Proteomics Study of Peripheral Blood Mononuclear Cells in Down Syndrome Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Samples Collection of Peripheral Blood Mononuclear Cells from HD and DS Subjects
2.3. Protein Sample Preparation
2.4. Protein Expression Analysis by nLC–HDMSE
2.5. Bioinformatics and Network Analysis
2.6. Western Blot
2.7. Slot Blot Analysis and Protein Carbonylation
2.8. Statistical Analysis
3. Results
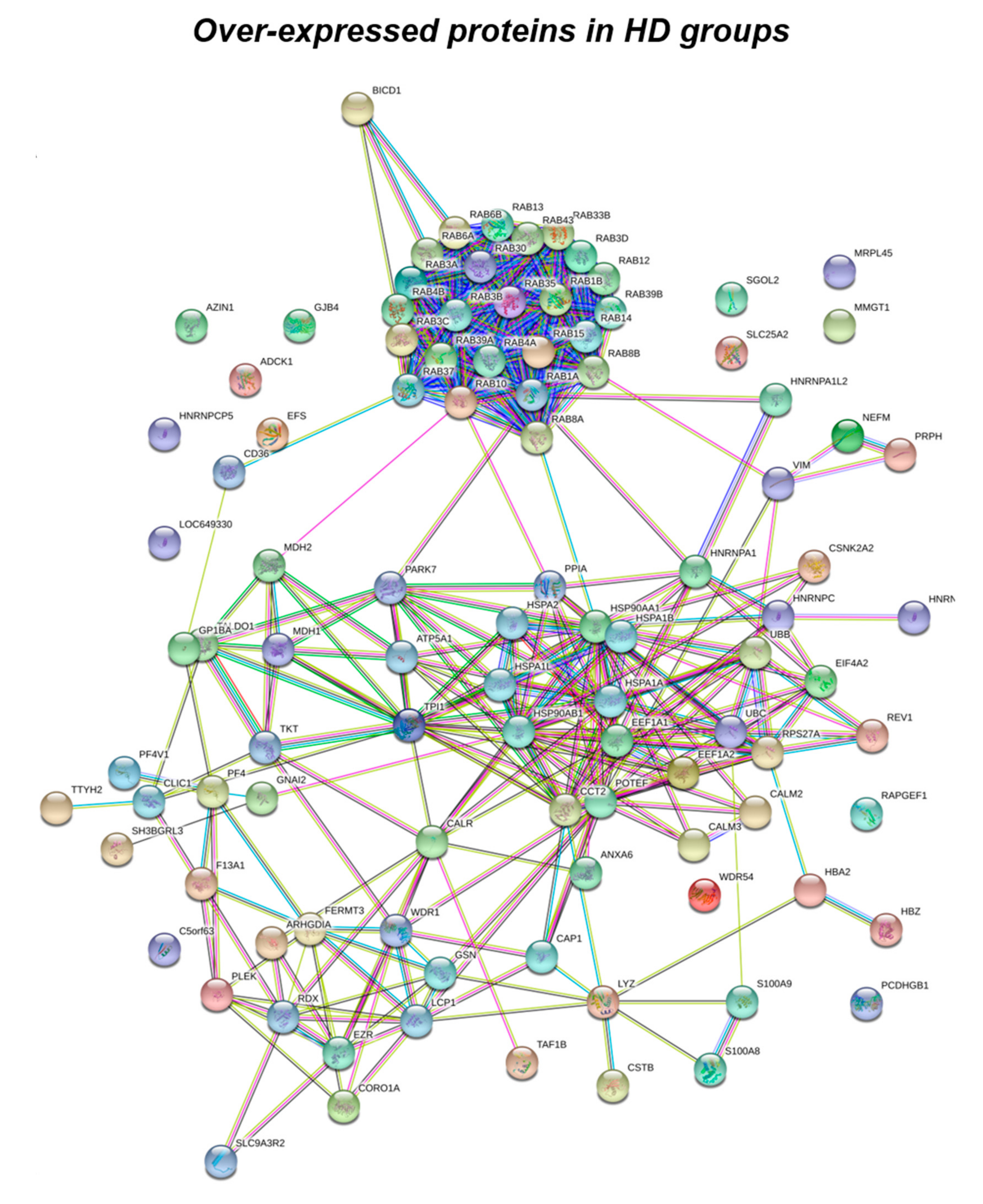
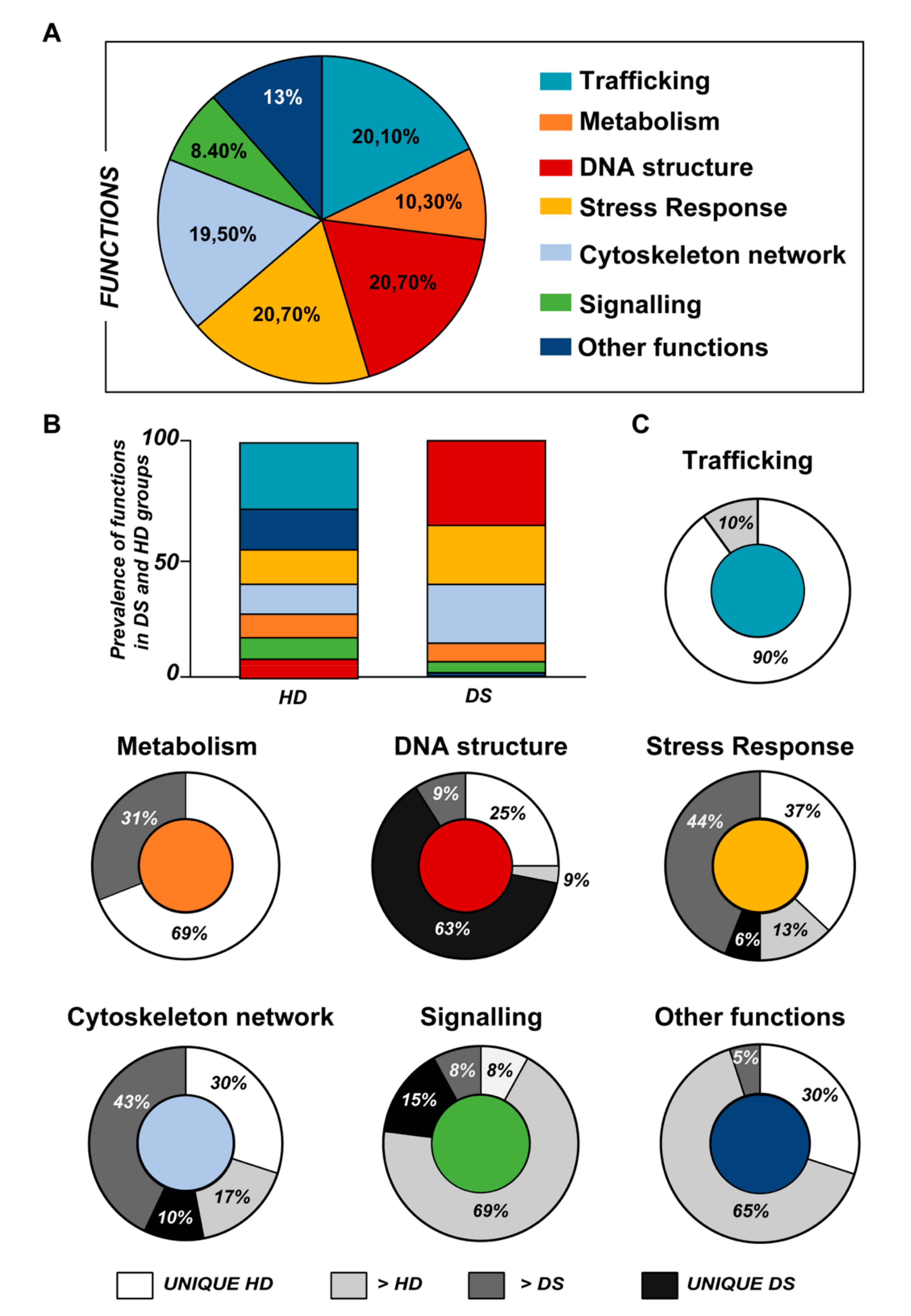
3.1. Proteomics Analysis
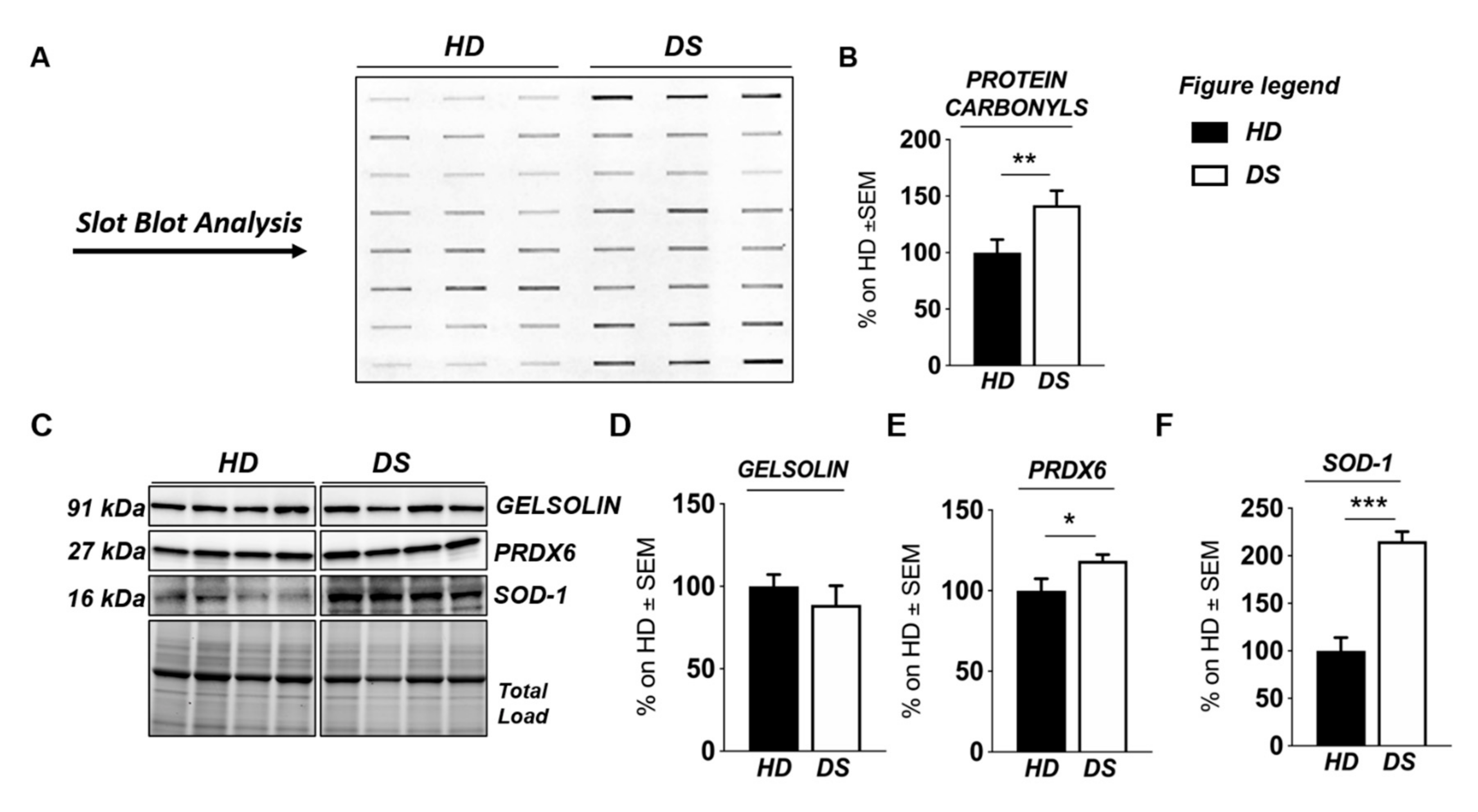
3.2. Western Blot Analysis
4. Discussion
5. Conclusions
Abbreviations
| Aβ | amyloid beta peptide |
| AD | Alzheimer disease |
| AOR | antioxidant response |
| APP | amyloid precursor protein |
| ARE | antioxidant response element |
| ATF6 | activating transcription factor 6 |
| ATP | adenosine triphosphate |
| BiP-GRP78 | Binding immunoglobulin—Glucose regulated protein 78 |
| CHOP (DDTI3) | DNA damage-inducible transcript 3 |
| Chr21 | chromosome 21 |
| CN | central nervous system |
| DS | Down syndrome |
| Dyrk1A | dual specificity tyrosine phosphorylation regulated kinase 1A |
| eIF2α | eukaryotic initiation factor 2 alpha |
| ER | endoplasmic reticulum |
| GAPDH | glyceraldehyde 3 phosphate dehydrogenase |
| H1A | histone 1A |
| HSP | heat shock protein |
| IRE1 | inositol-requiring enzyme 1 |
| ISR | integrated stress response |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OS | oxidative stress |
| PBMC | peripheral blood mononuclear cell |
| PERK | protein kinase R (PKR)-like endoplasmic reticulum kinase |
| PGK | phosphoglycerate kinase |
| PPP | pentose phosphate pathway |
| PKC | protein kinase C |
| PRDX | peroxiredoxin |
Author Contributions
Funding
Conflicts of Interest
References
- De Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the number of people with Down syndrome in the United States. Genet. Med. 2017, 19, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Weijerman, M.E.; van Furth, A.M.; Vonk Noordegraaf, A.; van Wouwe, J.P.; Broers, C.J.; Gemke, R.J. Prevalence, neonatal characteristics, and first-year mortality of Down syndrome: A national study. J. Pediatr. 2008, 152, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Lyle, R.; Dermitzakis, E.T.; Reymond, A.; Deutsch, S. Chromosome 21 and down syndrome: From genomics to pathophysiology. Nat. Rev. Genet. 2004, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.; Don, M.; Parekh, M.; Sarsoza, F.; Goodus, M.; Lopez, G.E.; Kawas, C.; Leverenz, J.; Doran, E.; Lott, I.T.; et al. Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain. Neurobiol. Aging 2007, 28, 1493–1506. [Google Scholar] [CrossRef]
- Lott, I.T.; Head, E. Alzheimer disease and Down syndrome: Factors in pathogenesis. Neurobiol. Aging 2005, 26, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Williams, R.S. A prospective study of Alzheimer disease in Down syndrome. Arch. Neurol. 1989, 46, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Lemere, C.A.; Blusztajn, J.K.; Yamaguchi, H.; Wisniewski, T.; Saido, T.C.; Selkoe, D.J. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: Implications for initial events in amyloid plaque formation. Neurobiol. Dis. 1996, 3, 16–32. [Google Scholar] [CrossRef]
- Baban, A.; Olivini, N.; Cantarutti, N.; Cali, F.; Vitello, C.; Valentini, D.; Adorisio, R.; Calcagni, G.; Alesi, V.; Di Mambro, C.; et al. Differences in morbidity and mortality in Down syndrome are related to the type of congenital heart defect. Am. J. Med. Genet. Part A 2020, 182, 1342–1350. [Google Scholar] [CrossRef]
- Valentini, D.; Alisi, A.; di Camillo, C.; Sartorelli, M.R.; Crudele, A.; Bartuli, A.; Nobili, V.; Villani, A. Nonalcoholic Fatty Liver Disease in Italian Children with Down Syndrome: Prevalence and Correlation with Obesity-Related Features. J. Pediatr. 2017, 189, 92–97.e1. [Google Scholar] [CrossRef]
- Bloemers, B.L.; van Bleek, G.M.; Kimpen, J.L.; Bont, L. Distinct abnormalities in the innate immune system of children with Down syndrome. J. Pediatr. 2010, 156, 804–809.e5. [Google Scholar] [CrossRef]
