Anticancer Properties of Carnosol: A Summary of In Vitro and In Vivo Evidence
Abstract
1. Introduction
2. Literature Review
2.1. Effects of Carnosol on Lung Cancer In Vitro
2.2. Effects of Carnosol on Colon Cancer In Vitro
2.3. Effects of Carnosol on Breast Cancer In Vitro
2.4. Effects of Carnosol on Breast Cancer In Vivo
2.5. Effects of Carnosol on Pancreatic Cancer In Vitro
2.6. Effects of Carnosol on Prostate Cancer In Vitro
2.7. Effects of Carnosol on Prostate Cancer In Vivo
2.8. Effects of Carnosol on Leukemia In Vitro
2.9. Effects of Carnosol on Brain Cancer In Vitro
2.10. Effects of Carnosol on Skin Cancer In Vitro
2.11. Effects of Carnosol on Skin Cancer In Vivo
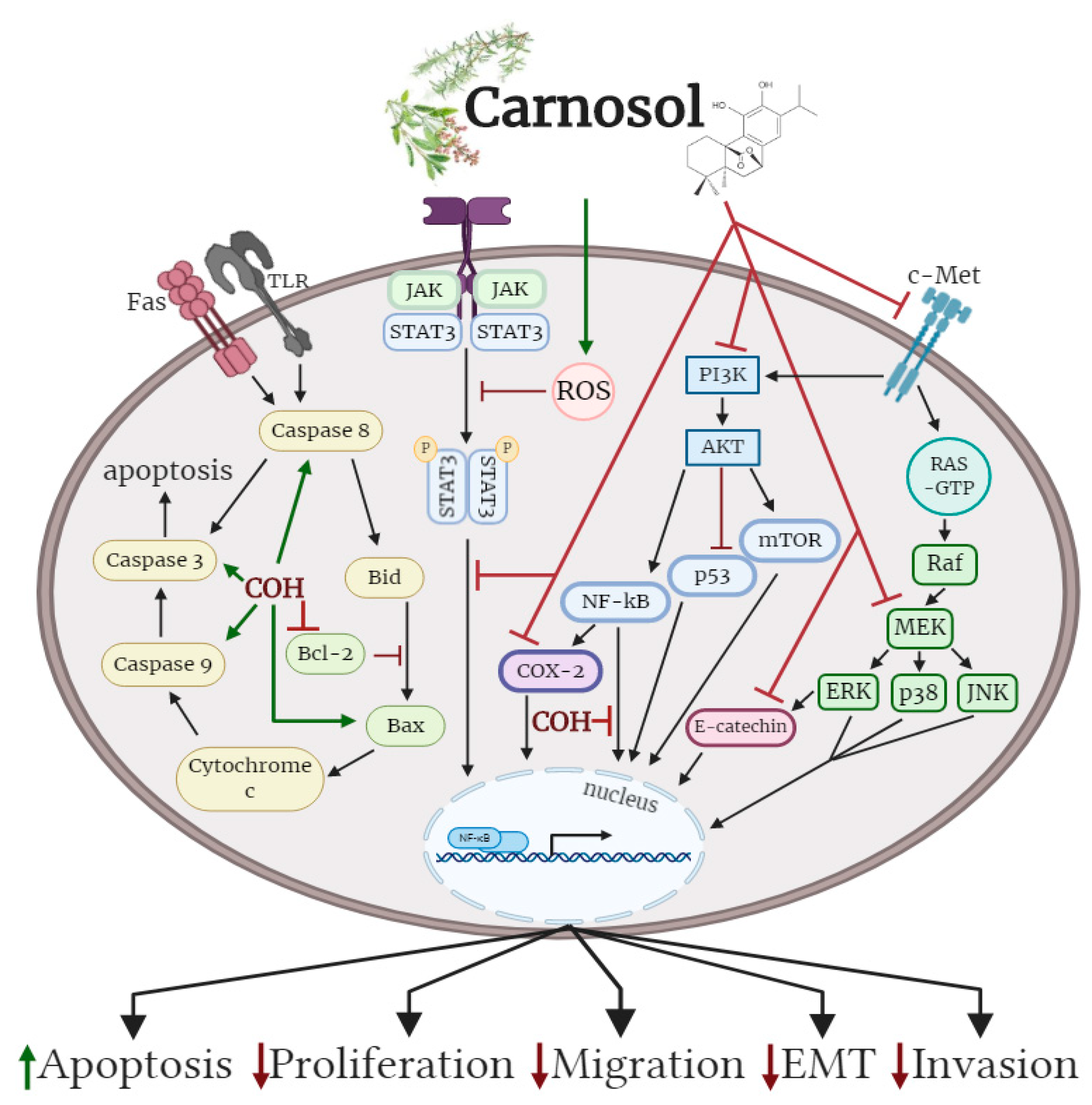
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, J.L. Modern Science Embraces Medicinal Plants. In Nature’s Medicine: Plants that Heal: A Chronicle of Mankind’s Search for Healing Plants through the Ages; National Geographic: Washington, DC, USA, 2000; pp. 110–157. ISBN 978-0-7922-7586-2. [Google Scholar]
- Barrett, B.; Kiefer, D.; Rabago, D. Assessing the risks and benefits of herbal medicine: an overview of scientific evidence. Altern. Ther. Health Med. 1999, 5, 40–49. [Google Scholar] [PubMed]
- Bent, S. Herbal Medicine in the United States: Review of Efficacy, Safety, and Regulation. J. Gen. Intern. Med. 2008, 23, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Lesschaeve, I.; Noble, A.C. Polyphenols: factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-Y.; Lee, Y.-J.; Hong, J.T.; Lee, H.-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Gajhede, M.; Anthoni, U.; Per Nielsen, H.; Pedersen, E.J.; Christophersen, C. Carnosol. Crystal structure, absolute configuration, and spectroscopic properties of a diterpene. J. Crystallogr. Spectrosc. Res. 1990, 20, 165–171. [Google Scholar] [CrossRef]
- Pizzale, L.; Bortolomeazzi, R.; Vichi, S.; Überegger, E.; Conte, L.S. Antioxidant activity of sage (Salvia officinalis and S fruticosa) and oregano (Origanum onites and O indercedens) extracts related to their phenolic compound content. J. Sci. Food Agric. 2002, 82, 1645–1651. [Google Scholar] [CrossRef]
- Zeng, H.-H.; Tu, P.-F.; Zhou, K.; Wang, H.; Wang, B.-H.; Lu, J.-F. Antioxidant properties of phenolic diterpenes from Rosmarinus officinalis. Acta Pharmacol. Sin. 2016, 22, 1094–1098. [Google Scholar]
