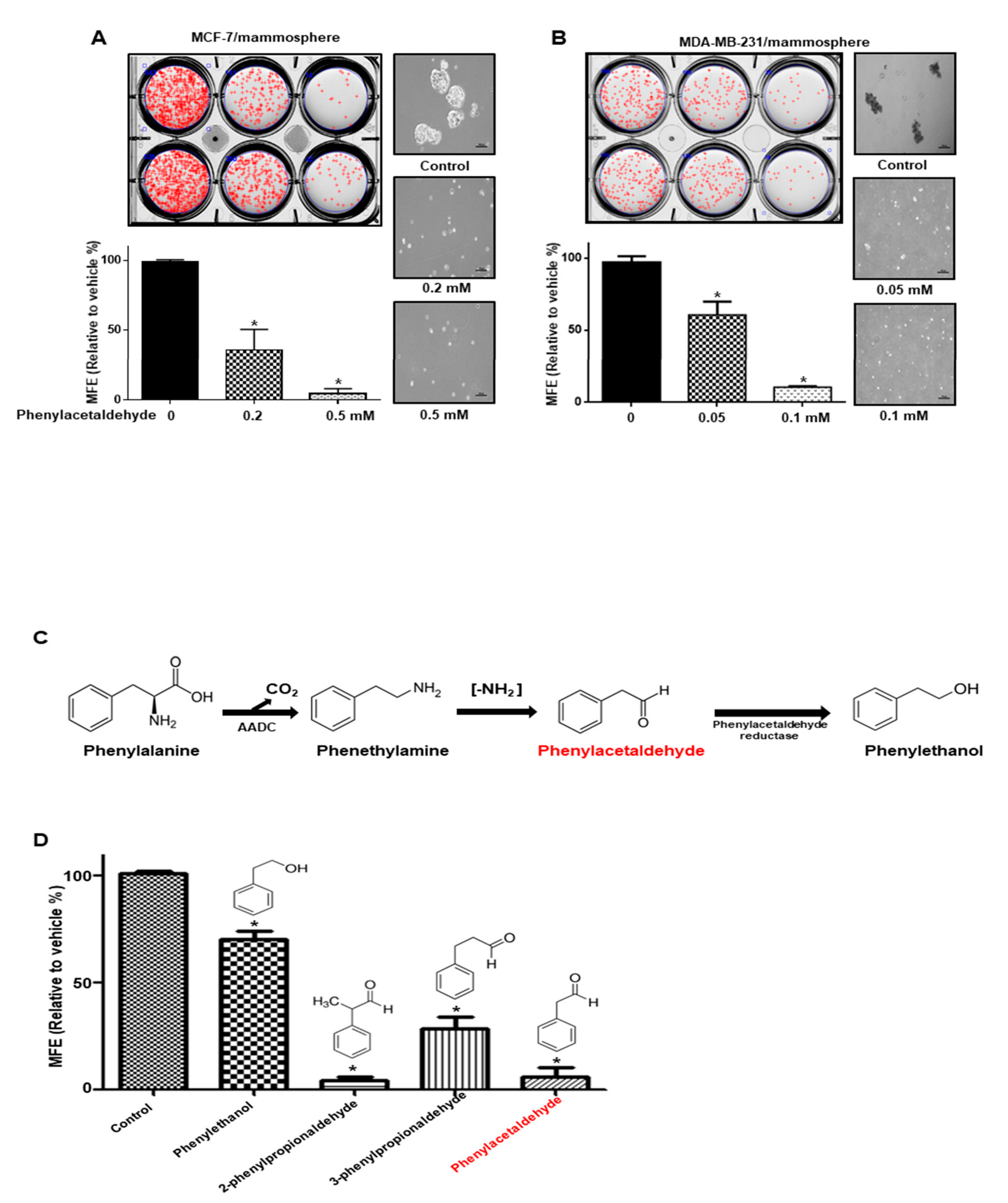
3.1. Screening of the Effect of Volatile Organic Compounds (VOCs) Derived from Plants on Mammosphere Formation
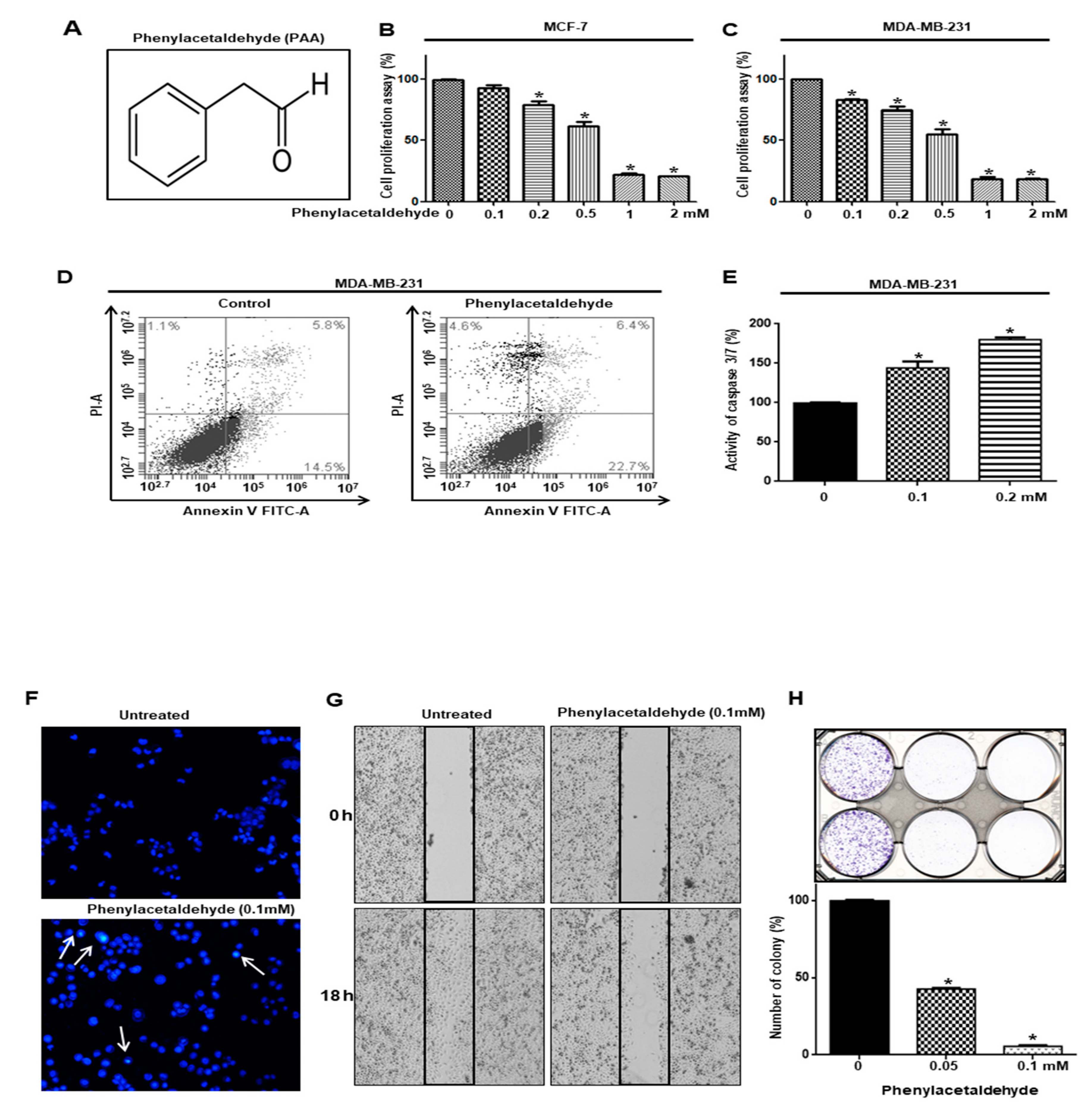
3.2. PAA Inhibits Cell Proliferation and Increased Apoptosis of Breast Cancer
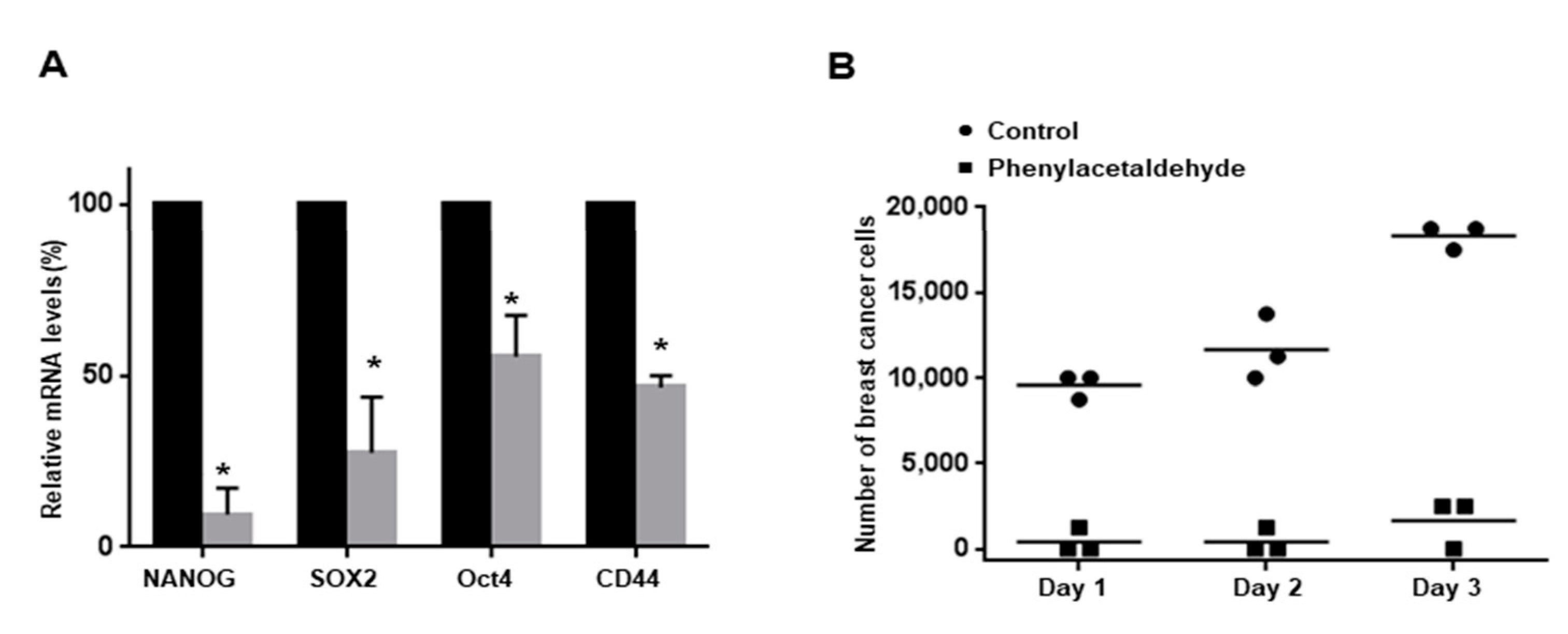
3.3. PAA Inhibits Tumor Growth in In Vivo
3.4. PAA Inhibits Breast CSCs
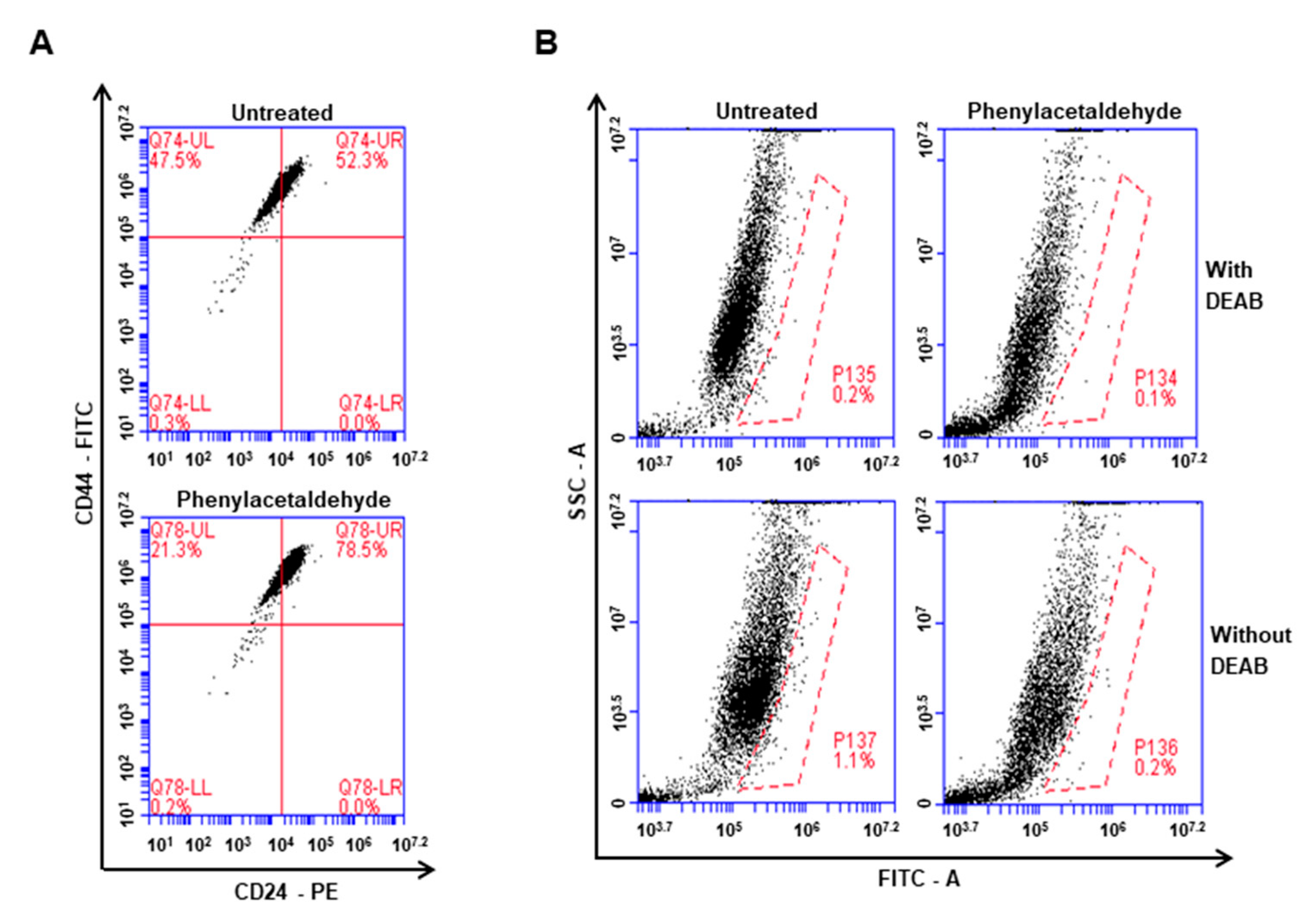
3.5. The PAA Reduced CD44+/CD24−-Expressing Population and ALDH-Positive Cells
3.6. PAA Inhibits CSC-Specific Gene Expression and Proliferation of Mammospheres
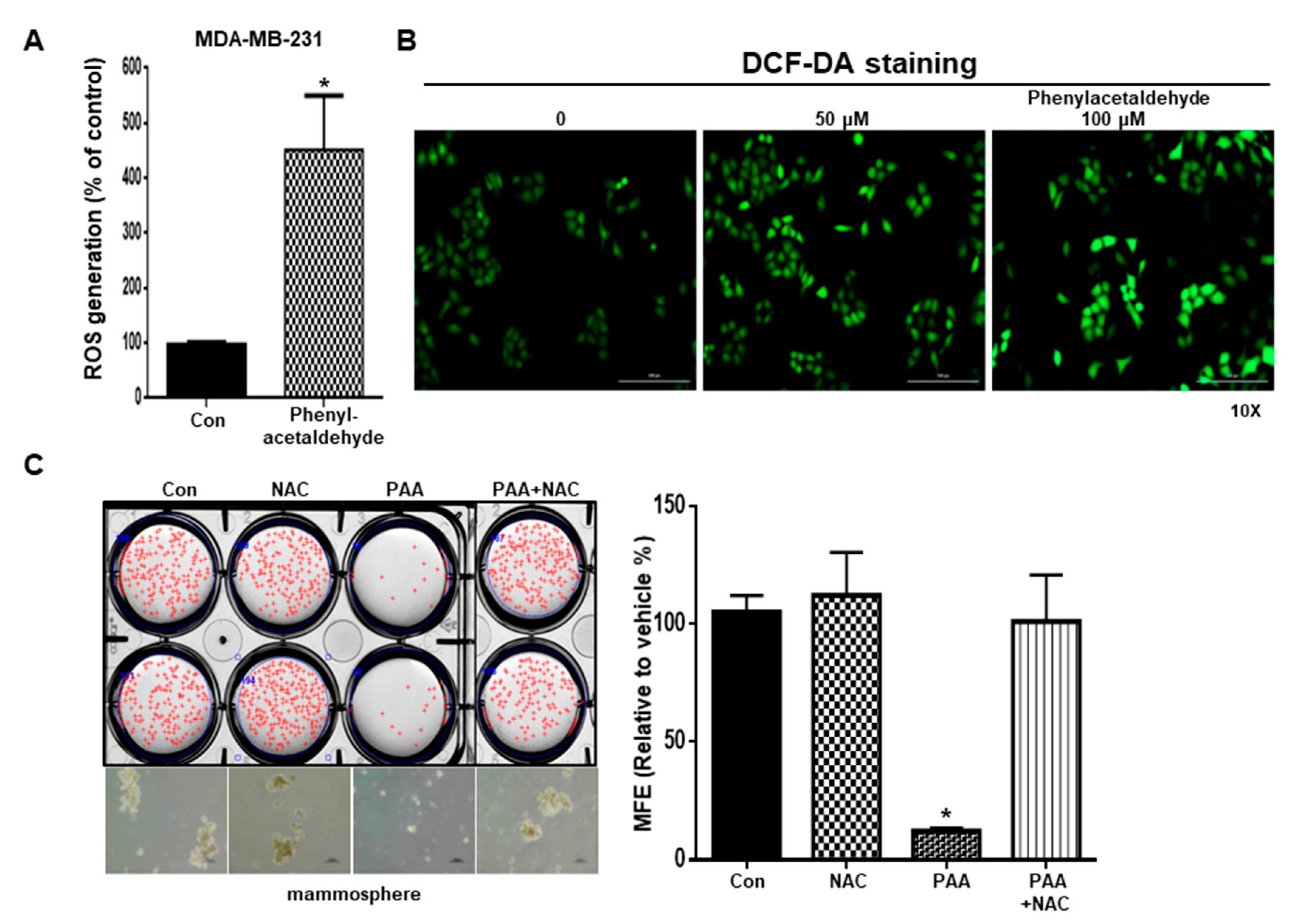
3.7. PAA Induce ROS and N-Acetycysteine (NAC) Reversed PAA-Induced Inhibition of Mammosphere Formation
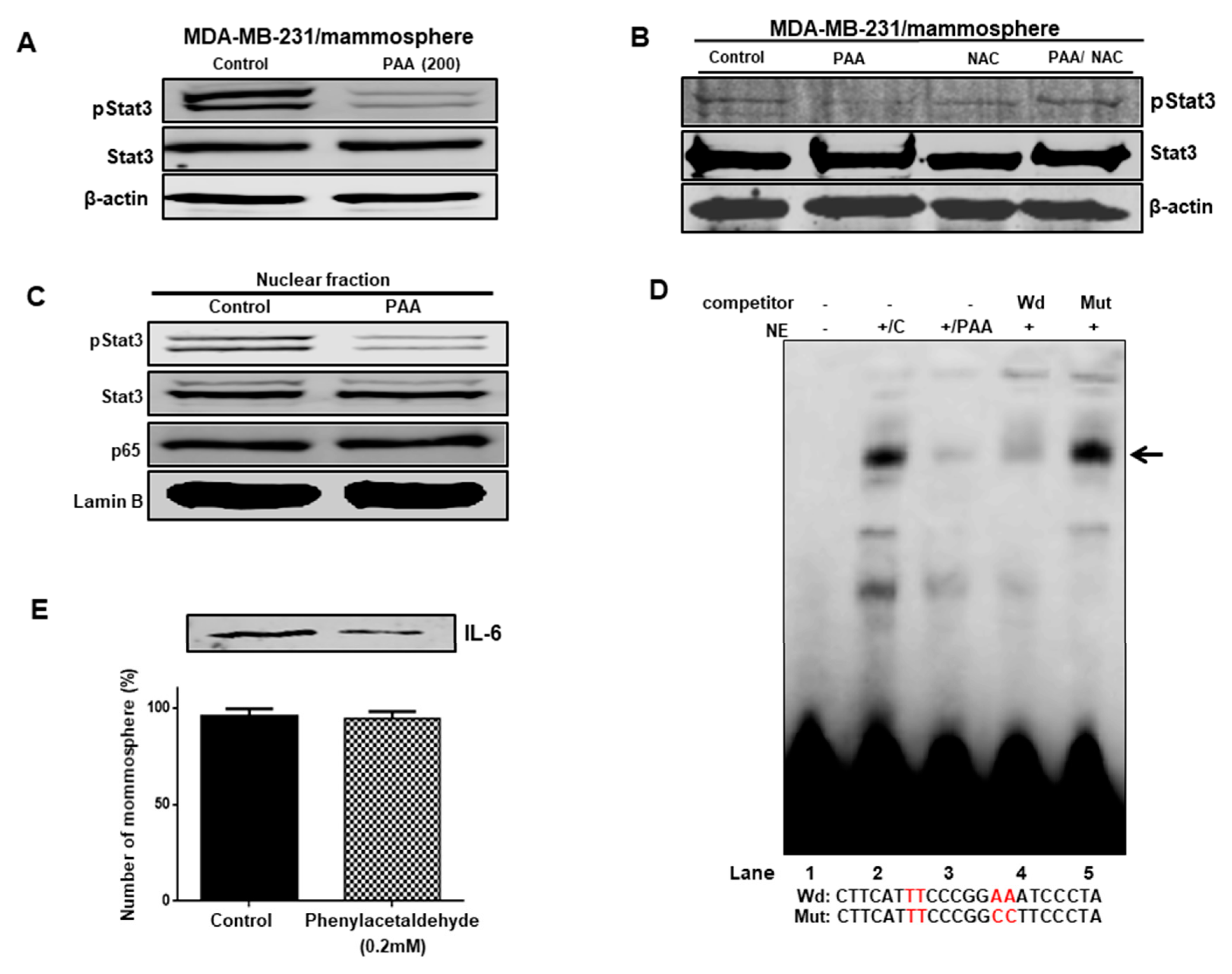
3.8. Effect of PAA on Stat3 Signal Pathway Regulation through ROS Production
3.9. The PAA Inhibits Stat3 Signal-Dependent IL-6 Production on Mammospheres
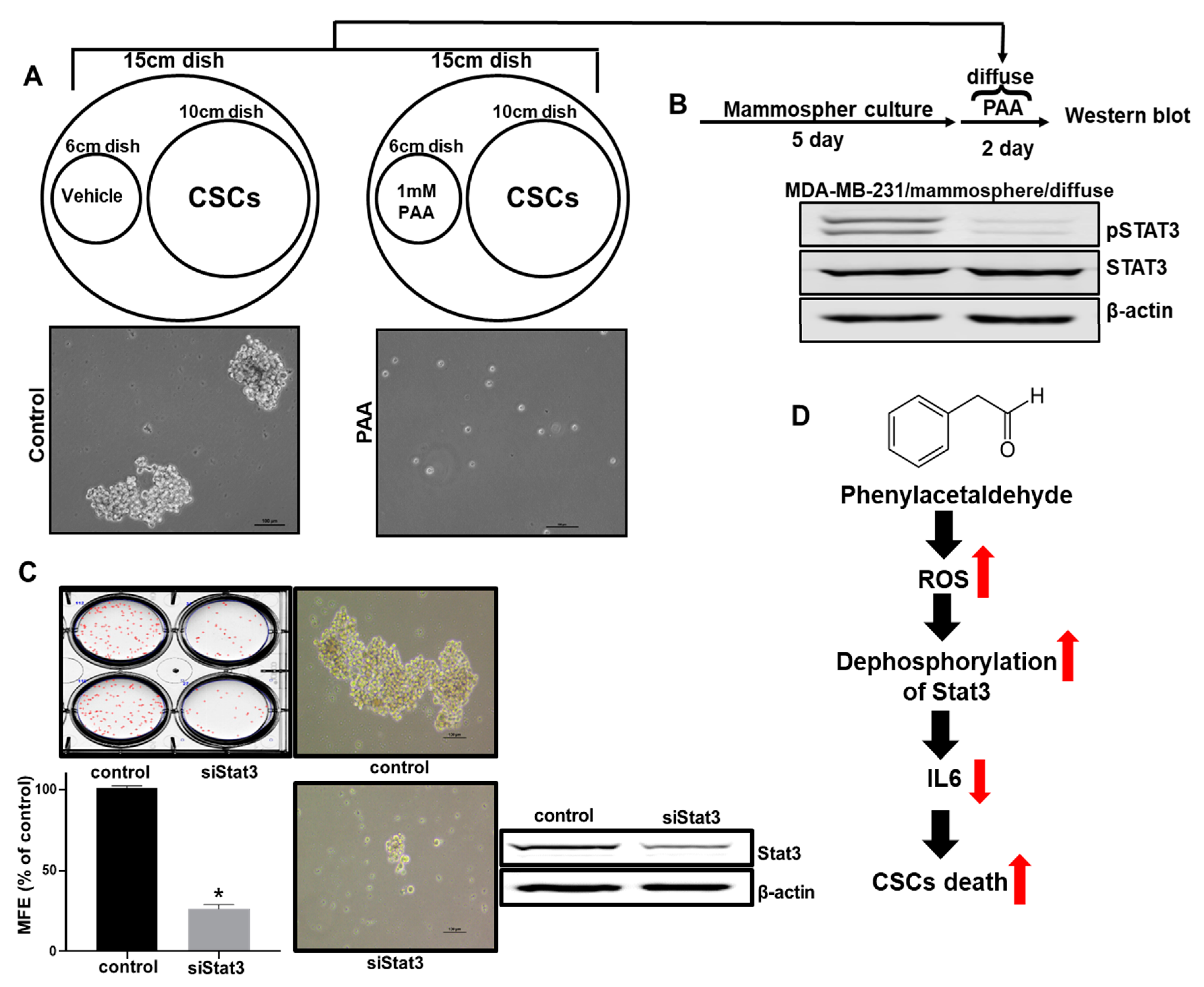
3.10. Effect of PAA Vapor on Breast CSCs Formation and pStat3 Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Peto, R.; Boreham, J.; Clarke, M.; Davies, C.; Beral, V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet 2000, 355, 1822. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet 1998, 352, 930–942. [Google Scholar]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, M.R.; Bagheri, V.; Razavi, M.S.; Momtazi, A.A.; Sahebkar, A.; Gholamin, M. Isolation, identification, and characterization of cancer stem cells: A review. J. Cell Physiol. 2017, 232, 2008–2018. [Google Scholar] [CrossRef]
- Islam, F.; Qiao, B.; Smith, R.A.; Gopalan, V.; Lam, A.K. Cancer stem cell: Fundamental experimental pathological concepts and updates. Exp. Mol. Pathol. 2015, 98, 184–191. [Google Scholar] [CrossRef]
- Matsui, W.H. Cancer stem cell signaling pathways. Medicine 2016, 95, S8–S19. [Google Scholar] [CrossRef]
- Glinka, Y.; Mohammed, N.; Subramaniam, V.; Jothy, S.; Prud’homme, G.J. Neuropilin-1 is expressed by breast cancer stem-like cells and is linked to NF-kappaB activation and tumor sphere formation. Biochem. Biophys. Res. Commun. 2012, 425, 775–780. [Google Scholar] [CrossRef]
- van Schaijik, B.; Davis, P.F.; Wickremesekera, A.C.; Tan, S.T.; Itinteang, T. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: A review. J. Clin. Pathol. 2018, 71, 88–91. [Google Scholar] [CrossRef]
- Marcato, P.; Dean, C.A.; Giacomantonio, C.A.; Lee, P.W. Aldehyde dehydrogenase: Its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle 2011, 10, 1378–1384. [Google Scholar] [CrossRef]