- Bloemers, B.L.; Broers, C.J.; Bont, L.; Weijerman, M.E.; Gemke, R.J.; van Furth, A.M. Increased risk of respiratory tract infections in children with Down syndrome: The consequence of an altered immune system. Microb. Infect. 2010, 12, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Capolunghi, F.; Rosado, M.M.; Sinibaldi, M.; Aranburu, A.; Carsetti, R. Why do we need IgM memory B cells? Immunol. Lett. 2013, 152, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Carsetti, R.; Valentini, D.; Marcellini, V.; Scarsella, M.; Marasco, E.; Giustini, F.; Bartuli, A.; Villani, A.; Ugazio, A.G. Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. Eur. J. Immunol. 2015, 45, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Ram, G.; Chinen, J. Infections and immunodeficiency in Down syndrome. Clin. Exp. Immunol. 2011, 164, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Korbel, J.O.; Tirosh-Wagner, T.; Urban, A.E.; Chen, X.N.; Kasowski, M.; Dai, L.; Grubert, F.; Erdman, C.; Gao, M.C.; Lange, K.; et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc. Natl. Acad. Sci. USA 2009, 106, 12031–12036. [Google Scholar] [CrossRef] [PubMed]
- Olson, L.E.; Richtsmeier, J.T.; Leszl, J.; Reeves, R.H. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science 2004, 306, 687–690. [Google Scholar] [CrossRef]
- Haydar, T.F.; Reeves, R.H. Trisomy 21 and early brain development. Trends Neurosci. 2012, 35, 81–91. [Google Scholar] [CrossRef] [PubMed]
- de Haan, J.B.; Susil, B.; Pritchard, M.; Kola, I. An altered antioxidant balance occurs in Down syndrome fetal organs: Implications for the “gene dosage effect” hypothesis. J. Neural. Transm. Suppl. 2003. [Google Scholar] [CrossRef]
- Di Domenico, F.; Pupo, G.; Mancuso, C.; Barone, E.; Paolini, F.; Arena, A.; Blarzino, C.; Schmitt, F.A.; Head, E.; Butterfield, D.A.; et al. Bach1 overexpression in Down syndrome correlates with the alteration of the HO-1/BVR-a system: Insights for transition to Alzheimer’s disease. J. Alzheimers Dis. 2015, 44, 1107–1120. [Google Scholar] [CrossRef]
- Busciglio, J.; Pelsman, A.; Wong, C.; Pigino, G.; Yuan, M.; Mori, H.; Yankner, B.A. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down’s syndrome. Neuron 2002, 33, 677–688. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Singh, I. Down’s syndrome, dementia, and superoxide dismutase. Br. J. Psychiatry 1993, 162, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Coskun, P.; Helguera, P.; Nemati, Z.; Bohannan, R.C.; Thomas, J.; Samuel, S.E.; Argueta, J.; Doran, E.; Wallace, D.C.; Lott, I.T.; et al. Metabolic and Growth Rate Alterations in Lymphoblastic Cell Lines Discriminate Between Down Syndrome and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Manente, G.A.; Moro, L.; Marra, E.; Vacca, R.A. Deficit of complex I activity in human skin fibroblasts with chromosome 21 trisomy and overproduction of reactive oxygen species by mitochondria: Involvement of the cAMP/PKA signalling pathway. Biochem. J. 2011, 435, 679–688. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Barone, E.; Arena, A.; Zuliani, I.; Mosca, L.; Blarzino, C.; Butterfield, D.A.; Perluigi, M.; Di Domenico, F. Intranasal rapamycin ameliorates Alzheimer-like cognitive decline in a mouse model of Down syndrome. Transl. Neurodegener. 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Tramutola, A.; Barone, E.; Lanzillotta, C.; Defever, O.; Arena, A.; Zuliani, I.; Foppoli, C.; Iavarone, F.; Vincenzoni, F.; et al. Restoration of aberrant mTOR signaling by intranasal rapamycin reduces oxidative damage: Focus on HNE-modified proteins in a mouse model of down syndrome. Redox. Biol. 2019, 23, 101162. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Tullo, A.; Caratozzolo, M.F.; Merafina, R.S.; Scartezzini, P.; Marra, E.; Vacca, R.A. Impairment of F1F0-ATPase, adenine nucleotide translocator and adenylate kinase causes mitochondrial energy deficit in human skin fibroblasts with chromosome 21 trisomy. Biochem. J. 2010, 431, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Helguera, P.; Seiglie, J.; Rodriguez, J.; Hanna, M.; Helguera, G.; Busciglio, J. Adaptive downregulation of mitochondrial function in down syndrome. Cell. Metab. 2013, 17, 132–140. [Google Scholar] [CrossRef]
- Di Domenico, F.; Barone, E.; Perluigi, M.; Butterfield, D.A. The Triangle of Death in Alzheimer’s Disease Brain: The Aberrant Cross-Talk Among Energy Metabolism, Mammalian Target of Rapamycin Signaling, and Protein Homeostasis Revealed by Redox Proteomics. Antioxid. Redox. Signal. 2017, 26, 364–387. [Google Scholar] [CrossRef]
- Druzhyna, N.; Nair, R.G.; LeDoux, S.P.; Wilson, G.L. Defective repair of oxidative damage in mitochondrial DNA in Down’s syndrome. Mutat. Res. 1998, 409, 81–89. [Google Scholar] [CrossRef]
- Coskun, P.E.; Busciglio, J. Oxidative Stress and Mitochondrial Dysfunction in Down’s Syndrome: Relevance to Aging and Dementia. Curr. Gerontol. Geriatr. Res. 2012, 2012, 383170. [Google Scholar] [CrossRef]