- Munné-Bosch, S.; Schwarz, K.; Alegre, L. Response of abietane diterpenes to stress in Rosmarinus officinalis L.: New insights into the function of diterpenes in plants. Free Radic. Res. 1999, 31 (Suppl. 1), S107–S112. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef]
- Gaté, L.; Paul, J.; Ba, G.N.; Tew, K.D.; Tapiero, H. Oxidative stress induced in pathologies: The role of antioxidants. Biomed. Pharmacother. 1999, 53, 169–180. [Google Scholar] [CrossRef]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; Ciucis, C.D.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Aeschbach, R.; Löligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Bains, S.K.; Pitchumony, T.S.; de Castro Brás, L.E.; Drago, F.; Dubois-Randé, J.-L.; Bucolo, C.; Motterlini, R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol. Res. 2013, 76, 132–148. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Hsieh, C.; Yang, Y.; Wung, B. Upregulation of NF-E2-related factor-2-dependent glutathione by carnosol provokes a cytoprotective response and enhances cell survival. Acta Pharmacol. Sin. 2011, 32, 62–69. [Google Scholar] [CrossRef][Green Version]
- Singletary, K.W. Rosemary extract and carnosol stimulate rat liver glutathione-S-transferase and quinone reductase activities. Cancer Lett. 1996, 100, 139–144. [Google Scholar] [CrossRef]
- Fang, J.; Seki, T.; Maeda, H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv. Drug Deliv. Rev. 2009, 61, 290–302. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Marín, F.R.; Santoyo, S.; García-Risco, M.R.; Señoráns, F.J.; Reglero, G. Testing and Enhancing the in Vitro Bioaccessibility and Bioavailability of Rosmarinus officinalis Extracts with a High Level of Antioxidant Abietanes. J. Agric. Food Chem. 2010, 58, 1144–1152. [Google Scholar] [CrossRef]
- Romo Vaquero, M.; Yáñez-Gascón, M.-J.; García Villalba, R.; Larrosa, M.; Fromentin, E.; Ibarra, A.; Roller, M.; Tomás-Barberán, F.; Espín de Gea, J.C.; García-Conesa, M.-T. Inhibition of Gastric Lipase as a Mechanism for Body Weight and Plasma Lipids Reduction in Zucker Rats Fed a Rosemary Extract Rich in Carnosic Acid. PLoS ONE 2012, 7, e39773. [Google Scholar] [CrossRef]
