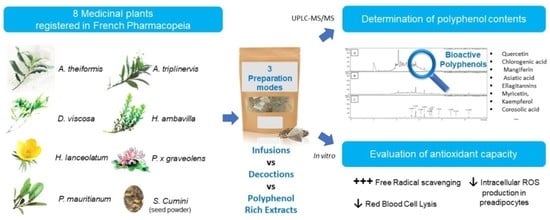
Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.1.1. Raw Material
2.1.2. Polyphenol-Rich Extracts (PRE)
2.1.3. Infusions and Decoctions
3. Determination of Antioxidant Polyphenol Content in Medicinal Plant Extracts, Infusions and Decoctions
3.1. Total Phenolic Content
3.2. Determination of Antioxidant Polyphenol Content in Medicinal Plant PRE, Infusions and Decoctions
4. Evaluation Antioxidant Activity of Polyphenol-Rich Extracts, Infusions and Decoctions from Medicinal Plants
4.1. Evaluation of the Free Radical-Scavenging Activity: DPPH Assay
4.2. Evaluation of the Protective Effects on Erythrocyte Damage Induced by the Radical AAPH: Hemolysis Test
4.3. Cell Culture
4.3.1. Evaluation of the Medicinal Plant Extracts, Infusions and Decoctions on Cell Viability
LDH Assay
Neutral Red Assay
4.3.2. Evaluation of the Effects of Medicinal Plants PRE, Infusions and Decoctions on Intracellular ROS Production by 3T3-L1 Preadipocytes Exposed to H2O2
5. Statistical Analysis
6. Results and Discussion
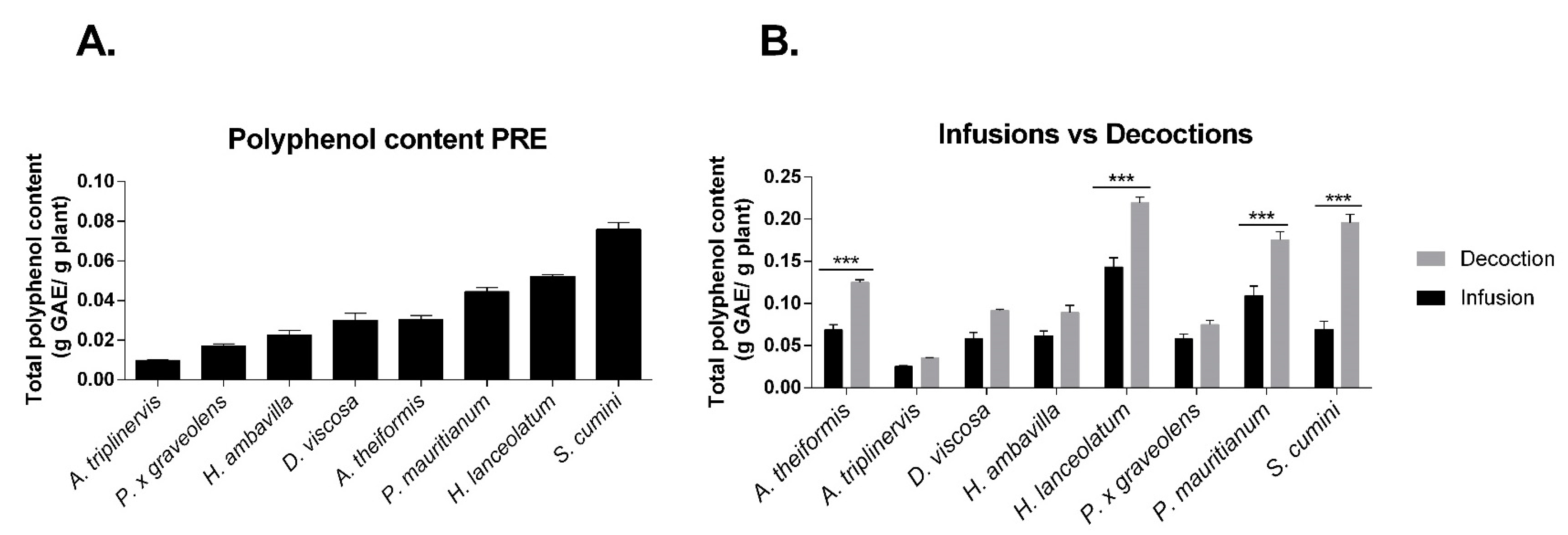
6.1. Determination of Polyphenol Content in Medicinal Plant Extracts
6.1.1. Total Phenolic Contents
6.1.2. Polyphenol Composition of Medicinal Plant PRE, Infusions and Decoctions by UPLC-MS/MS Analysis
7. Impact of Infusion and Decoction Process on the Antioxidant Polyphenolic Contents
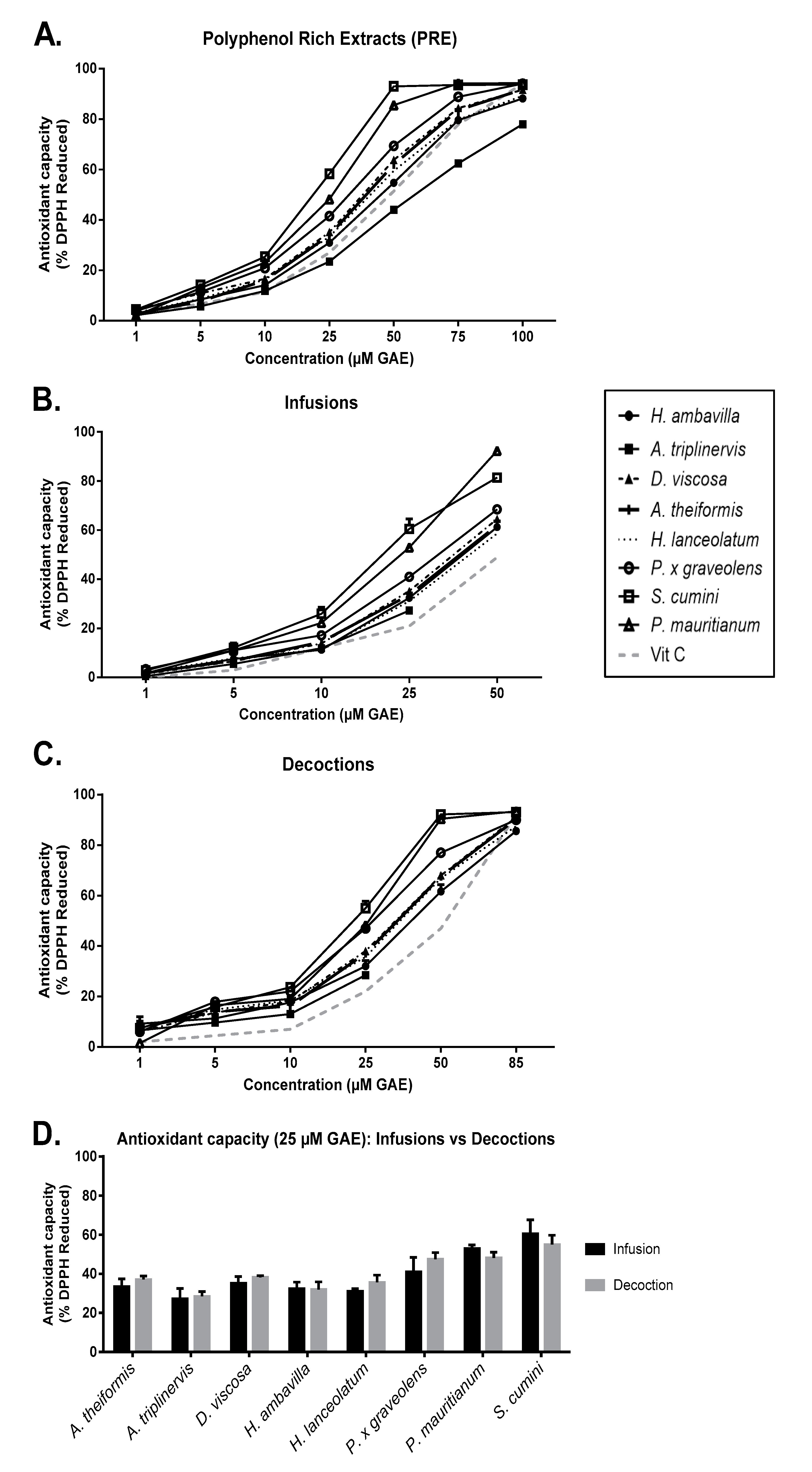
7.1. Impact of Infusion and Decoction Process on Antioxidant Activity
7.1.1. DPPH Assay
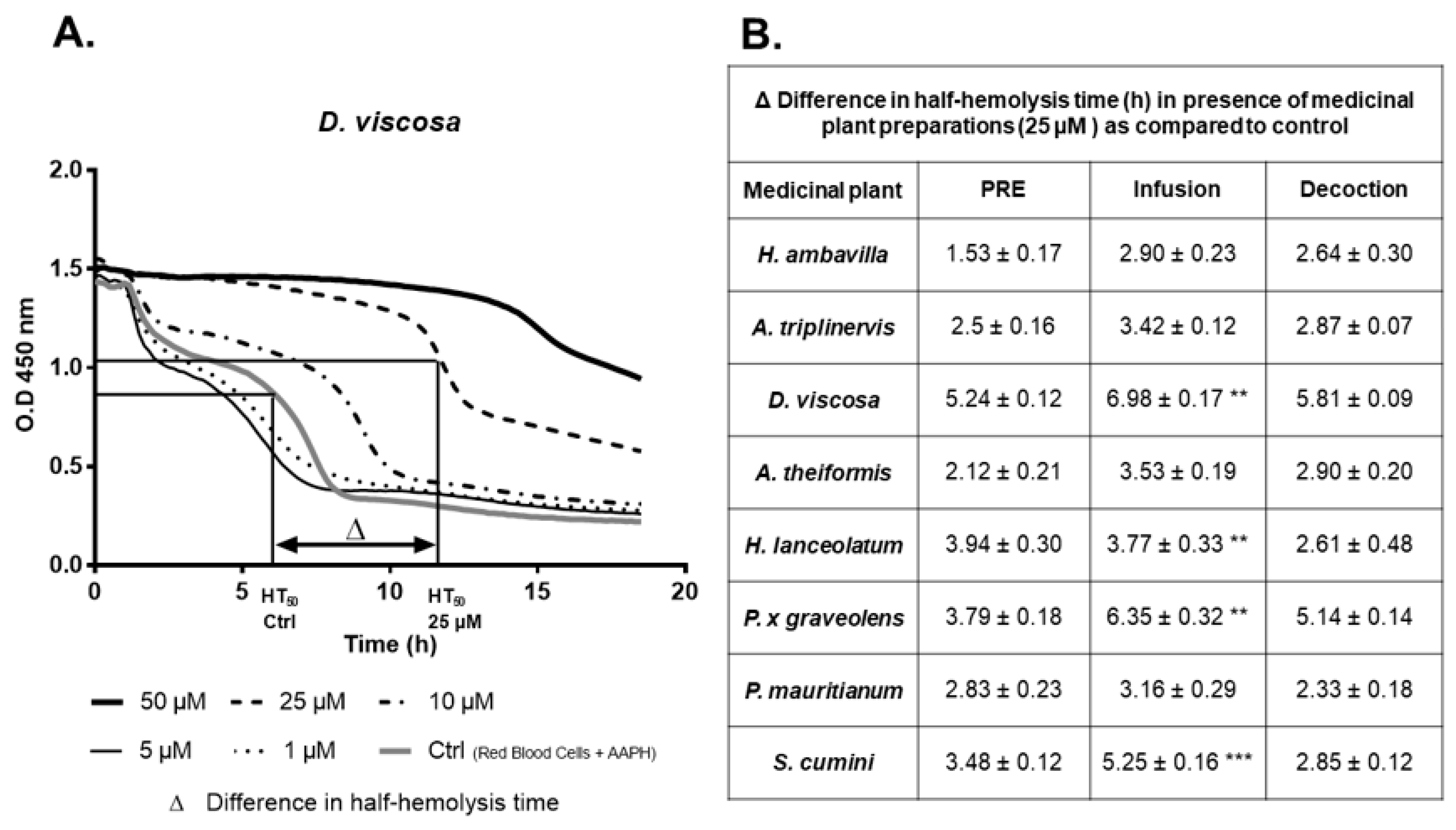
7.1.2. Evaluation of the Protective Effects of Medicinal Plant PRE, Infusions and Decoctions on Red Blood Cell Damage Induced by the Radical AAPH
7.2. Impact of Medicinal Plant Preparations on 3T3-L1 Preadipocytes
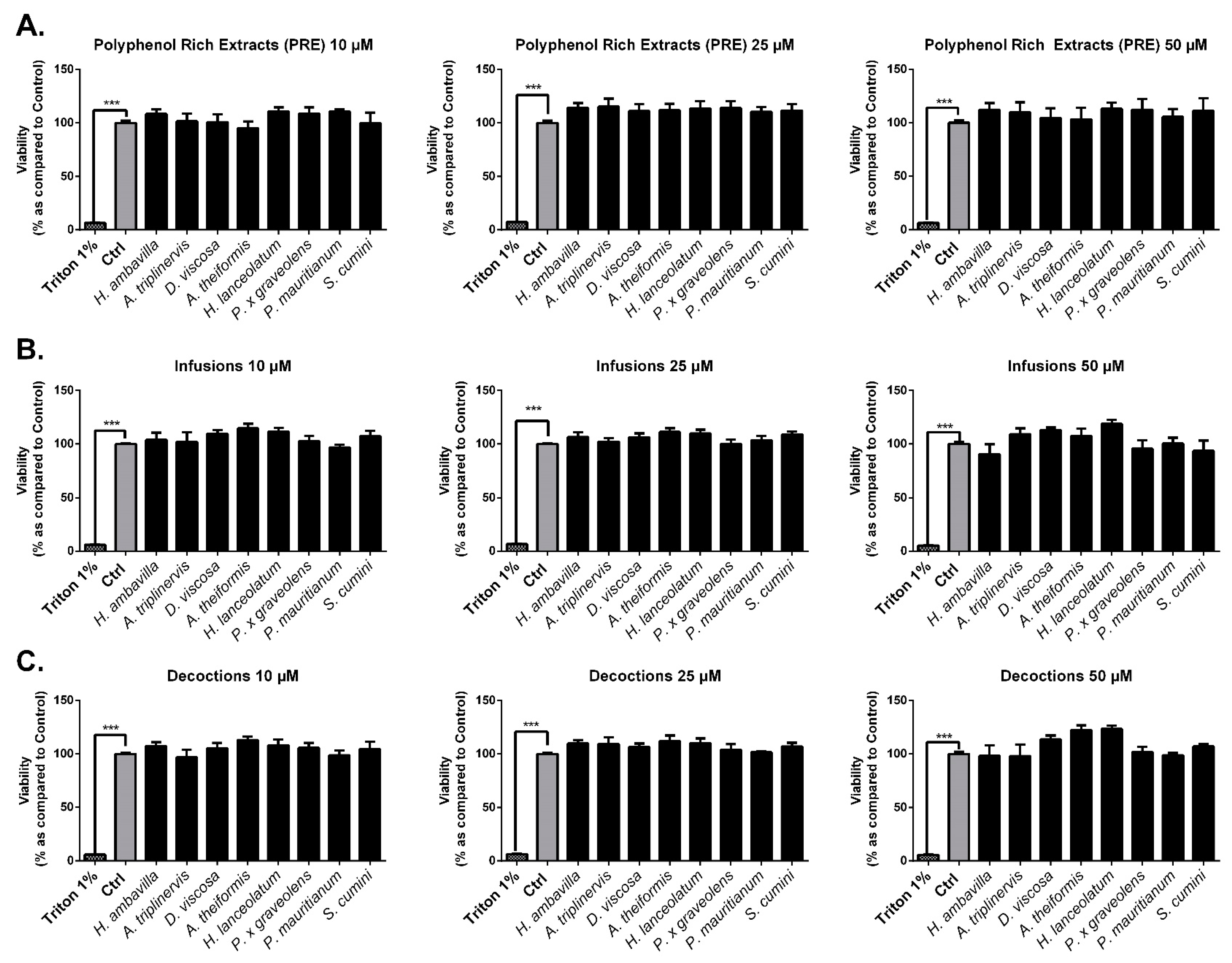
7.2.1. Evaluation of the Impact of Medicinal Plant Preparations on Cell Viability
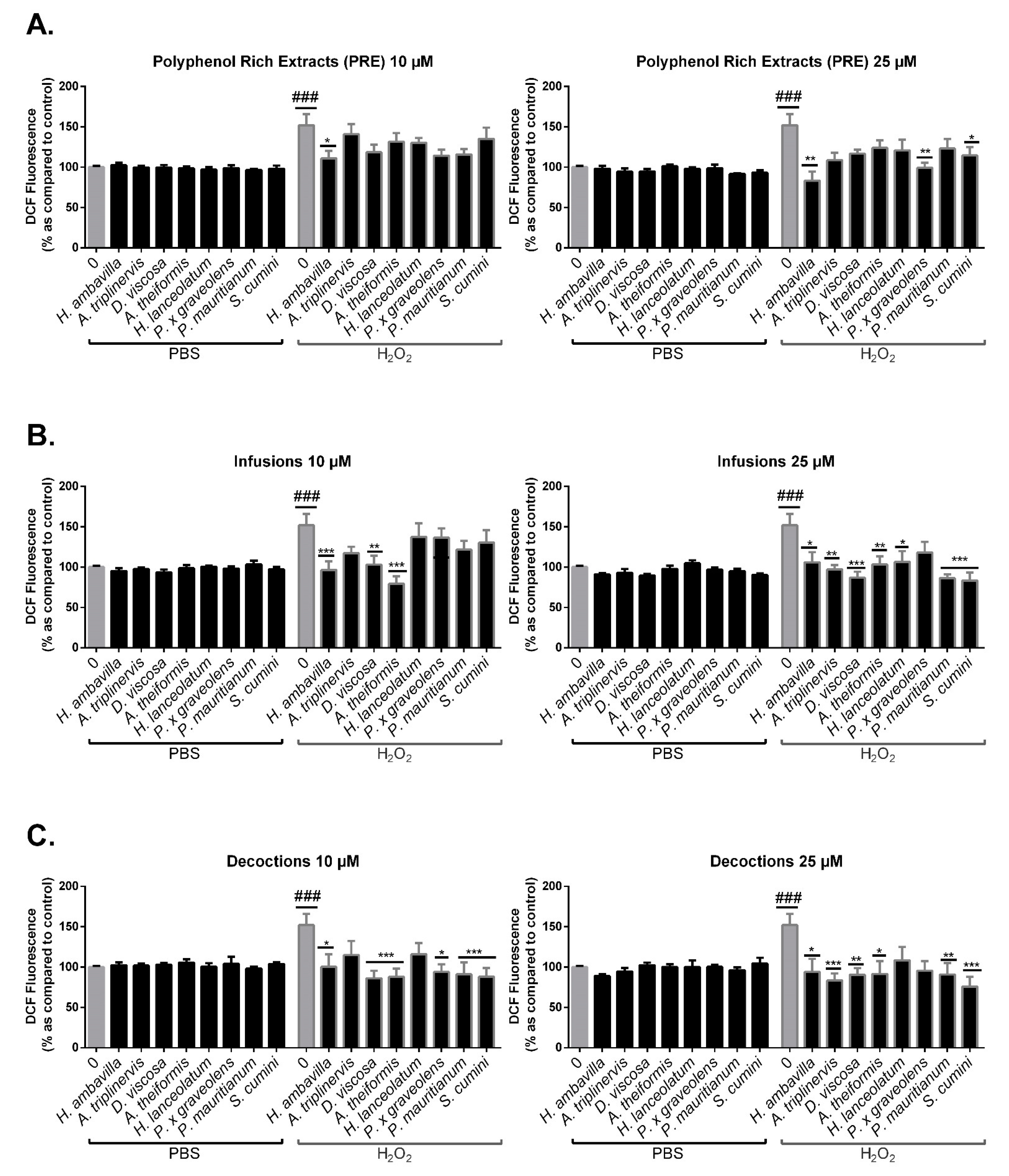
7.2.2. Evaluation of the Protective Effect of Medicinal Plant PRE, Infusions and Decoctions on 3T3-L1 Preadipocyte Intracellular Oxidative Stress in Response to H2O2
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lavergne, R. Plantes médicinales indigènes: Tisanerie et tisaneurs de la Réunion. Master’s Thesis, Université Montpellier II-Sciences et Techniques du Languedoc, Montpellier, France, 1989. [Google Scholar]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Annuzzi, G.; Varì, R.; Filesi, C.; Giacco, R.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Rivellese, A.A.; Masella, R. Predominant role of obesity/insulin resistance in oxidative stress development: OBESITY/INSULIN RESISTANCE AND OXIDATIVE STRESS. Eur. J. Clin. Investig. 2012, 42, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Innes, K.E.; Vincent, K.R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes. Metab. 2007, 9, 813–839. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- de Ferranti, S.; Mozaffarian, D. The Perfect Storm: Obesity, Adipocyte Dysfunction, and Metabolic Consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Gharras, H.E. Polyphenols: Food sources, properties and applications–a review. Int. J. Food Sci. Technol. 2009, 7, 2512–2518. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 13, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. An Overview of New Possible Treatments of Alzheimer’s Disease, Based on Natural Products and Semi-Synthetic Compounds. Curr. Med. Chem. 2017, 24, 3749–3773. [Google Scholar] [CrossRef]