- Sheridan, C.; Kishimoto, H.; Fuchs, R.K.; Mehrotra, S.; Bhat-Nakshatri, P.; Turner, C.H.; Goulet, R., Jr.; Badve, S.; Nakshatri, H. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: An early step necessary for metastasis. Breast Cancer Res. 2006, 8, R59. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Resat, H. Constitutive activation of STAT3 in breast cancer cells: A review. Int. J. Cancer 2016, 138, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kang, J.W.; Song, X.; Kim, B.K.; Yoo, Y.D.; Kwon, Y.T.; Lee, Y.J. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal 2013, 25, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, I.; Shimoda, L.A.; Park, Y.; Zhang, C.; Tran, L.; Zhang, H.; Semenza, G.L. Chemotherapy-Induced Ca(2+) Release Stimulates Breast Cancer Stem Cell Enrichment. Cell Rep. 2017, 18, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, L.N.; Chow, E.K. Mechanisms of chemoresistance in cancer stem cells. Clin. Transl. Med. 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar]
- Choi, H.S.; Kim, J.H.; Kim, S.L.; Deng, H.Y.; Lee, D.; Kim, C.S.; Yun, B.S.; Lee, D.S. Catechol derived from aronia juice through lactic acid bacteria fermentation inhibits breast cancer stem cell formation via modulation Stat3/IL-6 signaling pathway. Mol. Carcinog. 2018, 57, 1467–1479. [Google Scholar] [CrossRef]