- Weick, J.P.; Held, D.L.; Bonadurer, G.F., 3rd; Doers, M.E.; Liu, Y.; Maguire, C.; Clark, A.; Knackert, J.A.; Molinarolo, K.; Musser, M.; et al. Deficits in human trisomy 21 iPSCs and neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 9962–9967. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Sun, J.; Shepherd, J.; Ovchinnikov, D.A.; Chung, T.L.; Nayler, S.P.; Kao, L.P.; Morrow, C.A.; Thakar, N.Y.; Soo, S.Y.; et al. Integration-free induced pluripotent stem cells model genetic and neural developmental features of down syndrome etiology. Stem. Cells 2013, 31, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Mollo, N.; Cicatiello, R.; Aurilia, M.; Scognamiglio, R.; Genesio, R.; Charalambous, M.; Paladino, S.; Conti, A.; Nitsch, L.; Izzo, A. Targeting Mitochondrial Network Architecture in Down Syndrome and Aging. Int. J. Mol. Sci. 2020, 21, 3134. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Butterfield, D.A. The identification of protein biomarkers for oxidative stress in Down syndrome. Expert Rev. Proteom. 2011, 8, 427–429. [Google Scholar] [CrossRef][Green Version]
- Di Domenico, F.; Coccia, R.; Cocciolo, A.; Murphy, M.P.; Cenini, G.; Head, E.; Butterfield, D.A.; Giorgi, A.; Schinina, M.E.; Mancuso, C.; et al. Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer’s disease neuropathology: Redox proteomics analysis of human brain. Biochim. Biophys. Acta 2013, 1832, 1249–1259. [Google Scholar] [CrossRef]
- Perluigi, M.; Butterfield, D.A. Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr. Gerontol. Geriatr. Res. 2012, 2012, 724904. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; di Domenico, F.; Fiorini, A.; Cocciolo, A.; Giorgi, A.; Foppoli, C.; Butterfield, D.A.; Giorlandino, M.; Giorlandino, C.; Schinina, M.E.; et al. Oxidative stress occurs early in Down syndrome pregnancy: A redox proteomics analysis of amniotic fluid. Proteom. Clin. Appl. 2011, 5, 167–178. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Buttterfield, D.A. Unraveling the complexity of neurodegeneration in brains of subjects with Down syndrome: Insights from proteomics. Proteom. Clin. Appl. 2014, 8, 73–85. [Google Scholar] [CrossRef]
- Di Domenico, F.; Tramutola, A.; Foppoli, C.; Head, E.; Perluigi, M.; Butterfield, D.A. mTOR in Down syndrome: Role in Ass and tau neuropathology and transition to Alzheimer disease-like dementia. Free Radic Biol. Med. 2018, 114, 94–101. [Google Scholar] [CrossRef]
- Jaisson, S.; Gillery, P. Impaired proteostasis: Role in the pathogenesis of diabetes mellitus. Diabetologia 2014, 57, 1517–1527. [Google Scholar] [CrossRef]
- Kharabi Masouleh, B.; Geng, H.; Hurtz, C.; Chan, L.N.; Logan, A.C.; Chang, M.S.; Huang, C.; Swaminathan, S.; Sun, H.; Paietta, E.; et al. Mechanistic rationale for targeting the unfolded protein response in pre-B acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 2014, 111, E2219–E2228. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Bidasee, K.R.; Shinohara, T. Selenite cataracts: Activation of endoplasmic reticulum stress and loss of Nrf2/Keap1-dependent stress protection. Biochim. Biophys. Acta 2014, 1842, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Aivazidis, S.; Coughlan, C.M.; Rauniyar, A.K.; Jiang, H.; Liggett, L.A.; Maclean, K.N.; Roede, J.R. The burden of trisomy 21 disrupts the proteostasis network in Down syndrome. PLoS ONE 2017, 12, e0176307. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, C.; Tramutola, A.; Meier, S.; Schmitt, F.; Barone, E.; Perluigi, M.; Di Domenico, F.; Abisambra, J.F. Early and Selective Activation and Subsequent Alterations to the Unfolded Protein Response in Down Syndrome Mouse Models. J. Alzheimers Dis. 2018, 62, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, C.; Zuliani, I.; Tramutola, A.; Barone, E.; Blarzino, C.; Folgiero, V.; Caforio, M.; Valentini, D.; Villani, A.; Locatelli, F.; et al. Chronic PERK induction promotes Alzheimer-like neuropathology in Down syndrome: Insights for therapeutic intervention. Prog. Neurobiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tramutola, A.; Di Domenico, F.; Barone, E.; Arena, A.; Giorgi, A.; di Francesco, L.; Schinina, M.E.; Coccia, R.; Head, E.; Butterfield, D.A.; et al. Polyubiquitinylation Profile in Down Syndrome Brain Before and After the Development of Alzheimer Neuropathology. Antioxid. Redox. Signal. 2017, 26, 280–298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.J.; Khatiwada, S.; Cui, Y.; Reineke, L.C.; Dooling, S.W.; Kim, J.J.; Li, W.; Walter, P.; Costa-Mattioli, M. Activation of the ISR mediates the behavioral and neurophysiological abnormalities in Down syndrome. Science 2019, 366, 843–849. [Google Scholar] [CrossRef]