- Vaquero, M.R.; Villalba, R.G.; Larrosa, M.; Yáñez-Gascón, M.J.; Fromentin, E.; Flanagan, J.; Roller, M.; Tomás-Barberán, F.A.; Espín, J.C.; García-Conesa, M.-T. Bioavailability of the major bioactive diterpenoids in a rosemary extract: Metabolic profile in the intestine, liver, plasma, and brain of Zucker rats. Mol. Nutr. Food Res. 2013, 57, 1834–1846. [Google Scholar] [CrossRef]
- Kashyap, D.; Kumar, G.; Sharma, A.; Sak, K.; Tuli, H.S.; Mukherjee, T.K. Mechanistic insight into carnosol-mediated pharmacological effects: Recent trends and advancements. Life Sci. 2017, 169, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased Oral Bioavailability of Resveratrol by Its Encapsulation in Casein Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.-H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung Cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Offord, E.A.; Macé, K.; Ruffieux, C.; Malnoë, A.; Pfeifer, A.M. Rosemary components inhibit benzo[a]pyrene-induced genotoxicity in human bronchial cells. Carcinogenesis 1995, 16, 2057–2062. [Google Scholar] [CrossRef]
- Boyle, P.; Langman, J.S. ABC Of Colorectal Cancer: Epidemiology. BMJ 2000, 321, 805–808. [Google Scholar] [CrossRef]
- Cunningham, D.; Atkin, W.; Lenz, H.-J.; Lynch, H.T.; Minsky, B.; Nordlinger, B.; Starling, N. Colorectal cancer. Lancet 2010, 375, 1030–1047. [Google Scholar] [CrossRef]
- Park, K.-W.; Kundu, J.; Chae, I.-G.; Kim, D.-H.; Yu, M.-H.; Kundu, J.K.; Chun, K.-S. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int. J. Oncol. 2014, 44, 1309–1315. [Google Scholar] [CrossRef]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Cole, P.A.; Dannenberg, A.J. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and-independent mechanisms. Cancer Res. 2002, 62, 2522–2530. [Google Scholar] [PubMed]
- Johnson, J.J.; Syed, D.N.; Suh, Y.; Heren, C.R.; Saleem, M.; Siddiqui, I.A.; Mukhtar, H. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev. Res. (Phila. Pa.) 2010, 3, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Potter, D.A. CYP1A1 regulates breast cancer proliferation and survival. Mol. Cancer Res. 2013, 11, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Al Dhaheri, Y.; Attoub, S.; Ramadan, G.; Arafat, K.; Bajbouj, K.; Karuvantevida, N.; AbuQamar, S.; Eid, A.; Iratni, R. Carnosol Induces ROS-Mediated Beclin1-Independent Autophagy and Apoptosis in Triple Negative Breast Cancer. PLoS ONE 2014, 9, e109630. [Google Scholar] [CrossRef]