- Clarke, M.L.; Burton, R.L.; Hill, A.N.; Litorja, M.; Nahm, M.H.; Hwang, J. Low-cost, high-throughput, automated counting of bacterial colonies. Cytom. A 2010, 77, 790–797. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, D.A.; Chung, H.; Park, I.H.; Kim, B.H.; Oh, E.S.; Kang, D.H. Screening of breast cancer stem cell inhibitors using a protein kinase inhibitor library. Cancer Cell Int. 2017, 17, 25. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, S.L.; Kim, J.H.; Deng, H.Y.; Yun, B.S.; Lee, D.S. Triterpene Acid (3-O-p-Coumaroyltormentic Acid) Isolated From Aronia Extracts Inhibits Breast Cancer Stem Cell Formation through Downregulation of c-Myc Protein. Int. J. Mol. Sci. 2018, 19, 2528. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Hwang, C.K.; Kim, C.S.; Song, K.Y.; Law, P.Y.; Wei, L.N.; Loh, H.H. Transcriptional regulation of mouse mu opioid receptor gene: Sp3 isoforms (M1, M2) function as repressors in neuronal cells to regulate the mu opioid receptor gene. Mol. Pharmacol. 2005, 67, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Choi, H.S.; Kim, J.H.; Jeong, D.K.; Kim, K.S.; Lee, D.S. Dihydrotanshinone-Induced NOX5 Activation Inhibits Breast Cancer Stem Cell through the ROS/Stat3 Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 9296439. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.R.; Hanson, A.D.; Klee, H.J. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Zheng, J.; Pan, J. Reactive oxygen species in cancer stem cells. Antioxid. Redox Signal 2012, 16, 1215–1228. [Google Scholar] [CrossRef]