- Ciccone, L.; Tonali, N.; Nencetti, S.; Orlandini, E. Natural compounds as inhibitors of transthyretin amyloidosis and neuroprotective agents: Analysis of structural data for future drug design. J. Enzym. Inhib. Med. Chem. 2020, 35, 1145–1162. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, H.P.; He, Y.Y.; Chen, W.L.; Chen, J.W.; Gao, L.; Hu, H.Y.; Wang, J. Effects of Rich-Polyphenols Extract of Dendrobium loddigesii on Anti-Diabetic, Anti-Inflammatory, Anti-Oxidant, and Gut Microbiota Modulation in db/db Mice. Molecules 2018, 23, 3245. [Google Scholar] [CrossRef]
- Morand, C. Intérêt des aliments riches en flavonoïdes pour le maintien de la santé cardio-métabolique. Médecine Mal. Métaboliques 2014, 8, 477–482. [Google Scholar] [CrossRef]
- Wan, L.; Jiang, J.-G. Protective effects of plant-derived flavonoids on hepatic injury. J. Funct. Foods 2018, 44, 283–291. [Google Scholar] [CrossRef]
- Arya, V.S.; Kanthlal, S.K.; Linda, G. The role of dietary polyphenols in inflammatory bowel disease: A possible clue on the molecular mechanisms involved in the prevention of immune and inflammatory reactions. J. Food Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Delveaux, J.; Turpin, C.; Veeren, B.; Diotel, N.; Bravo, B.; Begue, F.; Álvarez, E.; Meilhac, O.; Bourdon, E.; Rondeau, P. Antirhea borbonica Aqueous Extract Protects Albumin and Erythrocytes From Glycoxidative Damages. Available online: https://pubmed.ncbi.nlm.nih.gov/32408712/?from_single_result=PMID%3A+32408712&expanded_search_query=PMID%3A+32408712 (accessed on 23 May 2020).
- dos Santos, M.M.; Prestes, A.S.; de Macedo, G.T.; Ecker, A.; Barcelos, R.P.; Boligon, A.A.; Souza, D.; de Bem, A.F.; da Rocha, J.B.T.; Barbosa, N.V. Syzygium cumini leaf extract inhibits LDL oxidation, but does not protect the liproprotein from glycation. J. Ethnopharmacol. 2018, 210, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Hatia, S.; Septembre-Malaterre, A.; Le Sage, F.; Badiou-Bénéteau, A.; Baret, P.; Payet, B.; Lefebvre d’hellencourt, C.; Gonthier, M.P. Evaluation of antioxidant properties of major dietary polyphenols and their protective effect on 3T3-L1 preadipocytes and red blood cells exposed to oxidative stress. Free Radic. Res. 2014, 48, 387–401. [Google Scholar] [CrossRef]
- Kang, M.R.; Park, K.H.; Oh, S.J.; Yun, J.; Lee, C.W.; Lee, M.Y.; Han, S.-B.; Kang, J.S. Cardiovascular protective effect of glabridin: Implications in LDL oxidation and inflammation. Int. Immunopharmacol. 2015, 29, 914–918. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, D.-S.; Kim, S.H.; Kim, H.K. Aqueous and ethanolic extracts of welsh onion, Allium fistulosum, attenuate high-fat diet-induced obesity. BMC Complementary Altern. Med. 2018, 18. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.-P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef]
