Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animal, Housing, and Diets
2.2. Sample Collection
2.3. Meat Quality
2.4. Antioxidant Enzyme Activity in Blood and Muscle
2.5. Radical Scavenging Activity
2.6. Hepatic Gene Expression
2.7. Feather Corticosterone
2.8. Statistical Analysis
3. Results
3.1. Antioxidant Enzymes
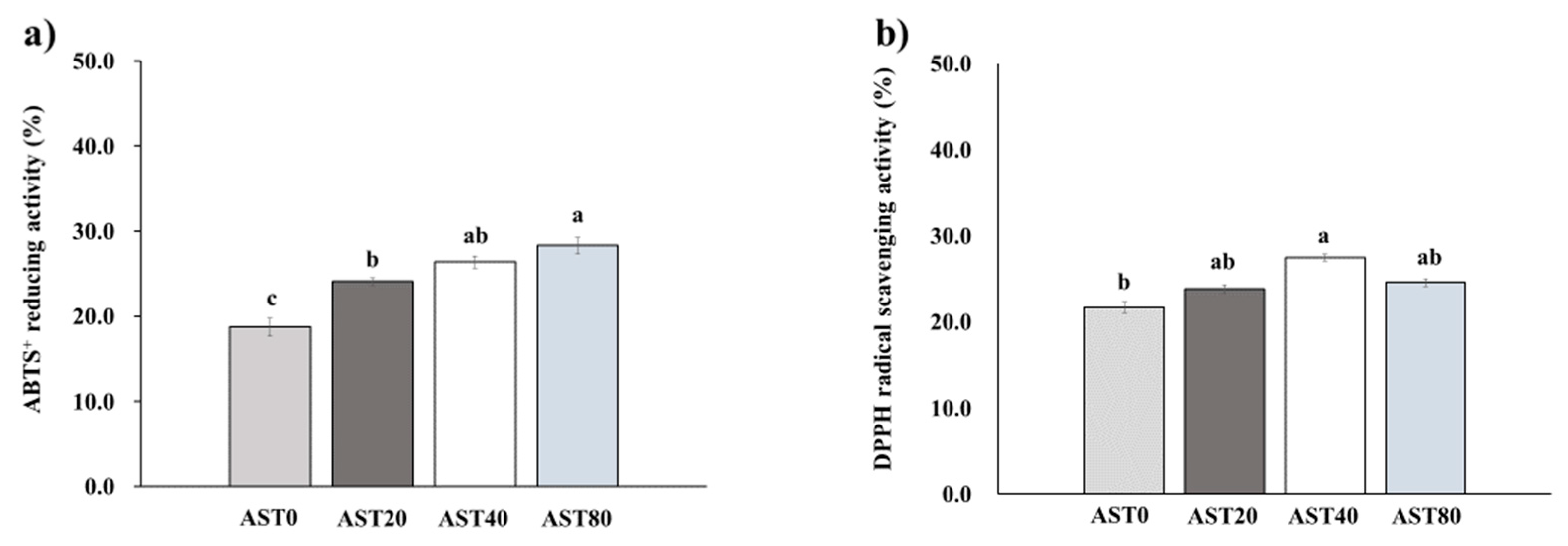
3.2. DPPH Radical Scavenging Activity and ABTS+ Reducing Activity
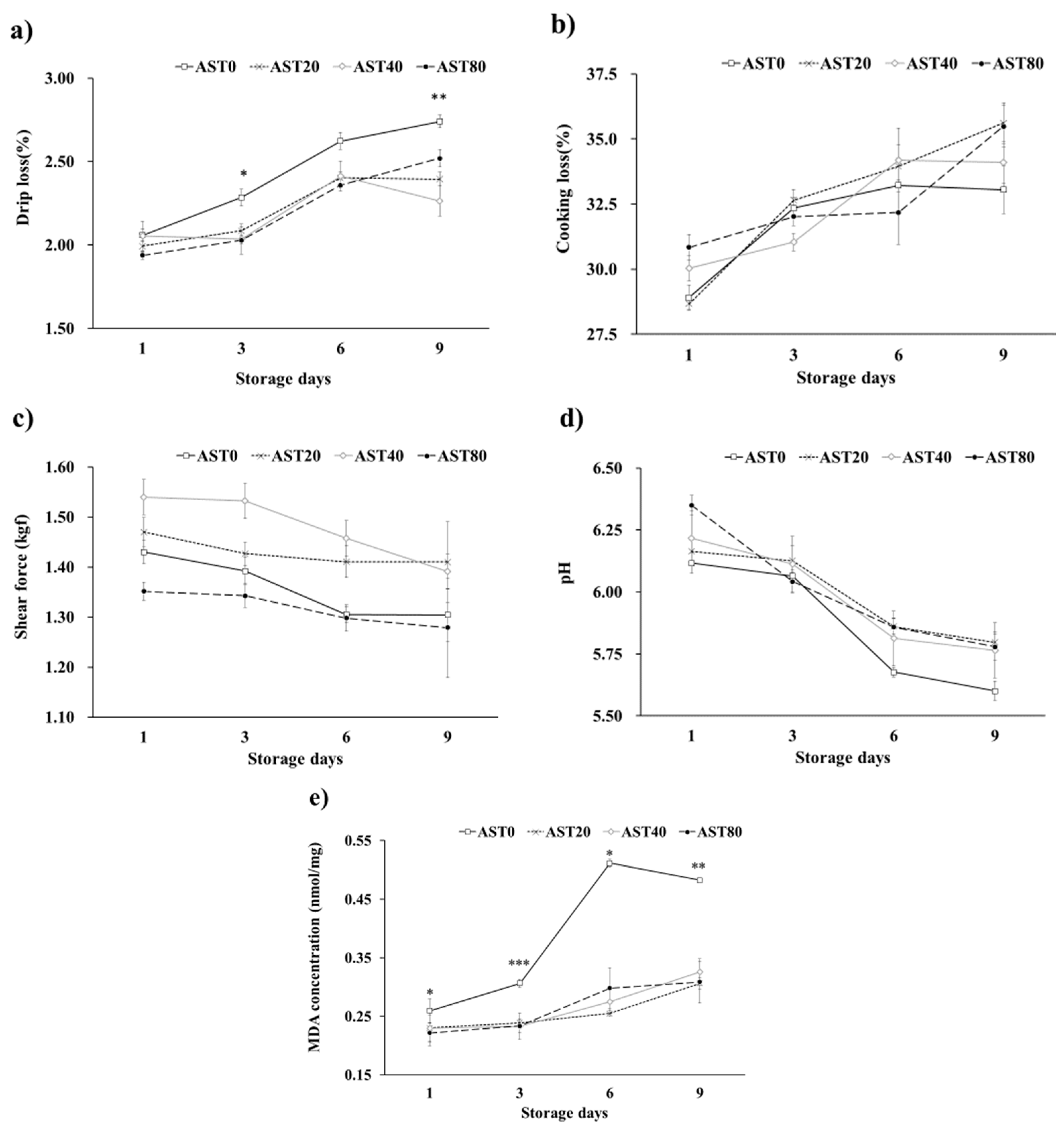
3.3. Growth Performance, Carcass Quality, Immune Organ Ratio, Meat Color and Meat Quality
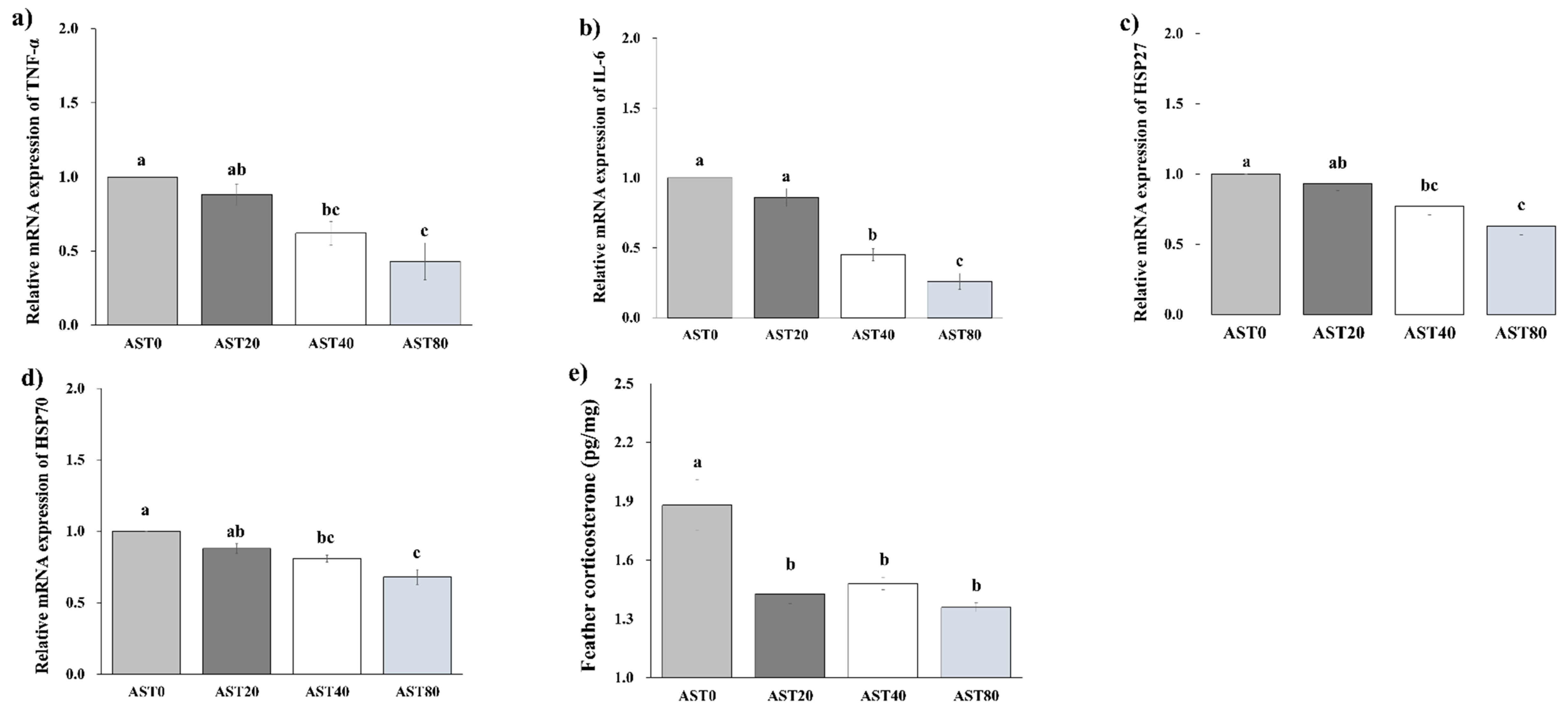
3.4. Hepatic Gene Expression and Feather Corticosterone
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Estevez, M.; Petracci, M. Benefits of Magnesium Supplementation to Broiler Subjected to Dietary and Heat Stress: Improved Redox Status, Breast Quality and Decreased Myopathy Incidence. Antioxidants 2019, 8, 456. [Google Scholar] [CrossRef]
- Sun, T.; Yin, R.; Magnuson, A.D.; Tolba, S.A.; Liu, G.; Lei, X.G. Dose-Dependent Enrichments and Improved Redox Status in Tissues of Broiler Chicks under Heat Stress by Dietary Supplemental Microalgal Astaxanthin. J. Agric. Food Chem. 2018, 66, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, E.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Tavaniello, S.; Maiorano, G.; Slawinska, A. Splenic Gene Expression Signatures in Slow-Growing Chickens Stimulated in Ovo with Galactooligosaccharides and Challenged with Heat. Animals 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.; Warner, R.D.; Dunshea, F.R. Growth performance and characterization of meat quality of broiler chickens supplemented with betaine and antioxidants under cyclic heat stress. Antioxidants 2019, 8, 336. [Google Scholar] [CrossRef]
- Hofmann, T.; Schmucker, S.S.; Bessei, W.; Grashorn, M.; Stefanski, V. Impact of Housing Environment on the Immune System in Chickens: A Review. Animals 2020, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, I.; Che Norma, M.T.; Israf, D.A.; Omar, A.R. The effect of early-age food restriction on heat shock protein 70 response in heat-stressed female broiler chickens. Br. Poult. Sci. 2002, 43, 141–145. [Google Scholar] [CrossRef]
- Dominguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-a mini-review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef]
- Arslan, M.; Yang, X.; Santhakumar, D.; Liu, X.; Hu, X.; Munir, M.; Li, Y.; Zhang, Z. Dynamic expression of interferon lambda regulated genes in primary fibroblasts and immune organs of the chicken. Genes 2019, 10, 145. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- Hu, R.; He, Y.; Arowolo, M.A.; Wu, S.; He, J. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Ambra, R.; Virgili, F. Tocotrienols: A family of molecules with specific biological activities. Antioxidants 2017, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Manfellotto, F.; Stella, G.R.; Falciatore, A.; Brunet, C.; Ferrante, M.I. Engineering the Unicellular Alga Phaeodactylum tricornutum for Enhancing Carotenoid Production. Antioxidants 2020, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, C.T.; Larroquet, L.; Véron, V.; Robaina, L.; Izquierdo, M.S.; Panserat, S.; Kaushik, S.; Fontagné-Dicharry, S. Influence of dietary astaxanthin on the hepatic oxidative stress response caused by episodic hyperoxia in rainbow trout. Antioxidants 2019, 8, 626. [Google Scholar] [CrossRef] [PubMed]
- Escobedo del Bosque, C.I.; Altmann, B.A.; Ciulu, M.; Halle, I.; Jansen, S.; Nolte, T.; Weigend, S.; Mörlein, D. Meat Quality Parameters and Sensory Properties of One High-Performing and Two Local Chicken Breeds Fed with Vicia faba. Foods 2020, 9, 1052. [Google Scholar] [CrossRef]
- Pena-Saldarriaga, L.M.; Fernández-López, J.; Pérez-Alvarez, J.A. Quality of Chicken Fat by-Products: Lipid Profile and Colour Properties. Foods 2020, 9, 1046. [Google Scholar] [CrossRef]
- Aviagen. Ross Broiler Management Manual. Available online: http://pt.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross_Broiler_Manual_ (accessed on 20 February 2019).
