Pharmaceutical Advantages of GenoTX-407, A Combination of Extracts from Scutellaria baicalensis Root and Magnolia officinalis Bark
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Extract Separation
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Cell Culture
2.5. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Nitric Oxide Production in RAW 264.7 Cells
2.8. Test Microorganisms and Growth Conditions
2.9. Agar Disk Diffusion Assay
2.10. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination
2.11. Measurement of Radical Scavenging Activity
2.12. Measurement of Lipid Peroxidation
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. Preparation and Separation of Extracts
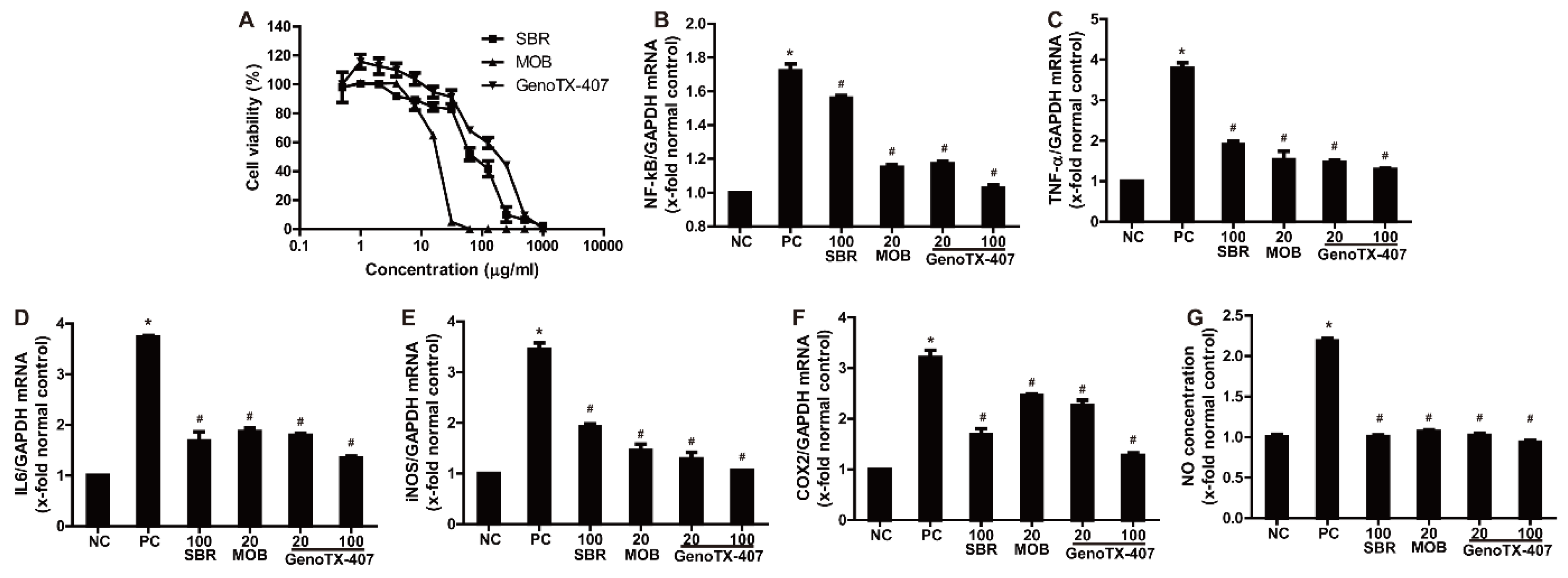
3.2. Anti-Inflammatory Effects
3.3. Antimicrobial Activity
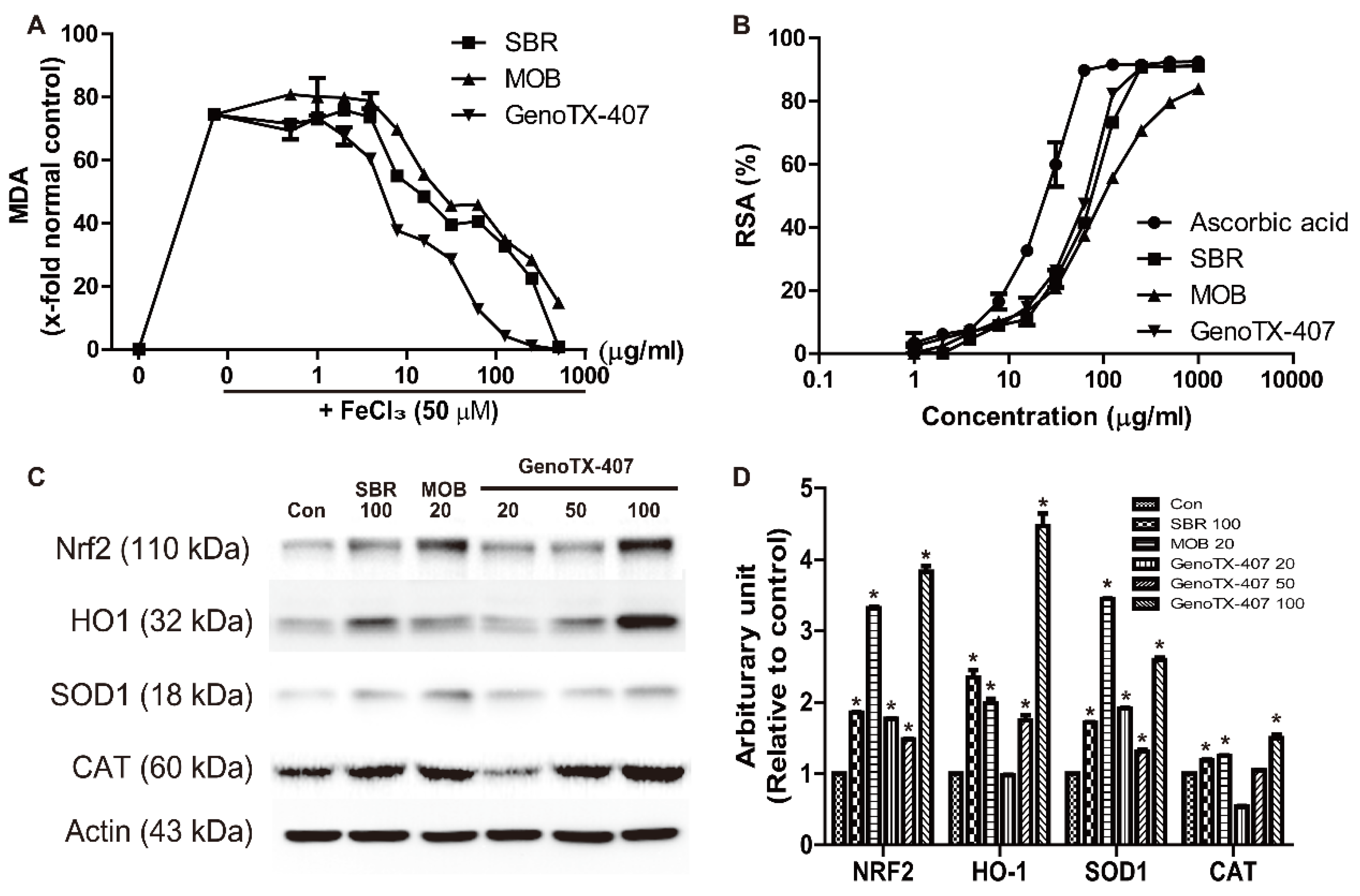
3.4. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinghorn, A.D.; Pan, L.; Fletcher, J.N.; Chai, H. The relevance of higher plants in lead compound discovery programs. J. Nat. Prod. 2011, 74, 1539–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.W.; Park, D.; Kwon, K.S.; Lee, D.H.; Jang, M.J.; Park, S.K.; Kim, J.Y. BST-104, a Water Extract of Lonicera japonica, Has a Gastroprotective Effect via Antioxidant and Anti-Inflammatory Activities. J. Med. Food 2019, 22, 140–151. [Google Scholar] [CrossRef]
- Yon, J.M.; Kim, Y.B.; Park, D. The Ethanol Fraction of White Rose Petal Extract Abrogates Excitotoxicity-Induced Neuronal Damage In Vivo and In Vitro through Inhibition of Oxidative Stress and Proinflammation. Nutrients 2018, 10, 1375. [Google Scholar] [CrossRef] [Green Version]
- Bochorakova, H.; Paulova, H.; Slanina, J.; Musil, P.; Taborska, E. Main flavonoids in the root of Scutellaria baicalensis cultivated in Europe and their comparative antiradical properties. Phytother. Res. 2003, 17, 640–644. [Google Scholar] [CrossRef]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Ishfaq, M.; Chen, C.; Bao, J.; Zhang, W.; Wu, Z.; Wang, J.; Liu, Y.; Tian, E.; Hamid, S.; Li, R.; et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-kappaB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult. Sci. 2019, 98, 6296–6310. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Cui, W.; Zhang, X.; Li, N.; Chen, J.; Wong, A.W.; Roberts, A. Evaluation of short-term and subchronic toxicity of magnolia bark extract in rats. Regul. Toxicol. Pharmacol. 2007, 49, 160–171. [Google Scholar] [CrossRef]