- Yang, C.S.; Sang, S.; Lambert, J.D.; Lee, M.J. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 2008. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Danthu, P.; Lubrano, C.; Flavet, L.; Rahajanirina, V.; Behra, O.; Fromageot, C.; Rabevohitra, R.; Roger, E. Biological Factors Influencing Production of Xanthones in Aphloia theiformis. Chem. Biodivers. 2010, 7, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.M.; Rathod, V.K. Exploring the potential of Mangifera indica leaves extract versus mangiferin for therapeutic application. Agric. Nat. Resour. 2018, 52, 155–161. [Google Scholar] [CrossRef]
- Lobo, F.A.; Nascimento, M.A.; Domingues, J.R.; Falcão, D.Q.; Hernanz, D.; Heredia, F.J.; de Lima Araujo, K.G. Foam mat drying of Tommy Atkins mango: Effects of air temperature and concentrations of soy lecithin and carboxymethylcellulose on phenolic composition, mangiferin, and antioxidant capacity. Food Chem. 2017, 221, 258–266. [Google Scholar] [CrossRef]
- Gauvin-Bialecki, A.; Marodon, C. Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether. Biochem. Syst. Ecol. 2008, 36, 853–858. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Tavares, I.M.D.C.; Lago-Vanzela, E.S.; Rebello, L.P.G.; Ramos, A.M.; Gómez-Alonso, S.; García-Romero, E.; Da-Silva, R.; Hermosín-Gutiérrez, I. Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels). Food Res. Int. 2016, 82, 1–13. [Google Scholar] [CrossRef]
- Rangasamy, O.; Mahomoodally, F.M.; Gurib-Fakim, A.; Quetin-Leclercq, J. Two anti-staphylococcal triterpenoid acids isolated from Psiloxylon mauritianum (Bouton ex Hook.f.) Baillon, an endemic traditional medicinal plant of Mauritius. S. Afr. J. Bot. 2014, 93, 198–203. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Carnat, A.; Carnat, A.P.; Fraisse, D.; Ricoux, L.; Lamaison, J.L. The aromatic and polyphenolic composition of Roman camomile tea. Fitoterapia 2004, 75, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Neergheen-Bhujun, V.S.; Munogee, N.; Coolen, V. Antioxidant and anti-inflammatory efficacies of polyherbal formulations and elixirs traditionally used in Mauritius for the treatment of rheumatoid arthritis. J. Herb. Med. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Giménez, R.; Rufián-Henares, J.A.; Pastoriza, S. Effect of brewing time and temperature on antioxidant capacity and phenols of white tea: Relationship with sensory properties. Food Chem. 2018, 248, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Callcott, E.T.; Santhakumar, A.B.; Luo, J.; Blanchard, C.L. Therapeutic potential of rice-derived polyphenols on obesity-related oxidative stress and inflammation. J. Appl. Biomed. 2018. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Pruchnik, H.; Cyboran, S.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Biophysical Mechanism of the Protective Effect of Blue Honeysuckle (Lonicera caerulea L. var. kamtschatica Sevast.) Polyphenols Extracts Against Lipid Peroxidation of Erythrocyte and Lipid Membranes. J. Membr. Biol. 2014, 247, 611–625. [Google Scholar] [CrossRef]
- Yang, Q.; Noviana, M.; Zhao, Y.; Chen, D.; Wang, X. Effect of curcumin extract against oxidative stress on both structure and deformation capability of red blood cell. J. Biomech. 2019, 95, 109301. [Google Scholar] [CrossRef]
- Dai, F.; Miao, Q.; Zhou, B.; Yang, L.; Liu, Z.-L. Protective effects of flavonols and their glycosides against free radical-induced oxidative hemolysis of red blood cells. Life Sci. 2006, 78, 2488–2493. [Google Scholar] [CrossRef]