- Botte, A.; Potier, M.C. Focusing on cellular biomarkers: The endo-lysosomal pathway in Down syndrome. Prog. Brain. Res. 2020, 251, 209–243. [Google Scholar] [CrossRef]
- Cataldo, A.M.; Mathews, P.M.; Boiteau, A.B.; Hassinger, L.C.; Peterhoff, C.M.; Jiang, Y.; Mullaney, K.; Neve, R.L.; Gruenberg, J.; Nixon, R.A. Down syndrome fibroblast model of Alzheimer-related endosome pathology: Accelerated endocytosis promotes late endocytic defects. Am. J. Pathol. 2008, 173, 370–384. [Google Scholar] [CrossRef]
- Cataldo, A.M.; Peterhoff, C.M.; Troncoso, J.C.; Gomez-Isla, T.; Hyman, B.T.; Nixon, R.A. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: Differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 2000, 157, 277–286. [Google Scholar] [CrossRef]
- Cossec, J.C.; Lavaur, J.; Berman, D.E.; Rivals, I.; Hoischen, A.; Stora, S.; Ripoll, C.; Mircher, C.; Grattau, Y.; Olivomarin, J.C.; et al. Trisomy for synaptojanin1 in Down syndrome is functionally linked to the enlargement of early endosomes. Hum. Mol. Genet. 2012, 21, 3156–3172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Mullaney, K.A.; Peterhoff, C.M.; Che, S.; Schmidt, S.D.; Boyer-Boiteau, A.; Ginsberg, S.D.; Cataldo, A.M.; Mathews, P.M.; Nixon, R.A. Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc. Natl. Acad. Sci. USA 2010, 107, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Dierssen, M.; Fructuoso, M.; Martinez de Lagran, M.; Perluigi, M.; Barone, E. Down Syndrome Is a Metabolic Disease: Altered Insulin Signaling Mediates Peripheral and Brain Dysfunctions. Front. Neurosci. 2020, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Distler, U.; Kuharev, J.; Navarro, P.; Tenzer, S. Label-free quantification in ion mobility-enhanced data-independent acquisition proteomics. Nat. Protoc. 2016, 11, 795–812. [Google Scholar] [CrossRef]
- Greco, V.; Spalloni, A.; Corasolla Carregari, V.; Pieroni, L.; Persichilli, S.; Mercuri, N.B.; Urbani, A.; Longone, P. Proteomics and Toxicity Analysis of Spinal-Cord Primary Cultures upon Hydrogen Sulfide Treatment. Antioxidants (Basel) 2018, 7, 87. [Google Scholar] [CrossRef]
- Marini, F.; Carregari, V.C.; Greco, V.; Ronci, M.; Iavarone, F.; Persichilli, S.; Castagnola, M.; Urbani, A.; Pieroni, L. Exploring the HeLa Dark Mitochondrial Proteome. Front. Cell Dev. Biol. 2020, 8, 137. [Google Scholar] [CrossRef]
- Silva, J.C.; Gorenstein, M.V.; Li, G.Z.; Vissers, J.P.; Geromanos, S.J. Absolute quantification of proteins by LCMSE: A virtue of parallel MS acquisition. Mol. Cell Proteom. 2006, 5, 144–156. [Google Scholar] [CrossRef]
- Vissers, J.P.; Langridge, J.I.; Aerts, J.M. Analysis and quantification of diagnostic serum markers and protein signatures for Gaucher disease. Mol. Cell Proteom. 2007, 6, 755–766. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Gu, L.; Di Domenico, F.; Robinson, R.A. Mass spectrometry and redox proteomics: Applications in disease. Mass. Spectrom. Rev. 2014, 33, 277–301. [Google Scholar] [CrossRef]
- Epstein, C.J.; Korenberg, J.R.; Anneren, G.; Antonarakis, S.E.; Ayme, S.; Courchesne, E.; Epstein, L.B.; Fowler, A.; Groner, Y.; Huret, J.L.; et al. Protocols to establish genotype-phenotype correlations in Down syndrome. Am. J. Hum. Genet. 1991, 49, 207–235. [Google Scholar]
- Gardiner, K. Gene-dosage effects in Down syndrome and trisomic mouse models. Genom. Biol. 2004, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Gearhart, J.D.; Oster-Granite, M.L.; Reeves, R.H.; Coyle, J.T. Developmental consequences of autosomal aneuploidy in mammals. Dev. Genet. 1987, 8, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, K.; Herault, Y.; Lott, I.T.; Antonarakis, S.E.; Reeves, R.H.; Dierssen, M. Down syndrome: From understanding the neurobiology to therapy. J. Neurosci. 2010, 30, 14943–14945. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.H.; Baxter, L.L.; Richtsmeier, J.T. Too much of a good thing: Mechanisms of gene action in Down syndrome. Trends Genet. 2001, 17, 83–88. [Google Scholar] [CrossRef]
- Engidawork, E.; Lubec, G. Molecular changes in fetal Down syndrome brain. J. Neurochem. 2003, 84, 895–904. [Google Scholar] [CrossRef]
- Lott, I.T.; Head, E. Dementia in Down syndrome: Unique insights for Alzheimer disease research. Nat. Rev. Neurol. 2019, 15, 135–147. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Di Domenico, F.; Head, E.; Butterfield, D.A.; Perluigi, M.; Barone, E. Brain insulin resistance triggers early onset Alzheimer disease in Down syndrome. Neurobiol. Dis. 2020, 137, 104772. [Google Scholar] [CrossRef]