- Vergara, D.; Simeone, P.; Bettini, S.; Tinelli, A.; Valli, L.; Storelli, C.; Leo, S.; Santino, A.; Maffia, M. Antitumor activity of the dietary diterpene carnosol against a panel of human cancer cell lines. Food Funct. 2014, 5, 1261. [Google Scholar] [CrossRef]
- Telang, N. Anti-proliferative and pro-apoptotic effects of rosemary and constituent terpenoids in a model for the HER-2-enriched molecular subtype of clinical breast cancer. Oncol. Lett. 2018, 16, 5489–5497. [Google Scholar] [CrossRef]
- Alsamri, H.; El Hasasna, H.; Al Dhaheri, Y.; Eid, A.H.; Attoub, S.; Iratni, R. Carnosol, a Natural Polyphenol, Inhibits Migration, Metastasis, and Tumor Growth of Breast Cancer via a ROS-Dependent Proteasome Degradation of STAT3. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Singletary, K.; MacDonald, C.; Wallig, M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996, 104, 43–48. [Google Scholar] [CrossRef]
- Anderson, K.E.; Mack, T.M.; Silverman, D.T. Cancer of the pancreas. In Cancer Epidemiology and Prevention; Oxford University Press: New York, NY, USA; Oxford, UK, 2006; pp. 721–762. ISBN 9786610835089. [Google Scholar]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Aliebrahimi, S.; Kouhsari, S.M.; Arab, S.S.; Shadboorestan, A.; Ostad, S.N. Phytochemicals, withaferin A and carnosol, overcome pancreatic cancer stem cells as c-Met inhibitors. Biomed. Pharmacother. 2018, 106, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.S.; Tan, H.-J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Syed, D.N.; Heren, C.R.; Suh, Y.; Adhami, V.M.; Mukhtar, H. Carnosol, a Dietary Diterpene, Displays Growth Inhibitory Effects in Human Prostate Cancer PC3 Cells Leading to G2-Phase Cell Cycle Arrest and Targets the 5′-AMP-Activated Protein Kinase (AMPK) Pathway. Pharm. Res. 2008, 25, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Song, Q.; Yang, J.; Yu, S.; Zhao, J.; Yu, G. Carnosol inhibits Hedgehog signaling pathway in both LNCaP and DU145 prostate cancer cell lines. Cell. Mol. Biol. Noisy--Gd. Fr. 2017, 63, 104–108. [Google Scholar] [CrossRef]
- Linet, M.S.; Devesa, S.S.; Morgan, G.J. The leukemias. In Cancer Epidemiology and Prevention; Oxford University Press: New York, NY, USA; Oxford, UK, 2006; pp. 841–871. ISBN 9786610835089. [Google Scholar]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef]