- Poivre, M.; Duez, P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J. Zhejiang Univ. Sci. B 2017, 18, 194–214. [Google Scholar] [PubMed] [Green Version]
- Park, J.; Lee, J.; Jung, E.; Park, Y.; Kim, K.; Park, B.; Jung, K.; Park, E.; Kim, J.; Park, D. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur. J. Pharmacol. 2004, 496, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Park, J.; Jung, K.; Lee, S.; Hong, S.; Park, J.; Park, E.; Kim, J.; Park, S.; et al. Anti-inflammatory effects of magnolol and honokiol are mediated through inhibition of the downstream pathway of MEKK-1 in NF-kappaB activation signaling. Planta Med. 2005, 71, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Song, G.; Zhang, G.; Ye, Z.; Williamson, E.M. The classification and application of toxic Chinese materia medica. Phytother. Res. 2014, 28, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, M.; Baudoux, T.; Stevigny, C.; Nortier, J.; Colet, J.M.; Efferth, T.; Qu, F.; Zhou, J.; Chan, K.; Shaw, D.; et al. Review of current and “omics” methods for assessing the toxicity (genotoxicity, teratogenicity and nephrotoxicity) of herbal medicines and mushrooms. J. Ethnopharmacol. 2012, 140, 492–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, J.; Liu, X.; Ye, Z.; Yang, X.; Meyboom, R.; Chan, K.; Shaw, D.; Duez, P. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: Current status and future perspective. J. Ethnopharmacol. 2012, 140, 519–525. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Olajuyigbe, O.O.; Afolayan, A.J. Evaluation of combination effects of ethanolic extract of Ziziphus mucronata Willd. subsp. mucronata Willd. and antibiotics against clinically important bacteria. Sci. World J. 2013, 2013, 769594. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.R.; Baron, E.J.; Pfaller, M.A.; Tenover, F.C.; Yolken, R.H.; Morgan, D.R. Manual of Clinical Microbiology (6th edn). Trends Microbiol. 1995, 3, 449. [Google Scholar]
- Zgoda, J.R.; Porter, J.R. A Convenient Microdilution Method for Screening Natural Products Against Bacteria and Fungi. Pharm. Biol. 2001, 39, 221–225. [Google Scholar] [CrossRef]
- Olney, J.W.; Rhee, V.; Ho, O.L. Kainic acid: A powerful neurotoxic analogue of glutamate. Brain Res. 1974, 77, 507–512. [Google Scholar] [CrossRef]