- Perluigi, M.; Barone, E.; Di Domenico, F.; Butterfield, D.A. Aberrant protein phosphorylation in Alzheimer disease brain disturbs pro-survival and cell death pathways. Biochim. Biophys. Acta 2016, 1862, 1871–1882. [Google Scholar] [CrossRef]
- Mancuso, C.; Siciliano, R.; Barone, E. Curcumin and Alzheimer disease: This marriage is not to be performed. J. Biol. Chem. 2011, 286. [Google Scholar] [CrossRef]
- Touchot, N.; Chardin, P.; Tavitian, A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: Molecular cloning of YPT-related cDNAs from a rat brain library. Proc. Natl. Acad. Sci. USA 1987, 84, 8210–8214. [Google Scholar] [CrossRef]
- Weber, E.; Jilling, T.; Kirk, K.L. Distinct functional properties of Rab3A and Rab3B in PC12 neuroendocrine cells. J. Biol. Chem. 1996, 271, 6963–6971. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R. Rab GTPases: Master regulators that establish the secretory and endocytic pathways. Mol. Biol. Cell 2017, 28, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Stenmark, H. Cellular functions of Rab GTPases at a glance. J. Cell Sci. 2015, 128, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Fiorini, A.; Sultana, R.; Perluigi, M.; Cai, J.; Klein, J.B.; Head, E.; Butterfield, D.A. An investigation of the molecular mechanisms engaged before and after the development of Alzheimer disease neuropathology in Down syndrome: A proteomics approach. Free Radic Biol. Med. 2014, 76, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Mani, G.; Park, B.S.; Jacques, J.; Murdoch, G.; Whetsell, W., Jr.; Kaye, J.; Manczak, M. Differential loss of synaptic proteins in Alzheimer’s disease: Implications for synaptic dysfunction. J. Alzheimers Dis. 2005, 7, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Kiral, F.R.; Kohrs, F.E.; Jin, E.J.; Hiesinger, P.R. Rab GTPases and Membrane Trafficking in Neurodegeneration. Curr. Biol. 2018, 28, R471–R486. [Google Scholar] [CrossRef]
- English, A.R.; Voeltz, G.K. Rab10 GTPase regulates ER dynamics and morphology. Nat. Cell Biol. 2013, 15, 169–178. [Google Scholar] [CrossRef]
- Weitzdoerfer, R.; Stolzlechner, D.; Dierssen, M.; Ferreres, J.; Fountoulakis, M.; Lubec, G. Reduction of nucleoside diphosphate kinase B, Rab GDP-dissociation inhibitor beta and histidine triad nucleotide-binding protein in fetal Down syndrome brain. J. Neural Transm. Suppl. 2001, 347–359. [Google Scholar] [CrossRef]
- Shisheva, A.; Chinni, S.R.; DeMarco, C. General role of GDP dissociation inhibitor 2 in membrane release of Rab proteins: Modulations of its functional interactions by in vitro and in vivo structural modifications. Biochemistry 1999, 38, 11711–11721. [Google Scholar] [CrossRef]
- Schimmoller, F.; Simon, I.; Pfeffer, S.R. Rab GTPases, directors of vesicle docking. J. Biol. Chem. 1998, 273, 22161–22164. [Google Scholar] [CrossRef]
- D’Adamo, P.; Menegon, A.; Lo Nigro, C.; Grasso, M.; Gulisano, M.; Tamanini, F.; Bienvenu, T.; Gedeon, A.K.; Oostra, B.; Wu, S.K.; et al. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat. Genet. 1998, 19, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, J.; Prywes, R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J. Biol. Chem. 2002, 277, 13045–13052. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Dave, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Itoh, K.; Ohtsu, T.; Sasaki, Y.; Ogura, M.; Morishima, Y.; Kasai, M.; Chou, T.; Yoshida, K.; Ohno, T.; Mizorogi, F.; et al. Randomized comparison of mobilization kinetics of circulating CD34+ cells between biweekly CHOP and dose-escalated CHOP with the prophylactic use of lenograstim (glycosylated rHuG-CSF) in aggressive non-Hodgkin’s lymphoma. The lenograstim/Lymphoma Study Group. Leuk Lymphoma 2000, 38, 521–532. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Lisbona, F.; Rojas-Rivera, D.; Hetz, C. When ER stress reaches a dead end. Biochim. Biophys. Acta 2013, 1833, 3507–3517. [Google Scholar] [CrossRef]
- Uzi, D.; Barda, L.; Scaiewicz, V.; Mills, M.; Mueller, T.; Gonzalez-Rodriguez, A.; Valverde, A.M.; Iwawaki, T.; Nahmias, Y.; Xavier, R.; et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J. Hepatol. 2013, 59, 495–503. [Google Scholar] [CrossRef]
- Yang, H.; Niemeijer, M.; van de Water, B.; Beltman, J.B. ATF6 Is a Critical Determinant of CHOP Dynamics during the Unfolded Protein Response. iScience 2020, 23, 100860. [Google Scholar] [CrossRef]
- Fullerton, H.J.; Ditelberg, J.S.; Chen, S.F.; Sarco, D.P.; Chan, P.H.; Epstein, C.J.; Ferriero, D.M. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann. Neurol. 1998, 44, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Krapfenbauer, K.; Engidawork, E.; Cairns, N.; Fountoulakis, M.; Lubec, G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003, 967, 152–160. [Google Scholar] [CrossRef]