- Dörrie, J.; Sapala, K.; Zunino, S.J. Carnosol-induced apoptosis and downregulation of Bcl-2 in B-lineage leukemia cells. Cancer Lett. 2001, 170, 33–39. [Google Scholar] [CrossRef]
- Ishida, Y.; Yamasaki, M.; Yukizaki, C.; Nishiyama, K.; Tsubouchi, H.; Okayama, A.; Kataoka, H. Carnosol, rosemary ingredient, induces apoptosis in adult T-cell leukemia/lymphoma cells via glutathione depletion: proteomic approach using fluorescent two-dimensional differential gel electrophoresis. Hum. Cell 2014, 27, 68–77. [Google Scholar] [CrossRef]
- Lee, K.W.; Hur, H.J.; Lee, H.J.; Lee, C.Y. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. J. Agric. Food Chem. 2005, 53, 1990–1995. [Google Scholar] [CrossRef]
- Liu, R.H.; Sun, J. Antiproliferative activity of apples is not due to phenolic-induced hydrogen peroxide formation. J. Agric. Food Chem. 2003, 51, 1718–1723. [Google Scholar] [CrossRef]
- Ohgaki, H. Epidemiology of brain tumors. In Cancer Epidemiology: Modifiable Factors; Verma, M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 472, ISBN 978-1-60327-491-3. [Google Scholar]
- McNeill, K.A. Epidemiology of Brain Tumors. Neurol. Clin. 2016, 34, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Sasmita, A.O.; Wong, Y.P.; Ling, A.P.K. Biomarkers and therapeutic advances in glioblastoma multiforme. Asia Pac. J. Clin. Oncol. 2018, 14, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Parlato, C. Surgery, radiotherapy and temozolomide in treating high-grade gliomas. Front. Biosci. 2006, 11, 1280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giacomelli, C.; Natali, L.; Trincavelli, M.L.; Daniele, S.; Bertoli, A.; Flamini, G.; Braca, A.; Martini, C. New insights into the anticancer activity of carnosol: p53 reactivation in the U87MG human glioblastoma cell line. Int. J. Biochem. Cell Biol. 2016, 74, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Daniele, S.; Natali, L.; Iofrida, C.; Flamini, G.; Braca, A.; Trincavelli, M.L.; Martini, C. Carnosol controls the human glioblastoma stemness features through the epithelial-mesenchymal transition modulation and the induction of cancer stem cell apoptosis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Gordon, R. Skin Cancer: An Overview of Epidemiology and Risk Factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Ng, C.; Yen, H.; Hsiao, H.-Y.; Su, S.-C. Phytochemicals in Skin Cancer Prevention and Treatment: An Updated Review. Int. J. Mol. Sci. 2018, 19, 941. [Google Scholar] [CrossRef]