- Li, H.B.; Chen, F. Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1074, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Genovese, S.; Tammaro, F.; Menghini, L.; Carlucci, G.; Epifano, F.; Locatelli, M. Comparison of three different extraction methods and HPLC determination of the anthraquinones aloe-emodine, emodine, rheine, chrysophanol and physcione in the bark of Rhamnus alpinus L. (Rhamnaceae). Phytochem. Anal. 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zeng, Y.; Nie, K.; Luo, D.; Wang, Z. Extraction Optimization, Characterization and Bioactivities of a Major Polysaccharide from Sargassum thunbergii. PLoS ONE 2015, 10, e0144773. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Teng, C.M.; Chen, C.F.; Chen, C.C.; Hong, C.Y. Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem. Pharm. 1994, 47, 549–553. [Google Scholar] [PubMed]
- Huang, R.L.; Chen, C.C.; Huang, H.L.; Chang, C.G.; Chen, C.F.; Chang, C.; Hsieh, M.T. Anti-hepatitis B virus effects of wogonin isolated from Scutellaria baicalensis. Planta Med. 2000, 66, 694–698. [Google Scholar] [CrossRef]
- Huang, W.H.; Lee, A.R.; Yang, C.H. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci. Biotechnol. Biochem. 2006, 70, 2371–2380. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-C.; Chuah, A.M.; Yamaguchi, T.; Takamura, H.; Matoba, T. Antioxidant Activity of Traditional Chinese Medicinal Herbs. Food Sci. Technol. Res. 2008, 14, 205–210. [Google Scholar] [CrossRef]
- Chen, G.; Chen, X.; Niu, C.; Huang, X.; An, N.; Sun, J.; Huang, S.; Ye, W.; Li, S.; Shen, Y.; et al. Baicalin alleviates hyperglycemia-induced endothelial impairment 1 via Nrf2. J. Endocrinol. 2018, 240, 81–98. [Google Scholar] [CrossRef]
- Fang, J.; Zhu, Y.; Wang, H.; Cao, B.; Fei, M.; Niu, W.; Zhou, Y.; Wang, X.; Li, X.; Zhou, M. Baicalin Protects Mice Brain From Apoptosis in Traumatic Brain Injury Model Through Activation of Autophagy. Front. Neurosci. 2019, 12, 1006. [Google Scholar] [CrossRef]