- Kreuz, S.; Fischle, W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 2016, 8, 843–862. [Google Scholar] [CrossRef] [PubMed]
- Feser, J.; Truong, D.; Das, C.; Carson, J.J.; Kieft, J.; Harkness, T.; Tyler, J.K. Elevated histone expression promotes life span extension. Mol. Cell 2010, 39, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Juvekar, A.; Lyssiotis, C.A.; Lien, E.C.; Albeck, J.G.; Oh, D.; Varma, G.; Hung, Y.P.; Ullas, S.; Lauring, J.; et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 2016, 164, 433–446. [Google Scholar] [CrossRef]
- Shestov, A.A.; Liu, X.; Ser, Z.; Cluntun, A.A.; Hung, Y.P.; Huang, L.; Kim, D.; Le, A.; Yellen, G.; Albeck, J.G.; et al. Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. Elife 2014, 3. [Google Scholar] [CrossRef]
- Antonaros, F.; Ghini, V.; Pulina, F.; Ramacieri, G.; Cicchini, E.; Mannini, E.; Martelli, A.; Feliciello, A.; Lanfranchi, S.; Onnivello, S.; et al. Plasma metabolome and cognitive skills in Down syndrome. Sci. Rep. 2020, 10, 10491. [Google Scholar] [CrossRef]
- Caracausi, M.; Ghini, V.; Locatelli, C.; Mericio, M.; Piovesan, A.; Antonaros, F.; Pelleri, M.C.; Vitale, L.; Vacca, R.A.; Bedetti, F.; et al. Plasma and urinary metabolomic profiles of Down syndrome correlate with alteration of mitochondrial metabolism. Sci. Rep. 2018, 8, 2977. [Google Scholar] [CrossRef]
- Barone, E.; Mosser, S.; Fraering, P.C. Inactivation of brain Cofilin-1 by age, Alzheimer’s disease and gamma-secretase. Biochim. Biophys. Acta 2014, 1842, 2500–2509. [Google Scholar] [CrossRef]
- Ori-McKenney, K.M.; McKenney, R.J.; Huang, H.H.; Li, T.; Meltzer, S.; Jan, L.Y.; Vale, R.D.; Wiita, A.P.; Jan, Y.N. Phosphorylation of beta-Tubulin by the Down Syndrome Kinase, Minibrain/DYRK1a, Regulates Microtubule Dynamics and Dendrite Morphogenesis. Neuron 2016, 90, 551–563. [Google Scholar] [CrossRef]
- Dowjat, K.; Adayev, T.; Kaczmarski, W.; Wegiel, J.; Hwang, Y.W. Gene dosage-dependent association of DYRK1A with the cytoskeleton in the brain and lymphocytes of down syndrome patients. J. Neuropathol. Exp. Neurol. 2012, 71, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Dowjat, K.; Adayev, T.; Wojda, U.; Brzozowska, K.; Barczak, A.; Gabryelewicz, T.; Hwang, Y.W. Abnormalities of DYRK1A-Cytoskeleton Complexes in the Blood Cells as Potential Biomarkers of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 1059–1075. [Google Scholar] [CrossRef] [PubMed]
- Weitzdoerfer, R.; Fountoulakis, M.; Lubec, G. Reduction of actin-related protein complex 2/3 in fetal Down syndrome brain. Biochem. Biophys. Res. Commun. 2002, 293, 836–841. [Google Scholar] [CrossRef]
- Wilson, C.H.; Ali, E.S.; Scrimgeour, N.; Martin, A.M.; Hua, J.; Tallis, G.A.; Rychkov, G.Y.; Barritt, G.J. Steatosis inhibits liver cell store-operated Ca(2)(+) entry and reduces ER Ca(2)(+) through a protein kinase C-dependent mechanism. Biochem. J. 2015, 466, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Siarey, R.J.; Kline-Burgess, A.; Cho, M.; Balbo, A.; Best, T.K.; Harashima, C.; Klann, E.; Galdzicki, Z. Altered signaling pathways underlying abnormal hippocampal synaptic plasticity in the Ts65Dn mouse model of Down syndrome. J. Neurochem. 2006, 98, 1266–1277. [Google Scholar] [CrossRef]


| Subject | Diagnosis | Age | Sex | BMI | Centile | Comorbidities |
|---|---|---|---|---|---|---|
| HD 1 | Healthy donors | 2 | F | n/a | 96 | Asthma |
| HD 2 | Healthy donors | 14 | F | 19.8 | 54 | Rash |
| HD 3 | Healthy donors | 9 | F | 17.6 | 68 | Abdominal pain |
| HD 4 | Healthy donors | 4 | M | 15.7 | 54 | Kawasaki disease |
| HD 5 | Healthy donors | 8 | F | 20 | 93 | Headache |
| HD 6 | Healthy donors | 3 | M | 16.2 | 49 | Kawasaki disease |
| HD 7 | Healthy donors | 12 | M | 19.6 | 54 | Abdominal pain |
| HD 8 | Healthy donors | 7 | F | 20 | 92 | Headache |
| DS 1 | Down Syndrome | 6 | M | 14.9 | 9 | Behavioral trouble |
| DS 2 | Down Syndrome | 4 | M | 20.6 | 40 | CAV surgery, behavioral trouble |
| DS 3 | Down Syndrome | 5 | M | 17.5 | 64 | sleep apnea |
| DS 4 | Down Syndrome | 17 | F | 24.5 | 35 | leukopenia |
| DS 5 | Down Syndrome | 1 | F | 21.9 | 48 | hypothyroidism |
| DS 6 | Down Syndrome | 1 | F | n/a | 0 | prematurity |
| DS 7 | Down Syndrome | 1 | F | n/a | 31 | FPIES |
| DS 8 | Down Syndrome | 3 | F | 16.1 | 35 | FPIES |
| (1) Group | (2) Protein Description | (3) Uni Prot Accession Number | (4) Highly Expressed | (5) DS/HD Ratio |
|---|---|---|---|---|
| Metabolism | ||||