- Huang, S.-C.; Ho, C.-T.; Lin-Shiau, S.-Y.; Lin, J.-K. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappaB and c-Jun. Biochem. Pharmacol. 2005, 69, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Mohebati, A.; Guttenplan, J.B.; Kochhar, A.; Zhao, Z.-L.; Kosinska, W.; Subbaramaiah, K.; Dannenberg, A.J. Carnosol, a Constituent of Zyflamend, Inhibits Aryl Hydrocarbon Receptor-Mediated Activation of CYP1A1 and CYP1B1 Transcription and Mutagenesis. Cancer Prev. Res. (Phila. Pa.) 2012, 5, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.; Achel, D.G.; Olivares, A.; Olmos, E.; Alcaraz-Saura, M.; Castillo, J. Carnosol, radiation and melanoma: a translational possibility. Clin. Transl. Oncol. 2013, 15, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.; Cicconi, R.; Mignogna, G.; Giorgi, A.; Mattei, M.; Graziani, G.; Ferracane, R.; Grosso, A.; Aducci, P.; Schininà, M.E.; et al. Anti-Proliferative Effect of Rosmarinus officinalis L. Extract on Human Melanoma A375 Cells. PLoS ONE 2015, 10, e0132439. [Google Scholar] [CrossRef]
- Tong, L.; Wu, S. The Mechanisms of Carnosol in Chemoprevention of Ultraviolet B-Light-Induced Non-Melanoma Skin Cancer Formation. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Huang, M.-T.; Ho, C.-T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.-R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of Skin Tumorigenesis by Rosemary and Its Constituents Carnosol and Ursolic Acid. Cancer Res. 1994, 54, 701–708. [Google Scholar]
- Núñez, M.J.; Reyes, C.P.; Jiménez, I.A.; Hayashi, H.; Tokuda, H.; Bazzocchi, I.L. ent-Rosane and abietane diterpenoids as cancer chemopreventive agents. Phytochemistry 2011, 72, 385–390. [Google Scholar] [CrossRef]
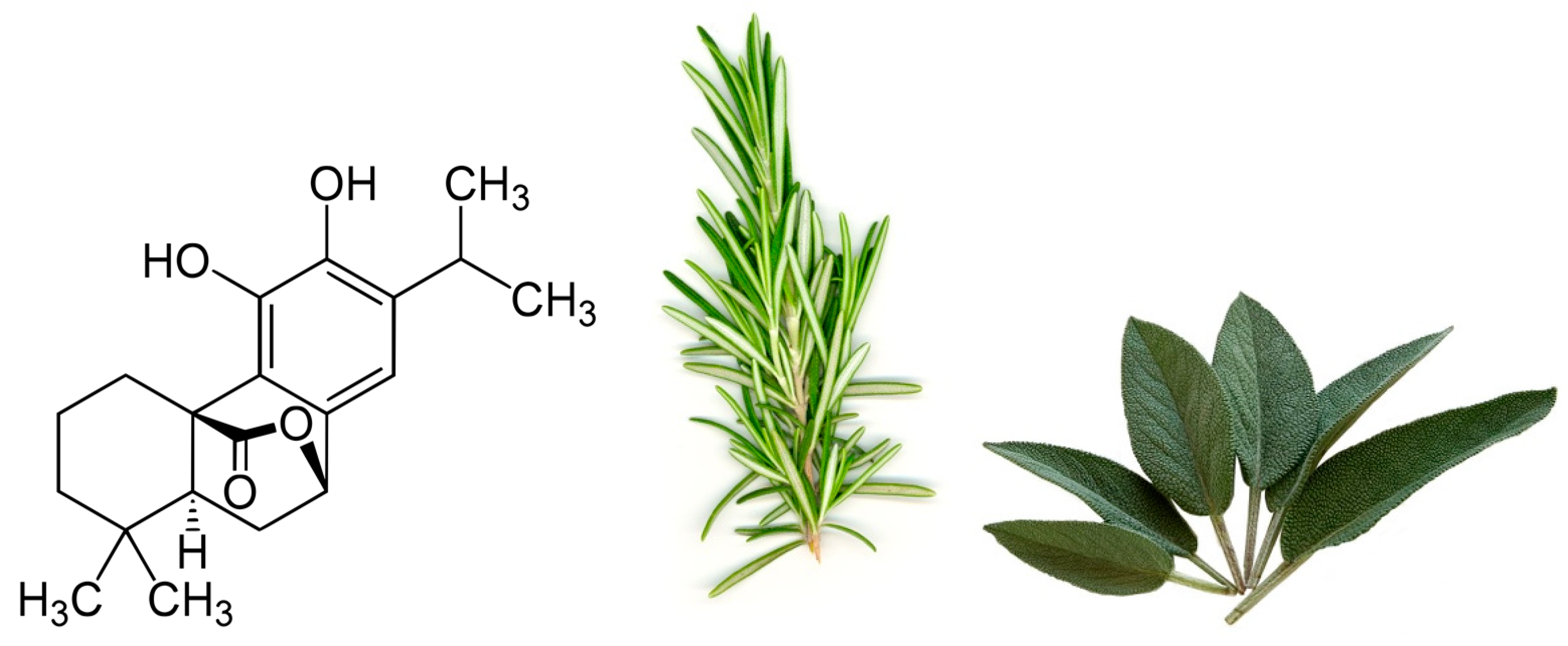
| Cell Line | Treatment | Effect | Reference |
|---|---|---|---|
| BEAS-2B (B[a]P-induced carcinogenesis) | 0.6–6 µg/mL; 7–24 h | ↓DNA adduct formation ↓CYP1A1 mRNA ↓CYP1A1 activity ↑GSTπ mRNA and protein ↑QR mRNA | [37] |
| Cell Line | Treatment | Effect | Reference |
|---|---|---|---|
| HCT116 | 5–100 µM; 24–72 h | ↓Viability ↑Apoptosis ↑c-Caspase-9 ↑c-Caspase-3 ↑c-PARP ↑p53 ↓Mdm2 ↓p-STAT3 ↓Cyclin-D1, -D2, and -D3 ↓Survivin ↓p-Jak2 ↓p-Src | [40] |
| Cell Line | Treatment | Effect | Reference |
|---|---|---|---|