- Gangwar, M.; Gautam, M.K.; Sharma, A.K.; Tripathi, Y.B.; Goel, R.K.; Nath, G. Antioxidant Capacity and Radical Scavenging Effect of Polyphenol Rich Mallotus philippenensis Fruit Extract on Human Erythrocytes: An In Vitro Study. Sci. World J. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Kumarappan, C.T.; Thilagam, E.; Mandal, S.C. Antioxidant activity of polyphenolic extracts of Ichnocarpus frutescens. Saudi J. Biol. Sci. 2012, 19, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhao, M.; Yang, K.; Lin, L.; Wang, Y. Enrichment of antioxidants in black garlic juice using macroporous resins and their protective effects on oxidation-damaged human erythrocytes. J. Chromatogr. B 2017, 1060, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Marcucci, R.; Mannucci, A.; Gori, A.; Giusti, B.; Sofi, F.; Mannini, L.; Cellai, A.; Alessandrello Liotta, A.; Mugnaini, M.; et al. Erythrocyte Membrane Fluidity Alterations in Sudden Sensorineural Hearing Loss Patients: The Role of Oxidative Stress. Thromb. Haemost. 2017, 117, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Aman, S.; Moin, S.; Owais, M.; Siddiqui, M.U. Antioxidant activity of thymol: Protective role in AAPH-induced hemolysis in diabetic erythrocytes. IJPSI 2013, 6, 55–60. [Google Scholar]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding Adipocyte Differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Brima, E.I. Toxic Elements in Different Medicinal Plants and the Impact on Human Health. Int. J. Environ. Res. Public Health 2017, 14, 1209. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Abdo, S.A.A.; Hasson, S.; Althawab, F.M.N.; Alaghbari, S.A.Z.; Lindequist, U. Antimicrobial, Antioxidant and Cytotoxic Activities and Phytochemical Screening of Some Yemeni Medicinal Plants. Evid. Based Complementary Altern. Med. 2010, 7, 323–330. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Vallejo, M.J.; Salazar, L.; Grijalva, M. Oxidative Stress Modulation and ROS-Mediated Toxicity in Cancer: A Review on In Vitro Models for Plant-Derived Compounds. Oxidative Med. Cell. Longev. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Pavan, V.; Ribaudo, G.; Zorzan, M.; Redaelli, M.; Pezzani, R.; Mucignat-Caretta, C.; Zagotto, G. Antiproliferative activity of Juglone derivatives on rat glioma. Nat. Prod. Res. 2017, 31, 632–638. [Google Scholar] [CrossRef]


| Medicinal Plant | Industrial Lot | GPS Coordinates |
|---|---|---|
| Ayapana triplinervis | PAY180111 | −21.037151, 55.687405″ |
| Hubertia ambavilla | FLAM20171127 | −21.131021, 55.640708″ |
| Psiloxylon mauritianum | FLBPM 20180427 | −20.947334, 55.548979″ |
| Dodonaea viscosa | FLBA20171106 | −21.059596, 55.507404″ |
| Aphloia theiformis | FLCE20171106 | −21.059596, 55.507404″ |
| Hypericum lanceolatum | FLFJ20171016 | −21.140988, 55.641630″ |
| Syzygium cumini | JAMBLON230617 | −20.540390, 55.26290″ |
| Pelargonium x graveolens | FLOGE171113 | −21.210391, 55.548644″ |
| Plant | Retention Time (min) | Molecular Weight (Da) | [M-H]−/[M-H]2− | [M-H]+ | Assigned Identity | Content in µg/mL | % of Total Composition | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extract | Infusion | Decoction | Extract | Infusion | Decoction | ||||||
