Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation
2.3. Cell Culture and Treatment
2.4. Assessment of Intracellular ROS
2.5. Real-Time Reverse Transcription PCR (qRT-PCR)
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blotting
2.8. Statistical Analysis
3. Results and Discussion
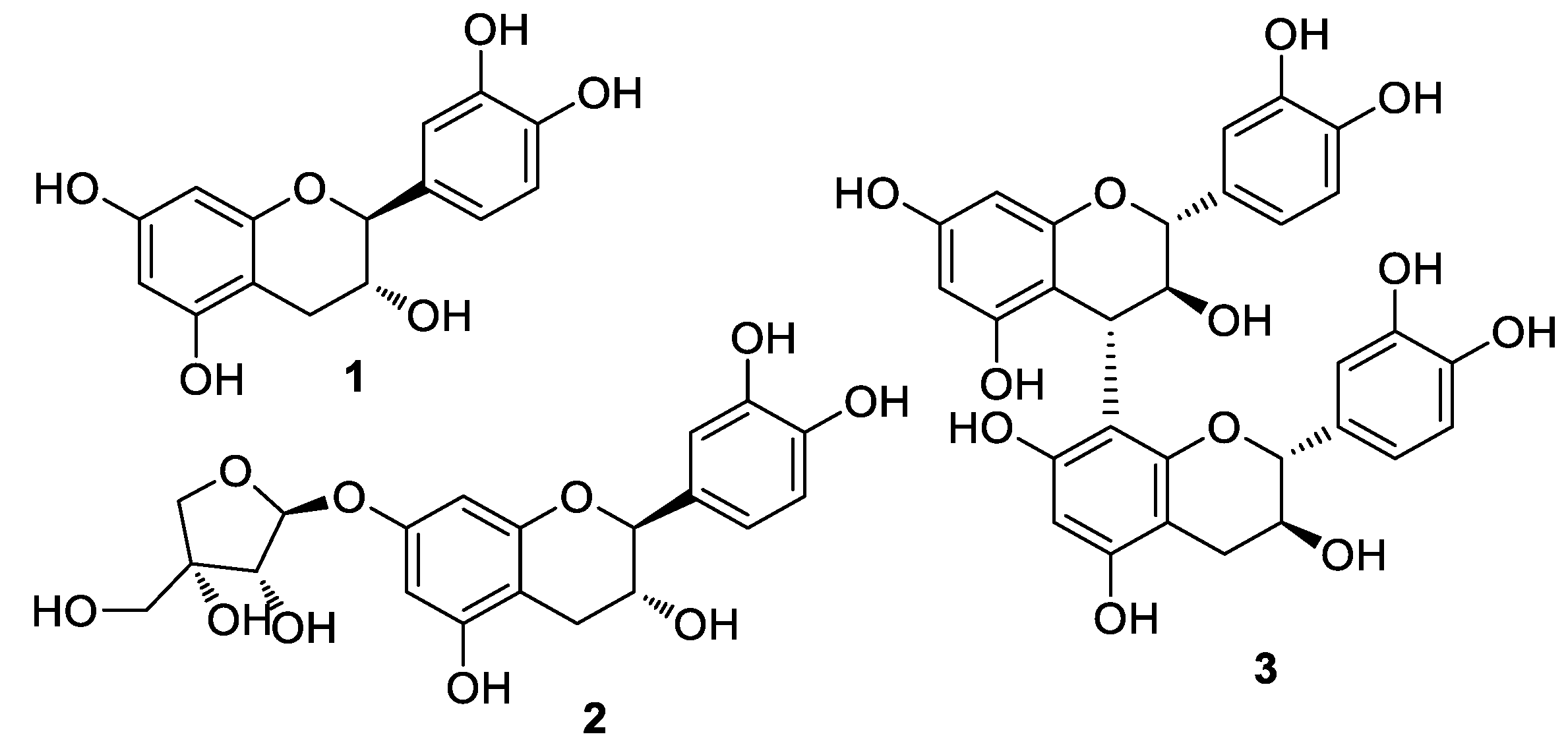
3.1. Isolation and Structural Identification of Compounds
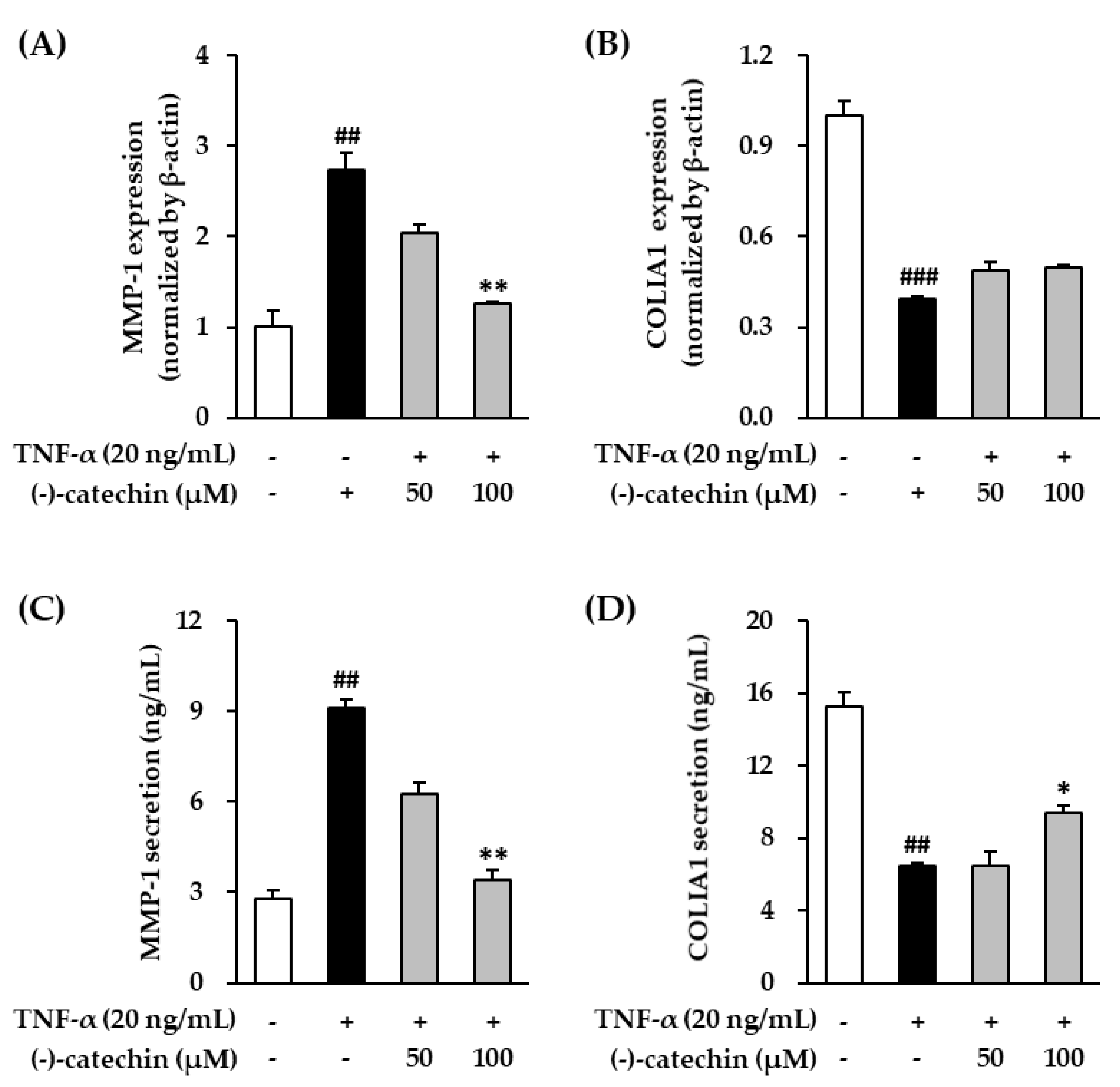
3.2. Effect of (-)-Catechin on MMP-1 and Procollagen I α1 mRNA and Protein Expression in TNF-α-Stimulated NHDFs
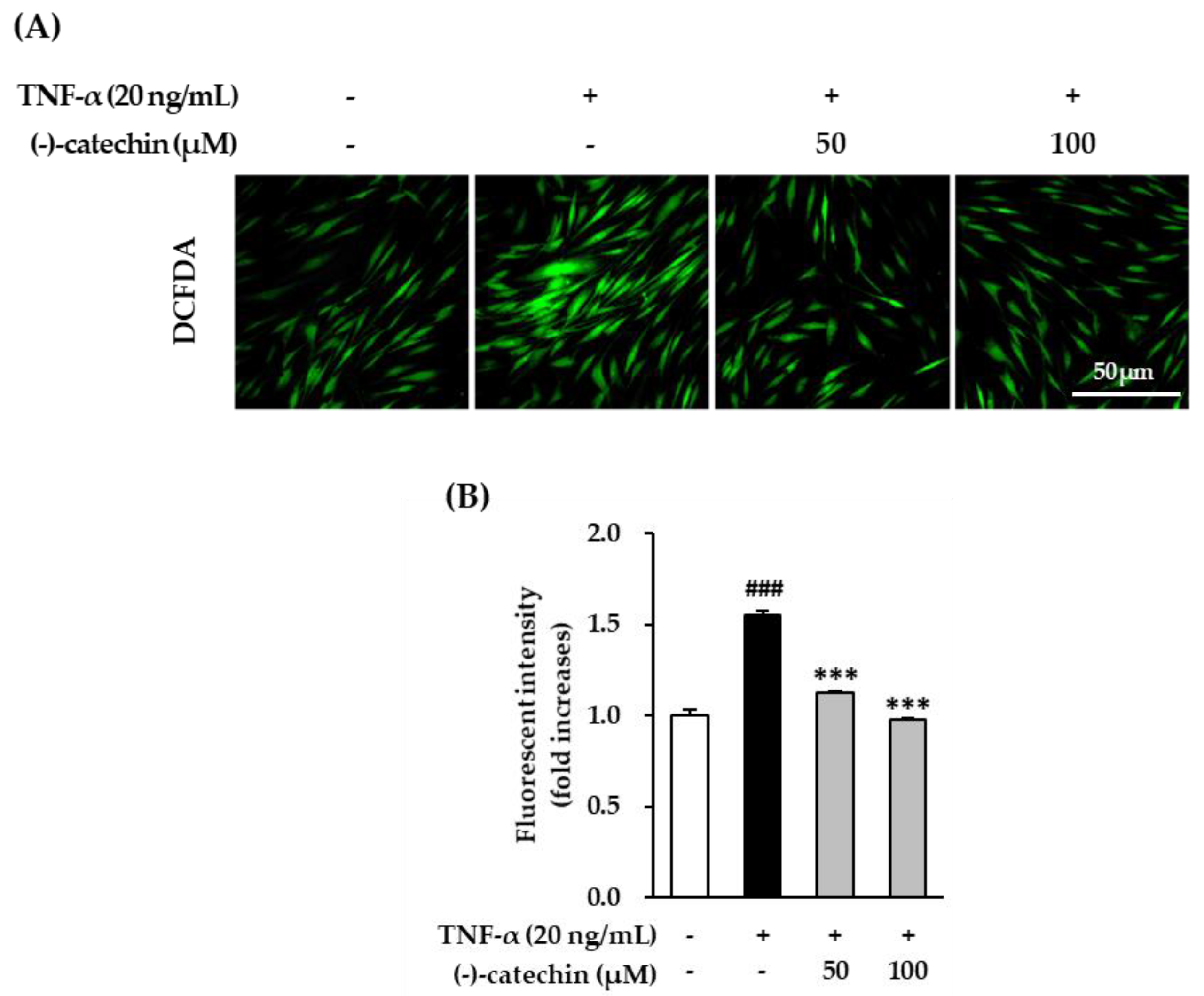
3.3. Inhibitory Effect of (-)-Catechin on Intracellular ROS Production in TNF-α-Stimulated NHDFs
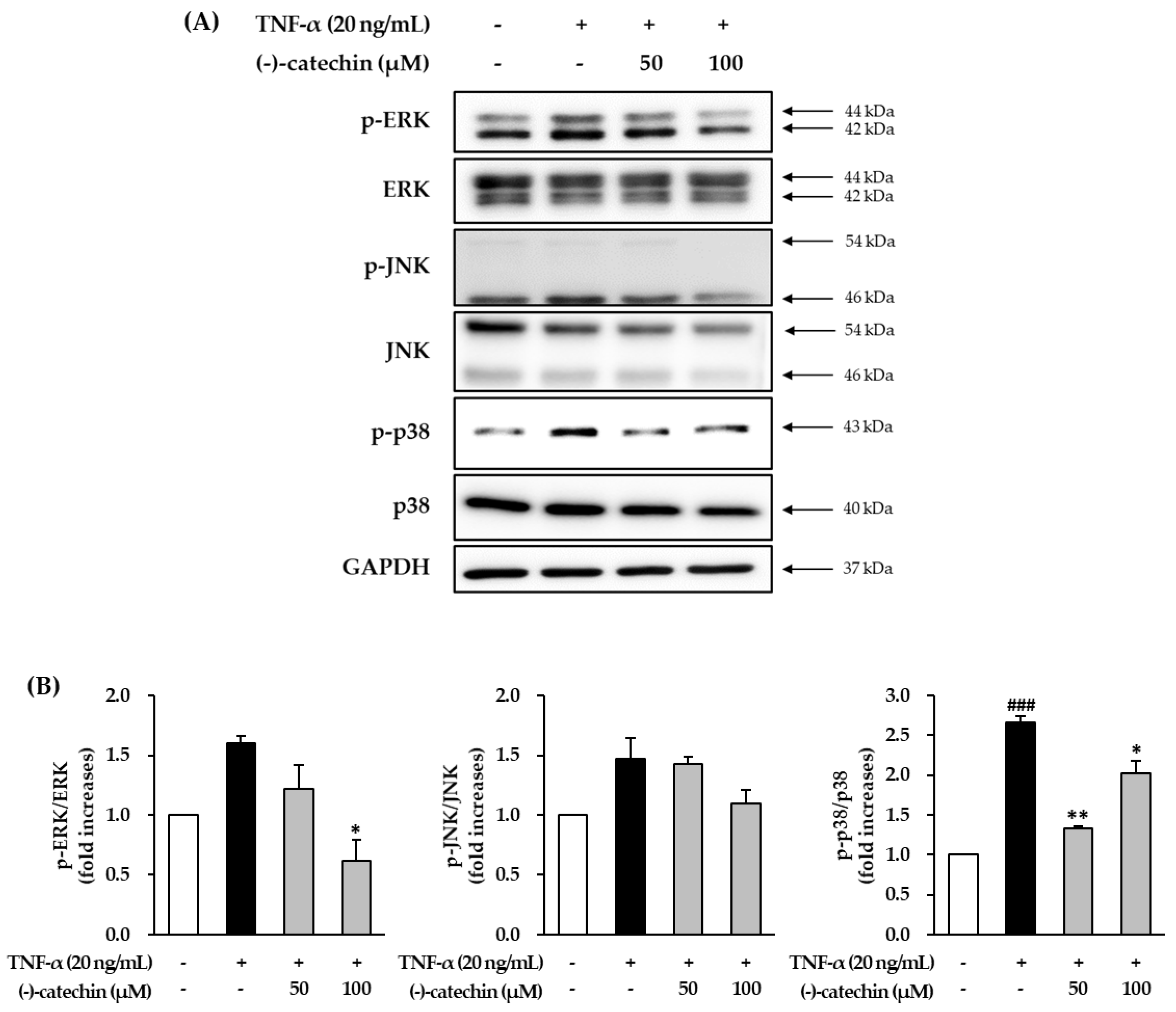
3.4. Effect of (-)-Catechin on TNF-α-Induced Phosphorylation of MAPKs in NHDFs
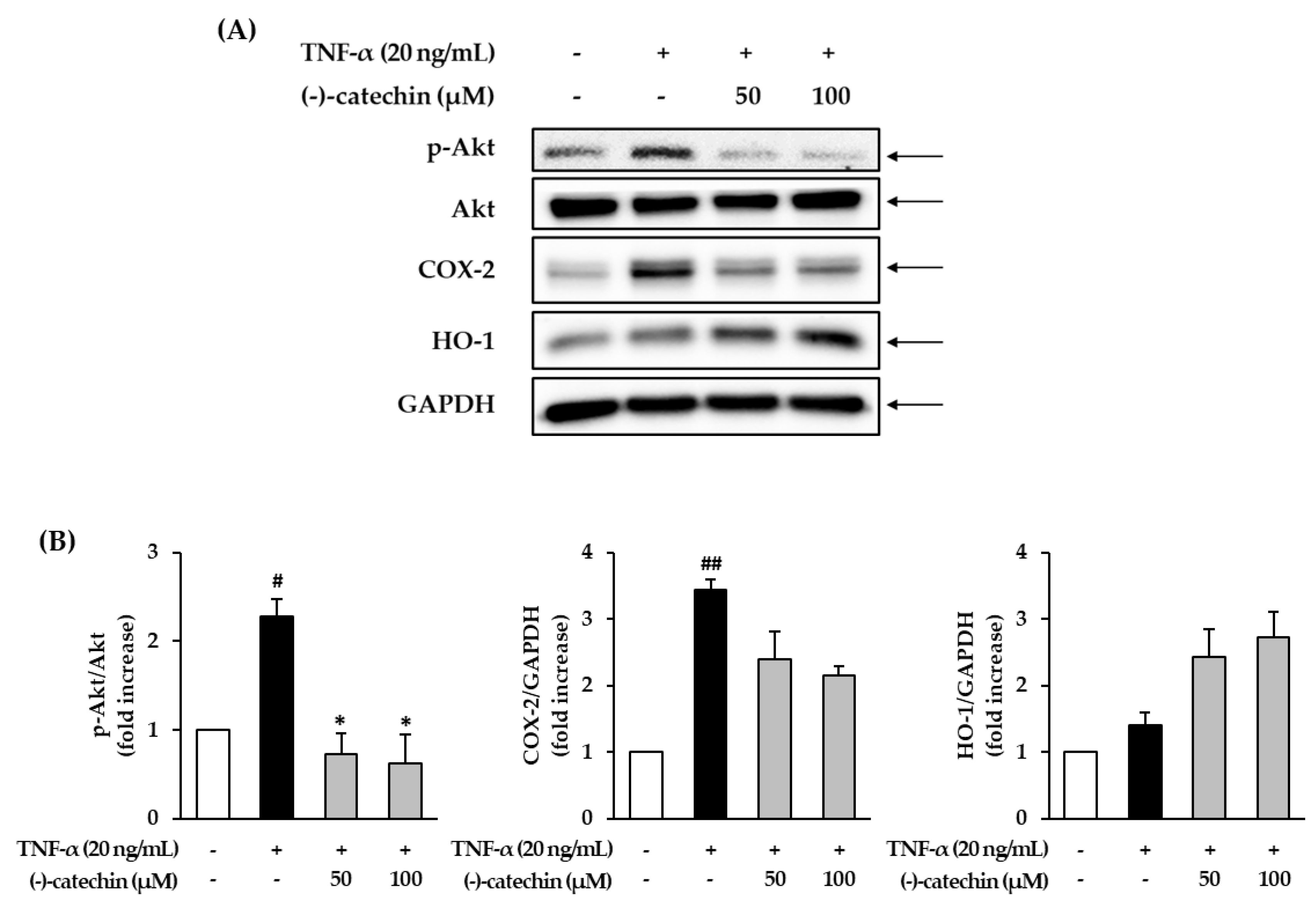
3.5. Effect of (-)-Catechin on the Phosphorylation of Akt and Expression of COX-2 and HO-1 in TNF-α-Stimulated NHDFs
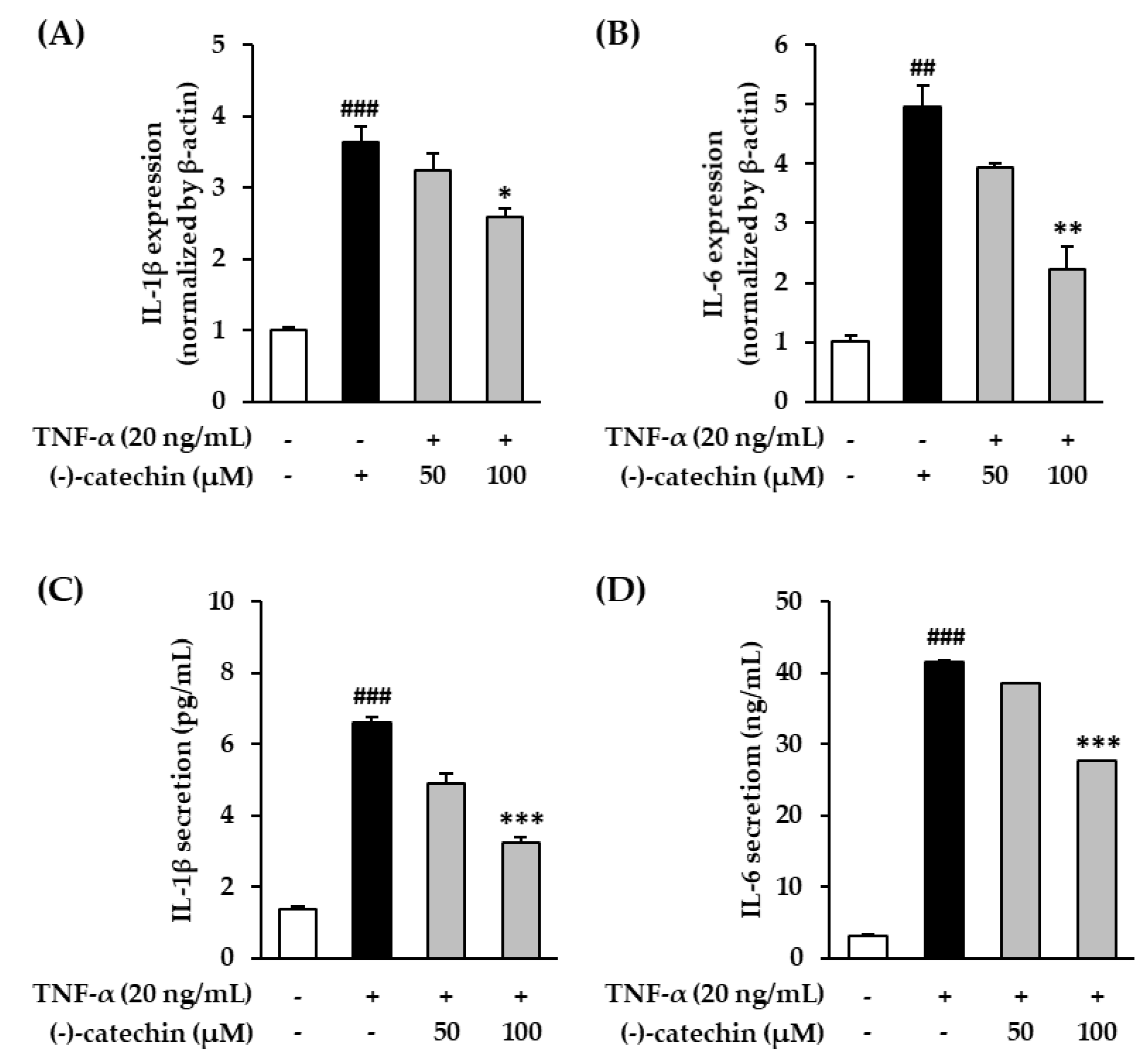
3.6. Effect of (-)-Catechin on Proinflammatory Cytokines in TNF-α-Stimulated NHDFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Aslani, B.A.; Ghobadi, S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016, 146, 163–173. [Google Scholar] [CrossRef]