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat. Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J.; Ma, Y.B.; Wu, S.G.; Qi, G.H.; Zhang, H.J. Effect of dietary β-alanine supplementation on growth performance, meat quality, carnosine content, and gene expression of carnosine-related enzymes in broilers. Poult. Sci. 2018, 97, 1220–1228. [Google Scholar] [CrossRef]
- Shim, Y.H.; Kim, J.S.; Hosseindoust, A.; Choi, Y.H.; Kim, M.J.; Oh, S.M.; Ham, H.B.; Kumar, A.; Kim, K.Y.; Jang, A.; et al. Investigating meat quality of broiler chickens fed on heat processed diets containing corn distillers dried grains with solubles. Korean J. Food. Sci. Anim. Sci. 2018, 38, 629–635. [Google Scholar]
- Kim, H.J.; Sujiwo, J.; Kim, H.J.; Jang, A. Effects of dipping chicken breast meat inoculated with Listeria monocytogenes in lyophilized scallion, garlic, and kiwi extracts on its physicochemical quality. Korean J. Food. Sci. Anim. Sci. 2019, 39, 418. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Calefi, A.S.; Cruz, D.S.G.; Aloia, T.P.A.; Zager, A.; Astolfi-Ferreira, C.S.; Piantino-Ferreira, J.A.; Sharif, S.; Palermo-Neto, J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunop. 2017, 186, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Park, J.; Shim, K. Heat Treatment at an Early Age Has Effects on the Resistance to Chronic Heat Stress on Broilers. Animals 2019, 9, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Papadomichelakis, G.; Pappas, A.C.; Theodorou, G.; Fegeros, K. Effects of Selenium and Cadmium on Breast Muscle Fatty-Acid Composition and Gene Expression of Liver Antioxidant Proteins in Broilers. Antioxidants 2019, 8, 147. [Google Scholar] [CrossRef]
- Carbajal, A.; Tallo-Parra, O.; Sabes-Alsina, M.; Mular, I.; Lopez-Bejar, M. Feather corticosterone evaluated by ELISA in broilers: A potential tool to evaluate broiler welfare. Poult. Sci. 2014, 93, 2884–2886. [Google Scholar] [CrossRef]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta BBA-Biomembr. 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Pontin, K.P.; Latorre, J.D.; Hernandez-Velasco, X.; Merino-Guzman, R.; Mendez-Albores, A.; Hargis, B.M.; Lopez-Arellano, R.; Tellez-Isaias, G. Evaluation of ascorbic acid or curcumin formulated in a solid dispersion on Salmonella enteritidis infection and intestinal integrity in broiler chickens. Pathogens 2019, 8, 229. [Google Scholar] [CrossRef]