| H. ambavilla | 3.4 | 354 | 353 | Chlorogenic acid isomer | 63.6 ± 0.2 | 0.40 ± 0.3 | 0.74 ± 0.01 | 0.51 ± 0.00 | 0.71 ± 0.01 | 1.21 ± 0.02 | |
| 3.6 | 370 | 369 | Unidentified | / | / | / | / | / | / | ||
| 3.9 | 354 | 353 | Chlorogenic acid | 1337.9 ± 5.5 | 7.18 ± 0.11 | 8.02 ± 0.21 | 10.98 ± 0.04 | 12.71 ± 0.19 | 13.06 ± 0.34 | ||
| 5.0 | 610 | 609 | Quercetin-hexose-rhamnose | 369.8 ± 4.4 | 0.54 ± 0.01 | 0.64 ± 0.01 | 2.95 ± 0.04 | 0.6 ± 0.02 | 1.04 ± 0.02 | ||
| 5.2 | 464 | 463 | Quercetin-hexose | 2473.5 ± 10.5 | 9.68 ± 0.03 | 11.43 ± 0.08 | 19.71 ± 0.08 | 17.13 ± 0.06 | 18.61 ± 0.13 | ||
| (5.4; 5.7; 5.8) | 516 | 515 | Di-caffeoyl quinic acid isomers | 4876.5 ± 24.7 | 23.34 ± 0.15 | 25.34 ± 0.20 | 38.85 ± 0.20 | 41.31 ± 0.27 | 41.27 ± 0.32 | ||
| 5.8 | 682 | 681 | Di-O-caffeoyl-O-[(hydroxy-oxocyclohe-dienyl)acetyl] quinic acid + hydroxy | 930.5 ± 13.9 | 4.34 ± 0.13 | 17.50 ± 0.32 | 7.41 ± 0.11 | 7.68 ± 0.23 | 6.37 ± 0.26 | ||
| (6.3; 6.6) | 666 | 665 | Di-O-caffeoyl-O-[(hydroxy-oxocyclohe-dienyl)acetyl] quinic acid isomers | 2280.8 ± 2.7 | 10.13 ± 0.16 | 10.24 ± 0.03 | 18.17 ± 0.02 | 17.93 ± 0.28 | 16.68 ± 0.06 | ||
| 7.0 | 650 | 649 | Di-O-caffeoyl-O-[(hydroxyphenyl)acetyl] quinic acid | 178.0 ± 1.1 | 0.88 ± 0.01 | 1.08 ± 0.01 | 1.42 ± 0.01 | 1.56 ± 0.02 | 1.76 ± 0.02 | ||
| A. triplinervis | (2.6; 2.9; 3.0; 3.2; 3.5) | 372 | 371 | Caffeoyl glucarate isomers | 58.6 ± 0.43 | 1.351 ± 0.008 | 1.359 ± 0.006 | 3.47 ± 0.03 | 12.55 ± 0.08 | 13.17 ± 0.07 | |
| 4.3 | 534 | 533 | Di-caffeoyl glucarate | 69.7 ± 0.6 | 1.251 ± 0.033 | 1.312 ± 0.003 | 4.13 ± 0.04 | 11.62 ± 0.31 | 12.72 ± 0.03 | ||
| 5.0 | 610 | 609 | Quercetin-hexose-rhamnose or feruoyl hexose | 478.8 ± 2.0 | 2.649 ± 0.009 | 2.472 ± 0.046 | 28.37 ± 0.12 | 24.61 ± 0.08 | 23.97 ± 0.45 | ||
| 356 | 355 | ||||||||||
| 5.2 | 464 | 463 | Quercetin hexose | 192.9 ± 2.8 | 1.121 ± 0.015 | 1.205 ± 0.011 | 11.43 ± 0.17 | 10.42 ± 0.14 | 11.68 ± 0.11 | ||
| 5.5 | 550 | 549 | Quercetin-hexose-malonate | 232.7 ± 1.7 | 1.303 ± 0.007 | 0.997 ± 0.034 | 13.79 ± 0.10 | 12.11 ± 0.07 | 9.67 ± 0.33 | ||
| 6.0 | 696 | 695 | Tri-caffeoyl glucarate | 212.6 ± 4.4 | 1.246 ± 0.023 | 1.278 ± 0.013 | 12.60 ± 0.26 | 11.58 ± 0.21 | 12.39 ± 0.13 | ||
| 6.4 | 194 | 193 | Isoferulic acid or thymohydroquinone dimethyl ether | 189.05 ± 0.5 | 0.898 ± 0.018 | 0.799 ± 0.018 | 11.20 ± 0.03 | 8.34 ± 0.17 | 7.75 ± 0.17 | ||
| 10.5 | / | / | / | Unidentified | / | / | / | / | / | / | |
| 6.9 | 190 | 191 | Ayapin | 31.1 ± 0.4 | 0.132 ± 0.006 | 0.122 ± 0.001 | 1.84 ± 0.02 | 1.23 ± 0.06 | 1.18 ± 0.01 | ||
| 7.5 | 176 | 177 | Herniarin | 222.3 ± 0.7 | 0.707 ± 0.015 | 0.671 ± 0.004 | 13.17 ± 0.04 | 6.57 ± 0.14 | 6.51 ± 0.04 | ||
| D. viscosa | (2.6; 3.0; 3.2; 3.5) | 372 | 371 | Caffeoyl glucarate isomers | 47 ± 0.15 | 0.759 ± 0.002 | 1.434 ± 0.003 | 1.43 ± 0.00 | 4.54 ± 0.01 | 4.15 ± 0.02 | |
| 3.4 | 354 | 353 | Chlorogenic acid isomer | 47.9 ± 0.6 | 0.331 ± 0.005 | 0.480 ± 0.003 | 1.45 ± 0.02 | 1.98 ± 0.03 | 2.66 ± 0.02 | ||
| (3.7; 3.8; 4.8) | 1152 | 1151 | Procyanidin tetramer type A isomers | 646.3 ± 2.47 | 4.538 ± 0.034 | 5.002 ± 0.049 | 19.59 ± 0.08 | 27.15 ± 0.21 | 27.73 ± 0.27 | ||