- Sansone, P.; Storci, G.; Tavolari, S.; Guarnieri, T.; Giovannini, C.; Taffurelli, M.; Ceccarelli, C.; Santini, D.; Paterini, P.; Marcu, K.B.; et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J. Clin. Investig. 2007, 117, 3988–4002. [Google Scholar] [CrossRef]
- St Helen, G.; Jacob, P., 3rd; Peng, M.; Dempsey, D.A.; Hammond, S.K.; Benowitz, N.L. Intake of toxic and carcinogenic volatile organic compounds from secondhand smoke in motor vehicles. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2774–2782. [Google Scholar] [CrossRef]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef]
- Bordoloi, M.; Saikia, S.; Kolita, B.; Sarmah, R.; Roy, S.; Narzary, B. Volatile Inhibitors of Phosphatidylinositol-3-Kinase (PI3K) Pathway: Anticancer Potential of Aroma Compounds of Plant Essential Oils. Anticancer Agents Med. Chem. 2018, 18, 87–109. [Google Scholar] [CrossRef]
- Quintans Jde, S.; Soares, B.M.; Ferraz, R.P.; Oliveira, A.C.; da Silva, T.B.; Menezes, L.R.; Sampaio, M.F.; Prata, A.P.; Moraes, M.O.; Pessoa, C.; et al. Chemical constituents and anticancer effects of the essential oil from leaves of Xylopia laevigata. Planta Med. 2013, 79, 123–130. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, Y.; Wang, Y.; Wang, C.; Fu, J.; Gao, L.; Liu, Y.; Feng, J.; Swamy, M.K.; Yogi, M.; et al. Anticancer Properties of Different Solvent Extracts of Cucumis melo L. Seeds and Whole Fruit and Their Metabolite Profiling Using HPLC and GC-MS. Biomed. Res. Int. 2020, 2020, 5282949. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Hirsch, H.A.; Wang, G.; Struhl, K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc. Natl. Acad. Sci. USA 2011, 108, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Bak, Y.; Park, Y.H.; Jang, G.B.; Nam, J.S.; Yoo, J.E.; Park, Y.N.; Bak, I.S.; Kim, J.M.; Yoon, D.Y.; et al. Peroxiredoxin II Is Essential for Maintaining Stemness by Redox Regulation in Liver Cancer Cells. Stem Cells 2016, 34, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl. Acad. Sci. USA 2013, 110, 972–977. [Google Scholar] [CrossRef]
- Korkaya, H.; Liu, S.; Wicha, M.S. Regulation of cancer stem cells by cytokine networks: Attacking cancer’s inflammatory roots. Clin. Cancer Res. 2011, 17, 6125–6129. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Hojfeldt, G.; Hojman, P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013, 138, 657–664. [Google Scholar] [CrossRef]

| Genes | Accession Number | Primers |