- Khan, T.A.; Hassan, I.; Ahmad, A.; Perveen, A.; Aman, S.; Quddusi, S.; Alhazza, I.M.; Ashraf, G.M.; Aliev, G. Recent updates on the dynamic association between oxidative stress and neurodegenerative disorders. CNS Neurol. Disord. Drug Targets Former. Curr. Drug Targets CNS Neurol. Disord. 2016, 15, 310–320. [Google Scholar] [CrossRef]
- Rahman, T.; Hosen, I.; Islam, M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997–1019. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. JDD 2008, 7, s12–s16. [Google Scholar]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Derm. ALP Pannonica Adriat 2012, 21, 33–36. [Google Scholar]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, J.J. Collagen synthesis and degradation in the uterine deciduoma: Regulation of collagenase activity by progesterone. Collagen Relat. Res. 1981, 1, 257–268. [Google Scholar] [CrossRef]
- Pan, J.H.; Lim, Y.; Kim, J.H.; Heo, W.; Lee, K.Y.; Shin, H.J.; Kim, J.K.; Lee, J.H.; Kim, Y.J. Root bark of Ulmus davidiana var. japonica restrains acute alcohol-induced hepatic steatosis onset in mice by inhibiting ROS accumulation. PLoS ONE 2017, 12, e0188381. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.-D.; Rho, Y.-S.; Kim, N.-J.; Kim, J.-S. A study on efficacy of Ulmi cortex. Korean J. Pharmacogn. 1990, 21, 217–222. [Google Scholar]