| 4.0 | 354 | 353 | Chlorogenic acid | 186.2 ± 2.9 | 0.888 ± 0.017 | 0.733 ± 0.008 | 5.64 ± 0.09 | 5.31 ± 0.10 | 4.06 ± 0.04 | ||
| 4.3 | 578 | 577 | Procyanidin dimer type B | 548.5 ± 5.4 | 2.431 ± 0.035 | 2.354 ± 0.096 | 16.63 ± 0.16 | 14.54 ± 0.21 | 13.05 ± 0.53 | ||
| 4.5 | 290 | 289 | Catechin or epicatechin | 388.0 ± 8.7 | 1.112 ± 0.025 | 1.118 ± 0.010 | 11.76 ± 0.26 | 6.65 ± 0.15 | 6.20 ± 0.06 | ||
| 4.5 | 338 | 337 | Coumaroyl quinic acid | 105.3 ± 2.5 | 0.449 ± 0.009 | 3.557 ± 0.044 | 3.19 ± 0.08 | 2.69 ± 0.05 | 2.64 ± 0.08 | ||
| (4.6; 5.0) | 864 | 863 | Procyanidin trimer type A isomers | 988.9 ± 6.25 | 4.389 ± 0.021 | 5.085 ± 0.05 | 29.98 ± 0.19 | 26.25 ± 0.13 | 28.19 ± 0.27 | ||
| 5.0 | 610 | 609 | Quercetin-hexose-rhamnose | 59.8 ± 0.9 | 0.307 ± 0.005 | 0.329 ± 0.004 | 1.81 ± 0.03 | 1.84 ± 0.03 | 1.82 ± 0.02 | ||
| 5.3 | 478 | 477 | Quercetin-glucuronide | 30.9 ± 0.5 | 0.173 ± 0.002 | 0.197 ± 0.001 | 0.94 ± 0.02 | 1.03 ± 0.01 | 1.09 ± 0.01 | ||
| 5.4 | 624 | 623 | Isorhamnetin-hexose-rhamnose | 175.4 ± 1.15 | 0.881 ± 0.010 | 0.991 ± 0.005 | 5.86 ± 0.03 | 5.26 ± 0.06 | 5.49 ± 0.02 | ||
| 5.7 | 492 | 491 | Isorhamnetin-glucuronide | 74.3 ± 0.9 | 0.464 ± 0.007 | 0.525 ± 0.005 | 2.25 ± 0.03 | 2.77 ± 0.04 | 2.91 ± 0.03 | ||
| (8.2; 9.3; 9.5; 9.6; 9.7; 10.7; 10.9) | (416; 330; 454; 484; 398; 436; 466) | (415; 329; 453; 483; 397; 435; 465) | Unidentified | / | / | / | / | / | / | ||
| A. theiformis | 3.5 | 584 | 583 | Neomangiferin | 243.1 ± 0.5 | 1.56 ± 0.02 | 1.93 ± 0.030 | 3.59 ± 0.01 | 2.76 ± 0.35 | 2.56 ± 0.04 | |
| 3.9 | 408 | 407 | Iriflophenone-C-hexoside | / | / | / | / | / | / | ||
| 4.2 | 422 | 421 | Mangiferin | 6488.6 ± 27.0 | 54.9 ± 0.10 | 73.4 ± 0.30 | 95.71 ± 0.40 | 97.24 ± 0.18 | 97.23 ± 0.40 | ||
| (4.4; 4.9) | 436 | 435 | Homomangiferin or O-methylisomangiferin isomers | 47.6 ± 0.85 | / | 0.16 ± 0.01 | 0.71 ± 0.01 | / | 0.21 ± 0.00 | ||
| 5.2 | 452 | 451 | Aspalatin | / | / | / | / | / | / | ||
| H. lanceolatum | 3.4 | 354 | 353 | Chlorogenic acid isomer | 598.2 ± 7.0 | 4.36 ± 0.04 | 4.11 ± 0.06 | 3.70 ± 0.04 | 4.06 ± 0.04 | 3.71 ± 0.05 | |
| 3.9 | 354 | 353 | Chlorogenic acid | 7022.3 ± 11.3 | 54.54 ± 0.27 | 53.17 ± 0.21 | 43.47 ± 0.07 | 50.78 ± 0.25 | 47.94 ± 0.19 | ||
| 4.3 | 578 | 577 | Procyanidin dimer type B | 669.1 ± 21.9 | 3.56 ± 0.26 | 4.33 ± 0.22 | 4.14 ± 0.14 | 3.31 ± 0.24 | 3.90 ± 0.20 | ||
| 4.4 | 180 | 179 | Caffeic acid | 240.5 ± 4.5 | 1.30 ± 0.02 | 2.82 ± 0.07 | 1.49 ± 0.03 | 1.21 ± 0.02 | 2.54 ± 0.06 | ||
| 4.6 | 866 | 865 | Procyanidin trimer type B | 642.3 ± 9.8 | 3.09 ± 0.29 | 3.51 ± 0.07 | 3.98 ± 0.06 | 2.88 ± 0.27 | 3.17 ± 0.06 | ||
| 4.8 | 1154 | 1153 | Procyanidin tetramer type B | 497.8 ± 29.6 | 2.36 ± 0.10 | 2.18 ± 0.16 | 3.08 ± 0.18 | 2.20 ± 0.09 | 1.97 ± 0.14 | ||
| 5.0 | 610 | 609 | Quercetin-hexose-rhamnose | 197.1 ± 1.7 | 1.16 ± 0.03 | 1.17 ± 0.04 | 1.22 ± 0.10 | 1.08 ± 0.03 | 1.06 ± 0.04 | ||
| (5.2; 5.3) | 464 | 463 | Quercetin-hexose isomers | 1041.8 ± 2.35 | 6.13 ± 0.03 | 6.90 ± 0.04 | 6.45 ± 0.02 | 5.71 ± 0.04 | 6.22 ± 0.05 | ||
| 5.5 | 550 | 549 | Quercetin-malonate-hexose | 747.2 ± 7.1 | 3.75 ± 0.04 | 3.52 ± 0.07 | 4.62 ± 0.04 | 3.49 ± 0.04 | 3.17 ± 0.06 | ||
| 5.7 | 448 | 447 | Quercetin-rhamnose | 427.8 ± 4.9 | 2.10 ± 0.03 | 2.36 ± 0.02 | 2.65 ± 0.03 | 1.96 ± 0.03 | 2.13 ± 0.02 | ||