- Xiong, Y.; Yin, Q.; Li, J.; He, S. Oxidative Stress and Endoplasmic Reticulum Stress Are Involved in the Protective Effect of Alpha Lipoic Acid Against Heat Damage in Chicken Testes. Animals 2020, 10, 384. [Google Scholar] [CrossRef]
- Taati, B.; Arazi, H.; Suzuki, K. Oxidative Stress and Inflammation Induced by Waterpipe Tobacco Smoking Despite Possible Protective Effects of Exercise Training: A Review of the Literature. Antioxidants 2020, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Shimamoto, S.; Takahashi, H.; Kawashima, Y.; Wataru, S.; Ijiri, D.; Ohtsuka, A. Effects of astaxanthin-rich dried cell powder from Paracoccus carotinifaciens on carotenoid composition and lipid peroxidation in skeletal muscle of broiler chickens under thermo-neutral or realistic high temperature conditions. Anim. Sci. J. 2019, 90, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Kim, I.H. Effects of astaxanthin produced by Phaffia rhodozyma on growth performance, antioxidant activities, and meat quality in Pekin ducks. Poult. Sci. 2019, 98, 4954–4960. [Google Scholar] [CrossRef]
- Perenlei, G.; Tojo, H.; Okada, T.; Kubota, M.; Kadowaki, M.; Fujimura, S. Effect of dietary astaxanthin rich yeast, P haffia rhodozyma, on meat quality of broiler chickens. Anim. Sci. J. 2014, 85, 895–903. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, I.H. Effect of astaxanthin produced by Phaffia rhodozyma on growth performance, meat quality, and fecal noxious gas emission in broilers. Poult. Sci. 2014, 93, 3138–3144. [Google Scholar] [CrossRef]
- Deng, J.J.; Mao, H.H.; Fang, W.; Li, Z.Q.; Shi, D.; Li, Z.W.; Zhou, T.; Luo, X.C. Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. J. Clean. Prod. 2020, 24, 122655. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Viña, J.; Ji, L.L. Role of redox signaling and inflammation in skeletal muscle adaptations to training. Antioxidants 2016, 5, 48. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef]