|---|---|---|
| CD44 | NP_0006013 | Forward: 5′-AGAAGGTGTGGGCAGAAGAA-3′ Reverse: 5′-AAATGCACCATTTCCTGAGA-3′ |
| Nanog | NP_0248654 | Forward: 5′-ATGCCTCACACGGAGACTGT-3′ Reverse: 5′-AAGTGGGTTGTTTGCCTTTG-3′ |
| Sox2 | NP_0030971 | Forward: 5′-TTGCTGCCTCTTTAAGACTAGGA-3′ Reverse: 5′-CTGGGGCTCAAACTTCTCTC-3′ |
| Oct4 | NP_1887022 | Forward: 5′-AGCAAAACCCGGAGGAGT-3′-3′ Reverse: 5′-CCACATCGGCCTGTGTATATC-3′ |
| β-actin | NP_0010921 | Forward: 5′-TGTTACCAACTGGGACGACA-3′ Reverse: 5′-GGGGTGTTGAAGGTCTCAAA-3′ |
| Number | Name | Molecular Weight | Final Concentration | Inhibitory Activity | Source (Plant) |
|---|---|---|---|---|---|
| 1 | Methyl salicylate | 152.15 | 100 μM | (−) | Wintergreen |
| 2 | Methyl jasmonate | 224.30 | 100 μM | (−) | Jasminum |
| 3 | Acetyl salicylic acid | 180.16 | 100 μM | (−) | Meadowsweet |
| 4 | Methyl cinnamic acid | 162.19 | 100 μM | (−) | Strawberry |
| 5 | Methyl trans-cinnamic acid | 162.19 | 100 μM | (−) | Strawberry |
| 6 | 2-Methyl cinnamic acid | 162.19 | 100 μM | (−) | Strawberry |
| 7 | Trans-cinnamic acid | 148.16 | 100 μM | (−) | Strawberry |
| 8 | Cis-3-hexen-1-ol | 100.16 | 100 μM | (−) | Strawberry |
| 9 | Trans-2-hexenal | 98.14 | 100 μM | (−) | Tomato |
| 10 | phenylacetaldehyde | 120.15 | 100 μM | (+) | Rose |
| 11 | Trans-2-hexenol | 100.16 | 100 μM | (−) | Banana |