| Unique HD | L-lactate dehydrogenase A, B, C | P00338; P07195; P07864 | ||
| Malate dehydrogenase_cytoplasmic | P40925 | HD | ||
| Malate dehydrogenase_mitochondrial | P40926 | HD | ||
| ATP synthase subunit alpha_mitochondrial | P25705 | HD | ||
| Transaldolase | P37837 | HD | ||
| Transketolase | P29401 | HD | ||
| Triosephosphate isomerase | P60174 | HD | ||
| Antizyme inhibitor 1 | O14977 | HD | ||
| Mitochondrial ornithine transporter 2 | Q9BXI2 | HD | ||
| Over-expressed in HD | N/A | N/A | N/A | N/A |
| Unique DS | N/A | N/A | N/A | N/A |
| Over-expressed in DS | Glyceraldehyde-3-phosphate dehydrogenase | P04406 | 5.2 | |
| Fructose-bisphosphate aldolase A, C | P04075; P09972 | 3.17 | ||
| Phosphoglycerate kinase 1, 2 | P00558; P07205 | 1.86 | ||
| Trafficking | ||||
| Unique HD | Rab1 (A, B, C) | P62820; Q9H0U4; Q92928 | HD | |
| Rab3 (A, B, C, D) | P20336; P20337; Q96E17; O95716 | HD | ||
| Rab4 (A, B) | P20338; P61018 | HD | ||
| Rab6 (A, B) | P20340; Q9NRW1 | HD | ||
| Rab8 (A, B) | P61006; Q92930 | HD | ||
| Rab10 | P61026 | HD | ||
| Rab12 | Q6IQ22 | HD | ||
| Rab13 | P51153 | HD | ||
| Rab14 | P61106 | HD | ||
| Rab15 | P59190 | HD | ||
| Rab30 | Q15771 | HD | ||
| Rab33 (B) | Q9H082 | HD | ||
| Rab35 | Q15286 | HD | ||
| Rab37 | Q96AX2 | HD | ||
| Rab39 (A, B) | Q14964; Q96DA2 | HD | ||
| Rab43 | Q86YS6 | HD | ||
| Peripherin | P41219 | HD | ||
| Chloride intracellular channel protein 1 | O00299 | HD | ||
| AarF domain-containing protein kinase 1 | Q86TW2 | HD | ||
| Over-expressed in HD | Rho GDP-dissociation inhibitor 1 | P52565 | 0.12 | |
| Protein bicaudal D homolog 1 | Q96G01 | 0.15 | ||
| Membrane magnesium transporter | Q8N4V1 | 0.3 | ||
| Unique DS | N/A | N/A | N/A | |
| Over-expressed in DS | N/A | N/A | N/A | |
| DNA Structure | ||||
| Unique HD | Heterogeneous nuclear ribonucleoprotein A1 | P09651 | HD | |
| Heterogeneous nuclear ribonucleoprotein A1-like 2 | Q32P51 | HD | ||
| Heterogeneous nuclear ribonucleoprotein C-like 1, 2, 3, 4 | O6081; B2RXH8; B7ZW38; P0DMR1 | HD | ||
| Heterogeneous nuclear ribonucleoproteins C1/C2 | P07910 | HD | ||
| TATA box-binding protein-associated factor RNA polymerase I subunit B | Q53T94 | HD | ||
| Over-expressed in HD | Putative male-specific lethal-3 protein-like 2 | P0C860 | 0.56 | |
| Unique DS | Histone H1.1,2, 3,4,5 | Q02539; P16403; P16402; P10412; P16401 | DS | |
| Histone H2B type 1-B, C, D, H, K, L, M, N, O | P33778; P62807; P58876; Q93079; O60814; Q99880; Q99879; Q99877; P23527 | DS | ||
| Histone H2B type 2- E, F | Q16778; Q5QNW6 | DS | ||
| Histone H2B type 3 | Q8N257 | DS | ||
| Histone H3.1,2,3 | Q16695; Q71DI3; P84243 | DS | ||
| Over-expressed in DS | Shieldin complex subunit 3 | Q6ZNX1 | 1.78 | |
| Ribonuclease H2 subunit C | Q8TDP1 | 2.4 | ||
| Histone H4 | P62805 | 2.51 | ||
| Stress Response | ||||
| Unique HD | Poliubiquitin B, C | P0CG47; P0CG48 | HD | |
| Heat shock 70 kDa protein 1A, 1B | P0DMV8; P0DMV9 | HD | ||
| Heat shock 70 kDa protein 1, 2 | P34931; P54652 | HD | ||
| Heat shock protein HSP 90-alpha, beta | P07900; P08238 | HD | ||
| Ubiquitin-40S ribosomal protein S27a | P62979 | HD | ||
| Calreticulin | P27797 | HD | ||
| Parkinson disease protein 7 | Q99497 | HD | ||
| Glutaredoxin-like protein C5orf63 | A6NC05 | HD | ||
| Over-expressed in HD | T-complex protein 1 subunit beta | P78371 | 0.3 | |
| Protein S100-A8, A9 | P05109; P06702 | 0.26 | ||
| Peptidyl-prolyl cis-trans isomerase A | P62937 | 0.4 | ||
| Unique DS | Nitric oxide synthase-interacting protein | Q9Y314 | DS | |
| Glutathione S-transferase | P09211 | DS | ||
| Over-expressed in DS | Cyclic AMP-dependent transcription factor ATF-6 alpha and beta | P18850; Q99941 | 2.2 | |
| DNA damage-inducible transcript 3 protein (CHOP) | P35638 | 3.4 | ||
| Endoplasmic reticulum chaperone BiP (GRP78) | P11021 | 2.07 | ||
| ERO1-like protein alpha | Q96HE7 | 3.04 | ||
| Protein disulfide-isomerase (PDI) | P07237 | 8.7 | ||
| Peroxiredoxin-1, 2, 4, 6 | Q06830; P32119; Q13162; P30041 | 2.88; 3.7; 4.2; 4.8 | ||
| Endoplasmin | P14625 | 2.4 | ||
| Superoxide dismutase [Cu-Zn] | P00441 | 6.7 | ||
| Extracellular superoxide dismutase [Cu-Zn] | P08294 | 5.4 | ||
| Cytoskeleton Network | ||||
| Unique HD | Gelsolin | P06396 | HD | |
| Annexin A6 | P08133 | HD | ||
| Calmodulin-1, 2, 3 | P0DP23; P0DP24; P0DP25 | HD | ||
| Protocadherin gamma | Q9Y5G3 | HD | ||
| Neurofilament medium polypeptide | P07197 | HD | ||
| Plastin-2 | P13796 | HD | ||
| Adenylyl cyclase-associated protein 1 | Q01518 | HD | ||
| Over-expressed in HD | POTE ankyrin domain family member F | A5A3E0 | 0.6 | |
| Radixin | P35241; P15311 | 0.7 | ||