- Zuluaga, M.; Barzegari, A.; Letourneur, D.; Gueguen, V.; Pavon-Djavid, G. Oxidative stress regulation on endothelial cells by hydrophilic astaxanthin complex: Chemical, biological, and molecular antioxidant activity evaluation. Oxid. Med. Cell. Longev. 2017, 2017, 8073798. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Sato, K.; Takahashi, K.; Matsushita, K.; Komiyama, H.; Tsunekawa, H.; Nagao, H. Meat color modification in broiler chickens by feeding yeast Phaffia rhodozyma containing high concentrations of astaxanthin. J. Appl. Poultry. Res. 2001, 10, 154–161. [Google Scholar] [CrossRef]
- Von Eugen, K.; Nordquist, R.E.; Zeinstra, E.; van der Staay, F.J. Stocking density affects stress and anxious behavior in the laying hen chick during rearing. Animals 2019, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, A.; Michiels, J.; Golian, A.; Buyse, J.; Wang, Y.; De Smet, S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fed Origanum compactum andCurcuma xanthorrhiza essential oils. Poult. Sci. 2014, 93, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Hasan Siddiqui, S.; Kang, D.; Park, J.; Choi, H.W.; Shim, K. Acute Heat Stress Induces the Differential Expression of Heat Shock Proteins in Different Sections of the Small Intestine of Chickens Based on Exposure Duration. Animals 2020, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Concannon, C.G.; Gorman, A.M.; Samali, A. On the role of Hsp27 in regulating apoptosis. Apoptosis 2003, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Seremelis, I.; Danezis, G.P.; Pappas, A.C.; Zoidis, E.; Fegeros, K. Avian stress-related transcriptome and selenotranscriptome: Role during exposure to heavy metals and heat stress. Antioxidants 2019, 8, 216. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, H.-W.; Song, W.-J.; Jeon, E.Y.; Bang, B.; Shim, E.-J.; Moon, H.-G.; Kim, Y.-K.; Kang, H.-R.; Min, K.-U. TNF-α enhance Th2 and Th17 immune responses regulating by IL23 during sensitization in asthma model. Cytokine 2016, 79, 23–30. [Google Scholar] [CrossRef]
| Genes | Primer Sequences (5′–3′) | Annealing Temp. (°C) | Product Size (bp) | Gene Accession Number | References |