- Lee, M.K.; Kim, Y.C. Five novel neuroprotective triterpene esters of Ulmus davidiana var. japonica. J. Nat. Prod. 2001, 64, 328–331. [Google Scholar] [CrossRef]
- Lee, S. Korean Folk Medicine, Monographs Series No. 3; Publishing Center of Seoul National University: Seoul, Korea, 1996; p. 39. [Google Scholar]
- Son, B.W.; Park, J.H.; Zee, O.-P. Catechin glycoside from Ulmus davidiana. Arch. Pharmacal Res. 1989, 12, 219–222. [Google Scholar] [CrossRef]
- Kim, K.-S.; Lee, S.-D.; Kim, K.-H.; Kil, S.-Y.; Chung, K.-H.; Kim, C.-H. Suppressive effects of a water extract of Ulmus davidiana Planch (Ulmaceae) on collagen-induced arthritis in mice. J. Ethnopharmacol. 2005, 97, 65–71. [Google Scholar] [CrossRef]
- Jung, H.-J.; Jeon, H.-J.; Lim, E.-J.; Ahn, E.-K.; Song, Y.S.; Lee, S.; Shin, K.H.; Lim, C.-J.; Park, E.-H. Anti-angiogenic activity of the methanol extract and its fractions of Ulmus davidiana var. japonica. J. Ethnopharmacol. 2007, 112, 406–409. [Google Scholar] [CrossRef]