| (5.7; 5.9) | 516 | 515 | Di-caffeoyl quinic acid isomers | 3440.3 ± 19.4 | 21.61 ± 0.12 | 23.00 ± 0.1 | 21.3 ± 0.24 | 20.12 ± 0.11 | 20.74 ± 0.09 | ||
| 6.0 | 520 | 519 | Isorhamnetin-acetyl-hexose | 38.1 ± 0.5 | 0.16 ± 0.01 | 0.21 ± 0.01 | 0.24 ± 0.00 | 0.15 ± 0.01 | 0.19 ± 0.01 | ||
| 6.2 | 500 | 499 | Caffoyl coumaroyl quinic acid | 252.6 ± 7.3 | 1.50 ± 0.05 | 1.70 ± 0.01 | 1.56 ± 0.05 | 1.40 ± 0.05 | 1.53 ± 0.01 | ||
| 6.4 | 530 | 529 | Feruoyl caffeoyl quinic acid | 153.7 ± 3.5 | 0.87 ± 0.01 | 0.99 ± 0.03 | 0.95 ± 0.02 | 0.81 ± 0.01 | 0.89 ± 0.03 | ||
| 7.1 | 302 | 301 | Quercetin | 187.4 ± 8.7 | 0.92 ± 0.01 | 0.93 ± 0.03 | 1.16 ± 0.05 | 0.01 ± 0.01 | 0.84 ± 0.03 | ||
| P. x graveolens | (2.9; 3.5) | 372 | 371 | Caffeoyl glucarate isomers | 28.2 ± 0.75 | 0.227 ± 0.004 | 0.146 ± 0.003 | 1.73 ± 0.05 | 2.41 ± 0.04 | 1.88 ± 0.04 | |
| 4.5 | 612 | 611 | Myricetin derivatives | 41.4 ± 1.1 | 0.198 ± 0.006 | 0.153 ± 0.005 | 2.55 ± 0.07 | 2.10 ± 0.06 | 1.97 ± 0.06 | ||
| 4.7 | 626 | 625 | Myricetin-rhamnose-hexose | 55.9 ± 1.1 | 0.263 ± 0.008 | 0.220 ± 0.005 | 3.44 ± 0.07 | 2.79 ± 0.08 | 2.83 ± 0.06 | ||
| 4.8 | 480 | 479 | Myricetin-hexose | 103.6 ± 0.9 | 0.520 ± 0.005 | 0.425 ± 0.011 | 6.37 ± 0.06 | 5.51 ± 0.05 | 5.47 ± 0.14 | ||
| 4.9 | 596 | 595 | Quercetin-pentose-hexose | 268.3 ± 2.1 | 1.574 ± 0.019 | 1.312 ± 0.010 | 16.51 ± 0.13 | 16.69 ± 0.20 | 16.87 ± 0.13 | ||
| 5.1 | 610 | 609 | Quercetin-hexose-rhamnose | 122.3 ± 2.2 | 0.761 ± 0.008 | 0.636 ± 0.002 | 7.52 ± 0.14 | 8.07 ± 0.08 | 8.18 ± 0.03 | ||
| 5.1 | 450 | 449 | Myricetin-pentose | 43.4 ± 1.3 | 0.184 ± 0.009 | 0.150 ± 0.002 | 2.67 ± 0.08 | 1.95 ± 0.10 | 1.93 ± 0.03 | ||
| 5.2 | 464 | 463 | Myricetin-rhamnose | 91.1 ± 0.7 | 0.456 ± 0.009 | 0.381 ± 0.004 | 5.60 ± 0.04 | 4.84 ± 0.10 | 4.90 ± 0.05 | ||
| (5.2; 5.3) | 464 | 463 | Quercetin-hexose isomers | 513.6 ± 2.9 | 3.058 ± 0.006 | 2.558 ± 0.009 | 31.6 ± 0.18 | 32.43 ± 0.06 | 32.9 ± 0.12 | ||
| 5.4 | 594 | 593 | Kaempferol-hexose-rhamnose | 16.4 ± 0.4 | 0.089 ± 0.002 | 0.067 ± 0.005 | 1.01 ± 0.02 | 0.94 ± 0.02 | 0.86 ± 0.06 | ||
| (5.5; 5.6) | 448 | 447 | Kaempferol-hexose isomers | 112.4 ± 0.8 | 0.655 ± 0.004 | 0.534 ± 0.004 | 5.07 ± 0.00 | 6.95 ± 0.04 | 6.87 ± 0.05 | ||
| 5.5 | 434 | 433 | Quercetin pentose | 189.2 ± 2.9 | 1.092 ± 0.010 | 0.877 ± 0.004 | 11.64 ± 0.18 | 11.58 ± 0.11 | 11.28 ± 0.05 | ||
| 5.8 | 418 | 417 | Kaempferol-pentose | 44.6 ± 0.6 | 0.252 ± 0.003 | 0.201 ± 0.003 | 2.74 ± 0.04 | 2.67 ± 0.03 | 2.58 ± 0.04 | ||
| 6.2 | 318 | 317 | Myricetin | / | / | / | / | / | / | ||
| 7.1 | 302 | 301 | Quercetin | 19.3 ± 0.4 | 0.090 ± 0.002 | 0.101 ± 0.002 | 1.19 ± 0.02 | 0.95 ± 0.02 | 1.30 ± 0.03 | ||
| 7.7 | 286 | 285 | Kaempferol | 5.8 ± 0.2 | 0.011 ± 0.001 | 0.015 ± 0.001 | 0.36 ± 0.01 | 0.12 ± 0.01 | 0.19 ± 0.01 | ||
| S. cumini | 2.4 | 170 | 169 | Gallic acid | 210.7 ± 6.8 | 1.931 ± 0.039 | 6.351 ± 0.084 | 19.54 ± 0.63 | 21.03 ± 0.42 | 27.82 ± 0.37 | |
| (2.5; 2.9) | 634 | 633 | HHDP-galloyl-hexose isomers | 59.5 ± 0.8 | 1.025 ± 0.016 | 2.969 ± 0.021 | 5.52 ± 0.07 | 11.16 ± 0.18 | 13.01 ± 0.18 | ||
| 2.6 | 484 | 483 | Di-galloyl-hexose | 7.7 ± 0.3 | 0.090 ± 0.002 | 0.143 ± 0.003 | 0.71 ± 0.03 | 0.98 ± 0.02 | 0.63 ± 0.01 | ||