|---|---|---|---|---|---|
| β-actin | F: CAACACAGTGCTGTCTGGTGGTA | 58 | 205 | X00182 | [25] |
| R: ATCGTACTCCTGCTTGCTGATCC | |||||
| TNF-α | F: AGATGGGAAGGGAATGAACC | 60 | 164 | NM_204267.1 | [26] |
| R: GACGTGTCACGATCATCTGG | |||||
| IL-6 | F: CGTGTGCGAGAACAGCATGGAGA | 60 | 110 | NM_204628.1 | [25] |
| R: TCAGGCATTTCTCCTCGTCGAAGC | |||||
| HSP27 | F: ACACGAGGAGAAACAGGATGAG | 60 | 158 | NM_205290.1 | [26] |
| R: ACTGGATGGCTGGCTTGG | |||||
| HSP70 | F: GGTAAGCACAAGCGTGACAATGCT | 55 | 116 | AY143693.1 | [26] |
| R: TCAATCTCAATGCTGGCTTGCGTG |
| Items | Dietary Astaxanthin Supplementation (ppm) | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 80 | Linear | Quadratic | ||
| Plasma (35 d) | |||||||
| Malodialdehyde (nmol/mL) | 16.38 | 14.08 | 11.37 | 9.50 | 0.896 | 0.023 | 0.889 |
| Glutathione (μmol/mL) | 5.73 | 4.99 | 6.28 | 5.61 | 0.196 | 0.572 | 0.932 |
| Superoxide dismutase (U/mL) | 50.63 | 54.77 | 62.10 | 78.08 | 3.641 | 0.027 | 0.354 |
| Catalase (nmol/min/mL) | 0.449 | 0.532 | 0.553 | 0.651 | 0.019 | 0.001 | 0.776 |
| Myeloperoxidase (U/mL) | 28.60 | 26.41 | 25.36 | 25.88 | 0.968 | 0.684 | 0.507 |
| Total antioxidant capacity (U/mL) | 0.563 | 0.593 | 0.607 | 0.650 | 0.212 | 0.185 | 0.880 |
| Leg muscle (35 d) | |||||||
| Malodialdehyde (nmol/mg) | 0.259 | 0.230 | 0.230 | 0.221 | 0.005 | 0.003 | 0.184 |
| Glutathione (μmol/mg) | 2.18 | 2.33 | 2.54 | 2.89 | 0.762 | 0.111 | 0.756 |
| Superoxide dismutase (U/mg) | 33.61 | 36.47 | 33.17 | 36.45 | 1.040 | 0.588 | 0.920 |
| Catalase (nmol/min/mg) | 0.604 | 0.644 | 0.762 | 0.653 | 0.027 | 0.284 | 0.175 |
| Myeloperoxidase (U/mL) | 3.88 | 2.74 | 3.26 | 3.73 | 0.234 | 0.971 | 0.097 |
| Total antioxidant capacity (U/mg) | 0.044 | 0.047 | 0.050 | 0.062 | 0.002 | 0.004 | 0.284 |
| Items | Dietary Astaxanthin Supplementation (ppm) | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|
| 0 | 20 | 40 | 80 | Linear | Quadratic | ||
| Growth performance (22–35 d) | |||||||
| Body weight gain (g/bird) | 1066 | 1127 | 1105 | 1076 | 9.38 | 0.858 | 0.001 |