- Lee, Y.; Park, H.; Ryu, H.S.; Chun, M.; Kang, S.; Kim, H.-S. Effects of elm bark (Ulmus davidiana var. japonica) extracts on the modulation of immunocompetence in mice. J. Med. Food 2007, 10, 118–125. [Google Scholar] [CrossRef]
- Trinh, T.A.; Park, E.-J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.-E. Estrogenic activity of sanguiin H-6 through activation of estrogen receptor α coactivator-binding site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- Lee, T.K.; Lee, D.; Lee, S.R.; Ko, Y.-J.; Kang, K.S.; Chung, S.J.; Kim, K.H. Sesquiterpenes from Curcuma zedoaria rhizomes and their cytotoxicity against human gastric cancer AGS cells. Bioorg. Chem. 2019, 87, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Yi, S.A.; Nam, K.H.; Ryoo, R.; Lee, J.; Kim, K.H. Pantheric Acids A–C from a Poisonous Mushroom, Amanita pantherina, Promote Lipid Accumulation in Adipocytes. J. Nat. Prod. 2019, 82, 3489–3493. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Eom, H.J.; Lee, D.; Kim, S.; Kang, K.S.; Lee, I.K.; Baek, K.-H.; Park, J.Y.; Kim, K.H. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharmacal Res. 2018, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- So, H.M.; Yu, J.S.; Khan, Z.; Subedi, L.; Ko, Y.-J.; Lee, I.K.; Park, W.S.; Chung, S.J.; Ahn, M.