| 184B5/HER | 20–60 μM; 4.5 h | ↓Tumorigenesis ↓COX-2 ↓AP-1 activity ↓PKC ↓ERK1/2 ↓P38 ↓JNK ↓PGE2 synthesis | [43] |
| MCF7 | 40 μM; 48 h | ↓Cell viability IC50 25.6 µM ↑Cytotoxic activity IC50 82 µM ↓AR ↓ER-α | [44] |
| MCF7 MDA-MB-231 | 10–100 μM; 2–12 h | ↓Proliferation IC50 40 μM AMPK ↓CYP1A1 ↓AhR | [45] |
| MDA-MB-231 | 25–100 μM; 48 h | ↓Cell Viability ↑ Apoptosis ↓Colony formation ↑Autophagy ↑ ROS generation ↑DNA damage ↑pERK1&2 ↑P21 ↑ Cleaved Parp Caspases 3,8,9 | [46] |
| HBL-100 MDA-231 MDA-361 MDA-435 MCF-7 | 25–200 μM; 72 h | ↓Cell Viability IC50 >50 μM ↓Cell adhesion ↓Growth | [47] |
| 184-B5/HER | 1–5 μM; 21 days | ↓Colony formation IC50 2.5 µM ↑G2 phase-specific cyclin B1 expression ↑G2/M phase arrest | [48] |
| MDA-MB-231 Hs578T MCF-7 T47D | 25–100 μM; 24 h | ↓Migration ↓Invasive potential MMP-9 ↓phosphorylated and total STAT3 | [49] |
| Model | Treatment | Effect | Reference |
|---|---|---|---|
| Female Sprague–Dawley rats (DMBA-induced tumorigenesis) | 200 mg/kg; 5 days | ↓DNA adduct formation ↓Mammary adenocarcinoma | [50] |
| Chick embryo (MDA-MB-231-GFP xenograph) | 50–100 µM | ↓Tumor mass ↓Metastases | [49] |
| Cell Line | Treatment | Effect | Reference |
|---|---|---|---|
| AsPC-1 | 0–75 µM; 8–48 h | ↑Apoptosis ↓Proliferation ↑S-phase cell cycle arrest ↓Sphere formation ↓Colony formation ↓oct-4 ↓nanog | [53] |
| Cell Line/Animal Model | Treatment | Effects | Reference |
|---|---|---|---|
| PC3 | 10–70 μM; 24–72 h | ↑Apoptosis ↓Cell Viability ↑BAX ↓Bcl-2 ↑Caspase-7 and -8 ↓p21 (Waf1/Cip1) ↓Cyclins A, D1, D2 ↓CDK6 and CDK 2 ↓mTOR (Ser2448) ↓AMPK-α (Thr172) ↓p70 S6K ↑4E-BP1 ↑PTEN ↓PI3K (p85 and p110) | [56] |
| 22 RV1 PCA LNCAP | 20–40 μM; 48 h | ↓Cell Viability ↓AR ↓ERα | [44] |
| LNCAP DU145 | 0.25–16 μM; 48 h | ↑Apoptosis ↓Cell survival ↓Proliferation ↓GLi1 ↓Shh ↑Caspase 3 activity | [57] |
| Athymic nude mice implanted with 22RV1 cells | 30 mg/kg; 28 days | ↓Tumor growth ↓Serum prostate-specific antigen ↓AR ↓ER-α | [44] |
| Cell Line | Treatment | Effect | Reference |
|---|---|---|---|
| SEM RS4;11 MV4;11 REH Nalm-6 | 9–27 µM; 24 h | ↑Apoptosis ↓Viability ↓Nuclear DNA ↑Depolarization of mitochondrial membranes ↓Bcl-2 | [60] |
| ED | 40 µM; 3–24 h | ↑Apoptosis ↑Caspase-3 ↑Caspase-7 ↓Glutathione | [61] |
| Cell Line | Treatment | Effect | Reference |
|---|---|---|---|