| 3.0 | 934 | 933/466 | Vescalagin/di-galloyl-hexose | 30.8 ± 0.7 | 0.255 ± 0.014 | 0.533 ± 0.009 | 2.86 ± 0.06 | 2.78 ± 0.15 | 2.33 ± 0.04 | ||
| 484 | 483 | ||||||||||
| (3.2; 3.7; 4.2; 4.5) | 784 | 783 | Bis-HHDP-hexose isomers | 63.1 ± 0.7 | 0.720 ± 0.006 | 1.253 ± 0.015 | 5.85 ± 0.06 | 3.14 ± 0.02 | 1.41 ± 0.03 | ||
| (3.2; 3.6) | 802 | 801 | Galloyl tannin isomers | 53.1 ± 0.9 | 0.775 ± 0.010 | 2.583 ± 0.023 | 4.92 ± 0.08 | 8.44 ± 0.11 | 11.32 ± 0.11 | ||
| 3.4 | 934 | 933/466 | Castalagin/ellagitannin | 50.7 ± 0.7 | 0.623 ± 0.007 | 0.968 ± 0.029 | 4.70 ± 0.06 | 6.79 ± 0.08 | 4.24 ± 0.013 | ||
| 1418 | 1417/708 | ||||||||||
| 3.9 | 952 | 951 | Trisgalloyl-HHDP-hexose/ellagitannin | 53.7 ± 1.7 | 0.420 ± 0.009 | 0.662 ± 0.027 | 4.98 ± 0.16 | 4.57 ± 0.10 | 2.90 ± 0.12 | ||
| 1418 | 1417/708 | ||||||||||
| 4.0 | 1086 | 1085 | Digalloyl-Gallagyl-hexose | 97.2 ± 0.85 | 0.802 ± 0.008 | 1.511 ± 0.013 | 9.01 ± 0.08 | 5.29 ± 0.13 | 4.18 ± 0.07 | ||
| 4.3 | 952 | 951 | Trisgalloyl-HHDP-hexose | 51.7 ± 2.3 | 0.370 ± 0.017 | 0.814 ± 0.035 | 4.79 ± 0.21 | 4.03 ± 0.19 | 3.57 ± 0.15 | ||
| (4.8; 5.0) | 434 | 433 | Ellagic acid -pentose isomers | 209.1 ± 2.8 | 1.086 ± 0.017 | 2.039 ± 0.033 | 19.39 ± 0.26 | 4.77 ± 0.10 | 3.86 ± 0.19 | ||
| 5.2 | 302 | 301 | Ellagic acid | 191.0 ± 3.5 | 1.083 ± 0.038 | 3.005 ± 0.072 | 17.71 ± 0.32 | 11.79 ± 0.41 | 13.16 ± 0.32 | ||
| P. mauritianum | 5.3 | 464 | 463 | Quercetin-hexose | 166.4 ± 6.6 | 0.796 ± 0.026 | 1.235 ± 0.026 | 21.74 ± 0.86 | 25.43 ± 0.83 | 32.85 ± 0.69 | |
| 5.7 | 448 | 447 | Kaempferol-hexose | 274.9 ± 10.6 | 1.094 ± 0.035 | 1.208 ± 0.053 | 35.91 ± 1.38 | 34.95 ± 1.12 | 32.13 ± 1.41 | ||
| 6.1 | 506 | 505 | Quercetin-acetyl- hexose | 66.7 ± 3.9 | 0.256 ± 0.003 | 0.283 ± 0.015 | 8.71 ± 0.51 | 8.18 ± 0.10 | 7.53 ± 0.40 | ||
| 6.4 | 490 | 489 | Kaempferol-acetyl-hexose | 165.3 ± 7.9 | 0.634 ± 0.012 | 0.662 ± 0.002 | 21.59 ± 1.03 | 20.26 ± 0.38 | 17.61 ± 0.59 | ||
| 6.9 | 460 | 459 | Kaempferol-acetyl-pentose | 13.2 ± 0.4 | 0.039 ± 0.002 | 0.042 ± 0.002 | 1.72 ± 0.05 | 1.25 ± 0.06 | 1.12 ± 0.05 | ||
| 7.0 | 474 | 473 | Kaempferol-acetyl-rhamnose | 49.6 ± 1.0 | 0.219 ± 0.005 | 0.230 ± 0.002 | 6.48 ± 0.13 | 7 ± 0.16 | 6.12 ± 0.06 | ||
| 7.7 | 286 | 285 | Kaempferol | 29.4 ± 1.5 | 0.092 ± 0.006 | 0.100 ± 0.005 | 3.84 ± 0.20 | 2.94 ± 0.19 | 2.66 ± 0.13 | ||
| 8.8 | 488 | 487 | Asiatic acid | / | / | / | / | / | / | ||
| (9.2; 10.3) | (238; 266) | (237; 265) | Unidentified | / | / | / | / | / | / | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checkouri, E.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes. Antioxidants 2020, 9, 959. https://doi.org/10.3390/antiox9100959
Checkouri E, Reignier F, Robert-Da Silva C, Meilhac O. Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes. Antioxidants. 2020; 9(10):959. https://doi.org/10.3390/antiox9100959
Chicago/Turabian StyleCheckouri, Eloïse, Franck Reignier, Christine Robert-Da Silva, and Olivier Meilhac. 2020. "Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes" Antioxidants 9, no. 10: 959. https://doi.org/10.3390/antiox9100959
APA StyleCheckouri, E., Reignier, F., Robert-Da Silva, C., & Meilhac, O. (2020). Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes. Antioxidants, 9(10), 959. https://doi.org/10.3390/antiox9100959