-J.; Kim, S.Y. Chemical constituents of the root bark of Ulmus davidiana var. japonica and their potential biological activities. Bioorg. Chem. 2019, 91, 103145. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Proksch, P.; Conn, E.E. Dhurrin,(−)-catechin, flavonol glycosides and flavones from Chamaebatia foliolosa. Phytochemistry 1987, 26, 1546–1547. [Google Scholar] [CrossRef]
- Na, M.K.; An, R.B.; Lee, S.M.; Min, B.S.; Kim, Y.H.; Bae, K.H.; Kang, S.S. Antioxidant compounds from the stem bark of Sorbus commixta. Nat. Prod. Sci. 2002, 8, 26–29. [Google Scholar]
- Tarascou, I.; Barathieu, K.; André, Y.; Pianet, I.; Dufourc, E.J.; Fouquet, E. An improved synthesis of procyanidin dimers: Regio-and stereocontrol of the interflavan bond. Eur. J. Org. Chem. 2006, 23, 5367–5377. [Google Scholar] [CrossRef]
- Köhler, N.; Wray, V.; Winterhalter, P. Preparative isolation of procyanidins from grape seed extracts by high-speed counter-current chromatography. J. Chromatogr. A 2008, 1177, 114–125. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.-J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-κB, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef]