| 12 | 1-octen-3-ol | 128.21 | 100 μM | (−) | Lemon balm |
| 13 | (+) β-citronellol | 156.27 | 100 μM | (−) | Cymbopogon |
| 14 | (+) Rose oxide | 154.25 | 100 μM | (−) | Rose |
| 15 | hexanal | 100.16 | 100 μM | (−) | Strawberry |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.S.; Kim, S.-L.; Kim, J.-H.; Ko, Y.-C.; Lee, D.-S. Plant Volatile, Phenylacetaldehyde Targets Breast Cancer Stem Cell by Induction of ROS and Regulation of Stat3 Signal. Antioxidants 2020, 9, 1119. https://doi.org/10.3390/antiox9111119
Choi HS, Kim S-L, Kim J-H, Ko Y-C, Lee D-S. Plant Volatile, Phenylacetaldehyde Targets Breast Cancer Stem Cell by Induction of ROS and Regulation of Stat3 Signal. Antioxidants. 2020; 9(11):1119. https://doi.org/10.3390/antiox9111119
Chicago/Turabian StyleChoi, Hack Sun, Su-Lim Kim, Ji-Hyang Kim, Yu-Chan Ko, and Dong-Sun Lee. 2020. "Plant Volatile, Phenylacetaldehyde Targets Breast Cancer Stem Cell by Induction of ROS and Regulation of Stat3 Signal" Antioxidants 9, no. 11: 1119. https://doi.org/10.3390/antiox9111119
APA StyleChoi, H. S., Kim, S.-L., Kim, J.-H., Ko, Y.-C., & Lee, D.-S. (2020). Plant Volatile, Phenylacetaldehyde Targets Breast Cancer Stem Cell by Induction of ROS and Regulation of Stat3 Signal. Antioxidants, 9(11), 1119. https://doi.org/10.3390/antiox9111119