| U87MG U343MG T98G | 0.1–100 µM; 24–72 h | ↓Proliferation ↑Apoptosis ↓Cell migration ↑G2-cell cycle arrest ↑p53 protein ↓p53/MDM2 complex ↑p21 transcription ↑PUMA transcription ↑MDM2 transcription ↑BAX transcription ↓Bcl-2 transcription | [68] |
| U87MG U87MG-CSC U343MG-CSC T98G-CSC | 10 nm–40 µM | ↓CSC viability ↑CSC apoptosis ↑G2-cell cycle arrest ↓Mesenchymal phenotype ↓N-cadherin ↑E-cadherin ↓CD44 ↓Nanog ↓Oct4 ↓BMI1 ↓SOX2 ↓Nestin ↓OLIG2 ↑GFAP ↑p21 transcription ↑PUMA transcription ↑MDM2 transcription ↑BAX transcription ↓Bcl-2 transcription | [69] |
| Cell Line | Treatment | Effect | Reference |
|---|---|---|---|
| B16/F10 | 1.25–20 μM; 6–24 h | ↓Migration ↓Invasion ↓Colony formation IC50 5 µM ↓MMP-9 ↓ERK1/2 ↓AKT ↓p38 ↓JNK ↓NF-κB ↓c-Jun | [75] |
| HaCaT | 1–10 μM; 2 h | ↓CYP1A1 ↓CYP1B1 | [76] |
| B16F10 | 20–40 µM; 24–48 h | ↑Radiosensitivity ↓Cell survival | [77] |
| A375 melanoma | 1–50 μM; 24–72 h | ↓Cell Viability | [78] |
| HaCaT | 10–30 μM; 12 h | ↓ROS ↓DNA damage ↓γH2AX ↓p-Chk1 ↓CDP ↓Transformation ↑IκB ↓NF-κB | [79] |
| Model | Treatment | Effect | Reference |
|---|---|---|---|
| Female CD-1 mice (DMBA and TPA-induced tumorigenesis) | 0.1–1 µmol in 20 µL acetone (5–50 mM) 1–10 µmol in 0.2 mL acetone (10–50 mM) | ↓Tumorigenesis ↓ Ornithine decarboxylase activity ↓TPA-induced ear inflammation | [80] |
| Female ICR mice (DMBA and TPA-induced tumorigenesis) | 85 nmol in 0.1 mL acetone (850 µM) | ↓Tumorigenesis ↓Rate of papilloma formation ↓Number of papillomata | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, E.J.; Den Hartogh, D.J.; Azizi, K.; Tsiani, E. Anticancer Properties of Carnosol: A Summary of In Vitro and In Vivo Evidence. Antioxidants 2020, 9, 961. https://doi.org/10.3390/antiox9100961
O’Neill EJ, Den Hartogh DJ, Azizi K, Tsiani E. Anticancer Properties of Carnosol: A Summary of In Vitro and In Vivo Evidence. Antioxidants. 2020; 9(10):961. https://doi.org/10.3390/antiox9100961
Chicago/Turabian StyleO’Neill, Eric J., Danja J. Den Hartogh, Karim Azizi, and Evangelia Tsiani. 2020. "Anticancer Properties of Carnosol: A Summary of In Vitro and In Vivo Evidence" Antioxidants 9, no. 10: 961. https://doi.org/10.3390/antiox9100961
APA StyleO’Neill, E. J., Den Hartogh, D. J., Azizi, K., & Tsiani, E. (2020). Anticancer Properties of Carnosol: A Summary of In Vitro and In Vivo Evidence. Antioxidants, 9(10), 961. https://doi.org/10.3390/antiox9100961