- Li, C.; Xie, B. Evaluation of the antioxidant and pro-oxidant effects of tea catechin oxypolymers. J. Agric. Food Chem. 2000, 48, 6362–6366. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Takada, K.; Tsunenaga, M.; Fukuda, M.; Matsunaga, Y.; Amano, S.; Kobayashi, K.; Nishiyama, T.; Kohno, Y. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.M.; Sharma, M.R.; Werth, V.P. UVB and proinflammatory cytokines synergistically activate TNF-α production in keratinocytes through enhanced gene transcription. J. Investig. Dermatol. 2009, 129, 994–1001. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduct. 2011, 2011. [Google Scholar] [CrossRef]
- McCubrey, J.A.; LaHair, M.M.; Franklin, R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Torres, M.; Forman, H.J. Redox signaling and the MAP kinase pathways. Biofactors 2003, 17, 287–296. [Google Scholar] [CrossRef]
- Reunanen, N.; Li, S.-P.; Ahonen, M.; Foschi, M.; Han, J.; Kähäri, V.-M. Activation of p38α MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J. Biol. Chem. 2002, 277, 32360–32368. [Google Scholar] [CrossRef]
- Westermarck, J.; Holmström, T.; Ahonen, M.; Eriksson, J.E.; Kähäri, V.-M. Enhancement of fibroblast collagenase-1 (MMP-1) gene expression by tumor promoter okadaic acid is mediated by stress-activated protein kinases Jun N-terminal kinase and p38. Matrix Biol. 1998, 17, 547–557. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Ravi, R.; Piva, T.J. The role of furin in the development of skin cancer. In Highlights in Skin Cancer; Intech: Rijeka, Croatia, 2013; pp. 271–299. [Google Scholar]
- Shetty, M.; Subbannayya, K.; Shivananda, P. Antibacterial activity of tea (Camellia sinensis) and coffee (Coffee arabica) with special reference to Salmonella typhimurium. J. Commun. Dis. 1994, 26, 147–150. [Google Scholar] [PubMed]
- Hirao, K.; Yumoto, H.; Nakanishi, T.; Mukai, K.; Takahashi, K.; Takegawa, D.; Matsuo, T. Tea catechins reduce inflammatory reactions via mitogen-activated protein kinase pathways in toll-like receptor 2 ligand-stimulated dental pulp cells. Life Sci. 2010, 86, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y.; Kawaguchi, K.; Takimoto, H. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor α. Curr. Pharm. Des. 2006, 12, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-H.; Jeong, G.-S. Fisetin inhibits TNF-α-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int. Immunopharmacol. 2015, 29, 246–253. [Google Scholar] [CrossRef]
- Kimura, S.; Warabi, E.; Yanagawa, T.; Ma, D.; Itoh, K.; Ishii, Y.; Kawachi, Y.; Ishii, T. Essential role of Nrf2 in keratinocyte protection from UVA by quercetin. Biochem. Biophys. Res. Commun. 2009, 387, 109–114. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative stress and human skin connective tissue aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Chen, Y.; Lyga, J. Brain-skin connection: Stress, inflammation and skin aging. Inflamm. Allergy Drug Targets Former. Curr. Drug Targets Inflamm. Allergy 2014, 13, 177–190. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2020, 9, 981. https://doi.org/10.3390/antiox9100981
Lee S, Yu JS, Phung HM, Lee JG, Kim KH, Kang KS. Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts. Antioxidants. 2020; 9(10):981. https://doi.org/10.3390/antiox9100981
Chicago/Turabian StyleLee, Sullim, Jae Sik Yu, Hung Manh Phung, Jeong Gun Lee, Ki Hyun Kim, and Ki Sung Kang. 2020. "Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts" Antioxidants 9, no. 10: 981. https://doi.org/10.3390/antiox9100981
APA StyleLee, S., Yu, J. S., Phung, H. M., Lee, J. G., Kim, K. H., & Kang, K. S. (2020). Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts. Antioxidants, 9(10), 981. https://doi.org/10.3390/antiox9100981