| Feed intake (g/bird) | 1981 | 2037 | 2026 | 1927 | 59.59 | 0.524 | 0.209 |
| Feed conversion ratio | 1.86 | 1.81 | 1.83 | 1.79 | 0.05 | 0.426 | 0.957 |
| Carcass characteristics (35 d) | |||||||
| Carcass rate (%) | 69.55 | 70.70 | 71.13 | 71.49 | 0.326 | 0.035 | 0.536 |
| Breast muscle (g/100 g BW) | 11.96 | 10.91 | 10.59 | 10.78 | 0.368 | 0.265 | 0.419 |
| Drumsticks (g/100 g BW) | 10.33 | 10.08 | 10.29 | 9.60 | 0.244 | 0.388 | 0.667 |
| Abdominal fat (g/100 g BW) | 1.02 | 0.72 | 0.76 | 0.61 | 0.051 | 0.005 | 0.430 |
| Relative weights of organs (35 d) | |||||||
| Liver (g/100 g BW) | 2.59 | 2.50 | 2.50 | 2.37 | 0.031 | 0.016 | 0.748 |
| Spleen (g/100 g BW) | 0.082 | 0.078 | 0.088 | 0.087 | 0.004 | 0.543 | 0.927 |
| bursa of Fabricius (g/100 g BW) | 0.088 | 0.093 | 0.098 | 0.100 | 0.002 | 0.074 | 0.729 |
| Meat color (35 d) | |||||||
| Lightness (L*) | 47.49 | 46.31 | 45.83 | 45.90 | 2.09 | 0.580 | 0.766 |
| Redness (a*) | 10.51 | 12.90 | 13.74 | 13.81 | 1.04 | 0.032 | 0.277 |
| Yellowness (b*) | 8.47 | 9.08 | 10.82 | 12.47 | 0.67 | 0.001 | 0.445 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseindoust, A.; Oh, S.M.; Ko, H.S.; Jeon, S.M.; Ha, S.H.; Jang, A.; Son, J.S.; Kim, G.Y.; Kang, H.K.; Kim, J.S. Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature. Antioxidants 2020, 9, 1032. https://doi.org/10.3390/antiox9111032
Hosseindoust A, Oh SM, Ko HS, Jeon SM, Ha SH, Jang A, Son JS, Kim GY, Kang HK, Kim JS. Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature. Antioxidants. 2020; 9(11):1032. https://doi.org/10.3390/antiox9111032
Chicago/Turabian StyleHosseindoust, Abdolreza, Seung Min Oh, Han Seo Ko, Se Min Jeon, Sang Hoon Ha, Aera Jang, Ji Seon Son, Gur Yoo Kim, Hwan Ku Kang, and Jin Soo Kim. 2020. "Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature" Antioxidants 9, no. 11: 1032. https://doi.org/10.3390/antiox9111032
APA StyleHosseindoust, A., Oh, S. M., Ko, H. S., Jeon, S. M., Ha, S. H., Jang, A., Son, J. S., Kim, G. Y., Kang, H. K., & Kim, J. S. (2020). Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature. Antioxidants, 9(11), 1032. https://doi.org/10.3390/antiox9111032