- He, P.; Wu, Y.; Shun, J.; Liang, Y.; Cheng, M.; Wang, Y. Baicalin Ameliorates Liver Injury Induced by Chronic plus Binge Ethanol Feeding by Modulating Oxidative Stress and Inflammation via CYP2E1 and NRF2 in Mice. Oxid. Med. Cell Longev. 2017, 2017, 4820414. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Hsu, Y.C.; Lin, M.T. Magnolol protects against cerebral ischaemic injury of rat heatstroke. Clin. Exp. Pharmacol. Physiol. 2003, 30, 387–392. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Li, M.; Jiao, G. Magnolol induces apoptosis via activation of both mitochondrial and death receptor pathways in A375-S2 cells. Arch. Pharmacal Res. 2009, 32, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Lee, J.-Y.; Park, J.; Hwang, W.; Lee, J.; Park, D. Synthesis and microbiological evaluation of honokiol derivatives as new antimicrobial agents. Arch. Pharmacal Res. 2010, 33, 61–65. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharm. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [Green Version]
- Novy, P.; Urban, J.; Leuner, O.; Vadlejch, J.; Kokoska, L. In vitro synergistic effects of baicalin with oxytetracycline and tetracycline against Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 1298–1300. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-H.; Hu, Z.-Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [Green Version]
- Bang, K.H.; Kim, Y.K.; Min, B.S.; Na, M.K.; Rhee, Y.H.; Lee, J.P.; Bae, K.H. Antifungal activity of magnolol and honokiol. Arch. Pharm. Res. 2000, 23, 46–49. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.; Jeong, S.I.; Jahng, K.Y.; Yu, K.Y. Antimicrobial Effects and Resistant Regulation of Magnolol and Honokiol on Methicillin-Resistant Staphylococcus aureus. Biomed. Res. Int. 2015, 2015, 283630. [Google Scholar] [CrossRef] [Green Version]

| Mouse Primer | |||
|---|---|---|---|
| Gene Name | Accession No. | Forward (5′–3′) | Reverse (5′–3′) |
| COX2 | NM_011198 | GAACCTGCAGTTTGCTGTGG | ACTCTGTTGTGCTCCCGAAG |
| IL-6 | NM_031168.1 | TCCAGTTGCCTTCTTGGGAC | AGTCTCCTCTCCGGACTTGT |
| iNOS | NM_010927.3 | CTATGGCCGCTTTGATGTGC | TTGGGATGCTCCATGGTCAC |
| NF-κB | NM_008689 | CACTGCTCAGGTCCACTGTC | CTGTCACTATCCCGGAGTTCA |
| TNFα | NM_013693 | TACCTTGTTGCCTCCTCTT | GTCACCAAATCAGCGTTATTAAG |
| GAPDH | NM_008084 | CGTGCCGCCTGGAGAAACC | TGGAAGAGTGGGAGTTGCTGTTG |
| Inhibition Zones Diameter (mm) * around Disks Impregnated with 20 μL of Each Extract (2 mg/disk) | ||||
|---|---|---|---|---|
| Microbial Strains | DMSO | SBR | MOB | GenoTX-407 |
| Escherichia coli ATCC 8739 | ND | ND | ND | 10.20 ± 0.131 |
| Staphylococcus aureus ATCC 6538 | ND | ND | 16.53 ± 0.361 | 18.90 ± 0.335 |