| Na(+)/H(+) exchange regulatory cofactor NHE-RF2 | Q15599 | 0.13 | ||
| Vimentin | P08670 | 0.8 | ||
| Unique DS | Actin-related protein 2/3 complex subunit 1, 4, 5 | O15143; P59998; O15511 | DS | |
| Over-expressed in DS | Cofilin-1, 2 | P23528; Q9Y281 | 1.67; 1.7 | |
| Tropomyosin alpha-1,3, 4 chain | P09493; P06753; P67936, | 2.21; 2.18; 2.46 | ||
| Tropomyosin beta chain | P07951 | 2.46 | ||
| TUBA4B Putative tubulin-like protein alpha-4B Iso 1 | Q9H853 | 2.43 | ||
| TUBA8 Tubulin alpha-8 chain Iso 2 | Q9NY65 | 5.13 | ||
| TUBB1 Tubulin beta-1 chain Iso 1 | Q9H4B7 | 4.28 | ||
| TUBB2B Tubulin beta-2B chain Iso 1 | Q9BVA1 | 3.8 | ||
| Myosin-9 | P35579 | 7.29 | ||
| Ezrin | P15311 | 2.8 | ||
| Profilin-1 | P07737 | 7.4 | ||
| Signaling | ||||
| Unique HD | Guanine nucleotide-binding protein G(i) subunit alpha-2 | P04899 | HD | |
| Over-expressed in HD | Pleckstrin | P08567 | 0.53 | |
| Platelet factor 4 | P02776; P10720 | 0.24 | ||
| Platelet glycoprotein Ib alpha chain | P07359 | 0.22 | ||
| Platelet glycoprotein 4 | P16671 | 0.26 | ||
| Embryonal Fyn-associated substrate | O43281 | 0.32 | ||
| SH3 domain-binding glutamic acid-rich-like protein | Q9H299 | 0.2 | ||
| Gap junction beta-4 protein | Q9NTQ9 | 0.4 | ||
| Casein kinase II subunit alpha | P19784 | 0.37 | ||
| Unique DS | SHC SH2 domain-binding protein 1 | Q8NEM2 | DS | |
| Protein kinase C gamma | P05129 | DS | ||
| Overexpressed in DS | GPR107 | Q5VW38 | 8.4 | |
| Other Functions | ||||
| Unique HD | WD repeat-containing protein 1, 54 | O75083; Q9H977 | HD | |
| Cystatin-B | P04080 | HD | ||
| Putative elongation factor 1-alpha-like 3 | Q5VTE0 | HD | ||
| Coagulation factor XIII A chain | P00488 | HD | ||
| Coronin-1A | P31146 | HD | ||
| Over-expressed in HD | Hemoglobin subunit alpha, zeta | P69905; P02008 | 0.64; 0.45 | |
| Protein tweety homolog 2 | Q9BSA4 | 0.4 | ||
| Elongation factor 1-alpha 1 | P68104; Q5VTE0; Q05639 | 0.09 | ||
| Eukaryotic initiation factor 4A-II | Q14240 | 0.2 | ||
| Lysozyme C | P61626 | 0.39 | ||
| Shugoshin 2 | Q562F6 | 0.46 | ||
| Fermitin family homolog 3 | Q86UX7; Q13905; Q9UBZ9 | 0.34; 0.46; 0.6 | ||
| 39S ribosomal protein L45_ mitochondrial | Q9BRJ2 | 0.14 | ||
| Unique DS | N/A | N/A | N/A | |
| Overexpressed in DS | N-alpha-acetyltransferase | Q6N069 | 2.21 | |
| Reactome Pathways in HD | |||
|---|---|---|---|
| Pathway | Description | Count in Gene Set | False Discovery Rate |
| HSA-8873719 | RAB geranylgeranylation | 23 of 63 | 3.35 × 10−31 |
| HSA-9007101 | Rab regulation of trafficking | 16 of 118 | 2.57 × 10−15 |
| HSA-8876198 | RAB GEFs exchange GTP for GDP on RABs | 14 of 86 | 2.18 × 10−14 |
| HSA-5653656 | Vesicle-mediated transport | 26 of 64 | 6.66 × 10−14 |
| HSA-199991 | Membrane Trafficking | 23 of 612 | 1.27 × 10−11 |
| Reactome Pathways in DS | |||
|---|---|---|---|
| Pathway | Description | Count in Gene Set | False Discovery Rate |
| HSA-2262752 | Cellular responses to stress | 27 of 384 | 1.17 × 10−27 |
| HSA-2559586 | Cellular responses to external stimuli | 27 of 459 | 5.50 × 10−26 |
| HSA-2559586 | DNA Damage Stress Induced Senescence | 15 of 61 | 3.98 × 10−22 |
| HSA-2559583 | Cellular Senescence | 15 of 161 | 1.70 × 10−16 |
| HSA-195258 | RHO GTPase effectors | 17 of 273 | 3.41 × 10−16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzillotta, C.; Greco, V.; Valentini, D.; Villani, A.; Folgiero, V.; Caforio, M.; Locatelli, F.; Pagnotta, S.; Barone, E.; Urbani, A.; et al. Proteomics Study of Peripheral Blood Mononuclear Cells in Down Syndrome Children. Antioxidants 2020, 9, 1112. https://doi.org/10.3390/antiox9111112
Lanzillotta C, Greco V, Valentini D, Villani A, Folgiero V, Caforio M, Locatelli F, Pagnotta S, Barone E, Urbani A, et al. Proteomics Study of Peripheral Blood Mononuclear Cells in Down Syndrome Children. Antioxidants. 2020; 9(11):1112. https://doi.org/10.3390/antiox9111112
Chicago/Turabian StyleLanzillotta, Chiara, Viviana Greco, Diletta Valentini, Alberto Villani, Valentina Folgiero, Matteo Caforio, Franco Locatelli, Sara Pagnotta, Eugenio Barone, Andrea Urbani, and et al. 2020. "Proteomics Study of Peripheral Blood Mononuclear Cells in Down Syndrome Children" Antioxidants 9, no. 11: 1112. https://doi.org/10.3390/antiox9111112
APA StyleLanzillotta, C., Greco, V., Valentini, D., Villani, A., Folgiero, V., Caforio, M., Locatelli, F., Pagnotta, S., Barone, E., Urbani, A., Di Domenico, F., & Perluigi, M. (2020). Proteomics Study of Peripheral Blood Mononuclear Cells in Down Syndrome Children. Antioxidants, 9(11), 1112. https://doi.org/10.3390/antiox9111112