| Candida albicans ATCC 10231 | ND | ND | 16.48 ± 0.055 | 19.18 ± 0.155 |
| Propionibacterium acnes ATCC 9027 | ND | ND | 30.54 ± 0.922 | 35.10 ± 0.676 |
| Staphylococcus epidermidis ATCC 12228 | ND | ND | 19.10 ± 0.259 | 24.04 ± 0.492 |
| Pseudomonas aeruginosa ATCC 9097 | ND | ND | ND | 9.89 ± 0.083 |
| Bacillus subtilis ATCC 33608 | ND | ND | 20.70 ± 0.516 | 24.41 ± 0.492 |
| Saccharomyces cerevisiae ATCC 13007 | ND | ND | 22.61 ± 1.266 | 28.12 ± 0.829 |
| Aspergillus niger ATCC 16404 | ND | ND | 17.03 ± 0.871 | 18.92 ± 0.472 |
| T. rubum ATCC 62345 | ND | ND | 49.77 ± 0.240 | 50.47 ± 0.200 |
| Microbial Strains | SBR | MOB | GenoTX-407 | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| Escherichia coli ATCC 8739 | 2500 | >5000 | 78.1 | 78.1 | 39.1 | 39.1 |
| Staphylococcus aureus ATCC 6538 | - * | NA | 39.1 | 39.1 | 1.5 | 19.5 |
| Candida albicans ATCC 10231 | - | NA | 9.77 | 9.77 | 2.44 | 2.44 |
| Propionibacterium acnes ATCC 9027 | - | NA | 2.44 | 9.77 | 2.44 | 9.77 |
| Staphylococcus epidermidis ATCC 12228 | - | NA | 4.88 | 4.88 | 4.88 | 4.88 |
| Pseudomonas aeruginosa ATCC 9097 | - | NA | - | NA | 2500 | 2500 |
| Bacillus subtilis ATCC 33608 | - | NA | 9.77 | 19.5 | 9.77 | 19.5 |
| Saccharomyces cerevisiae ATCC 13007 | - | NA | 9.77 | 19.5 | 9.77 | 19.5 |
| Aspergillus niger ATCC 16404 | - | NA | 78.1 | 78.1 | 78.1 | 78.1 |
| T. rubum ATCC 62345 | - | NA | 4.88 | 4.88 | 4.88 | 4.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, E.-J.; Lee, M.Y.; Choi, B.I.; Lim, K.J.; Hong, S.Y.; Park, D. Pharmaceutical Advantages of GenoTX-407, A Combination of Extracts from Scutellaria baicalensis Root and Magnolia officinalis Bark. Antioxidants 2020, 9, 1111. https://doi.org/10.3390/antiox9111111
Yoon E-J, Lee MY, Choi BI, Lim KJ, Hong SY, Park D. Pharmaceutical Advantages of GenoTX-407, A Combination of Extracts from Scutellaria baicalensis Root and Magnolia officinalis Bark. Antioxidants. 2020; 9(11):1111. https://doi.org/10.3390/antiox9111111
Chicago/Turabian StyleYoon, Eun-Jung, Mi Young Lee, Byoung Il Choi, Kyong Jin Lim, Seung Young Hong, and Dongsun Park. 2020. "Pharmaceutical Advantages of GenoTX-407, A Combination of Extracts from Scutellaria baicalensis Root and Magnolia officinalis Bark" Antioxidants 9, no. 11: 1111. https://doi.org/10.3390/antiox9111111




