Valorisation of Exhausted Olive Pomace by an Eco-Friendly Solvent Extraction Process of Natural Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemical Characterisation
2.2. Solvent Extraction of EOP
2.3. Experimental Design for Aqueous Extraction of EOP
2.4. Extraction Yield
2.5. Characterisation of the EOP Extracts
2.5.1. Phenolic and Flavonoid Contents
2.5.2. Antioxidant Capacity of EOP Extracts
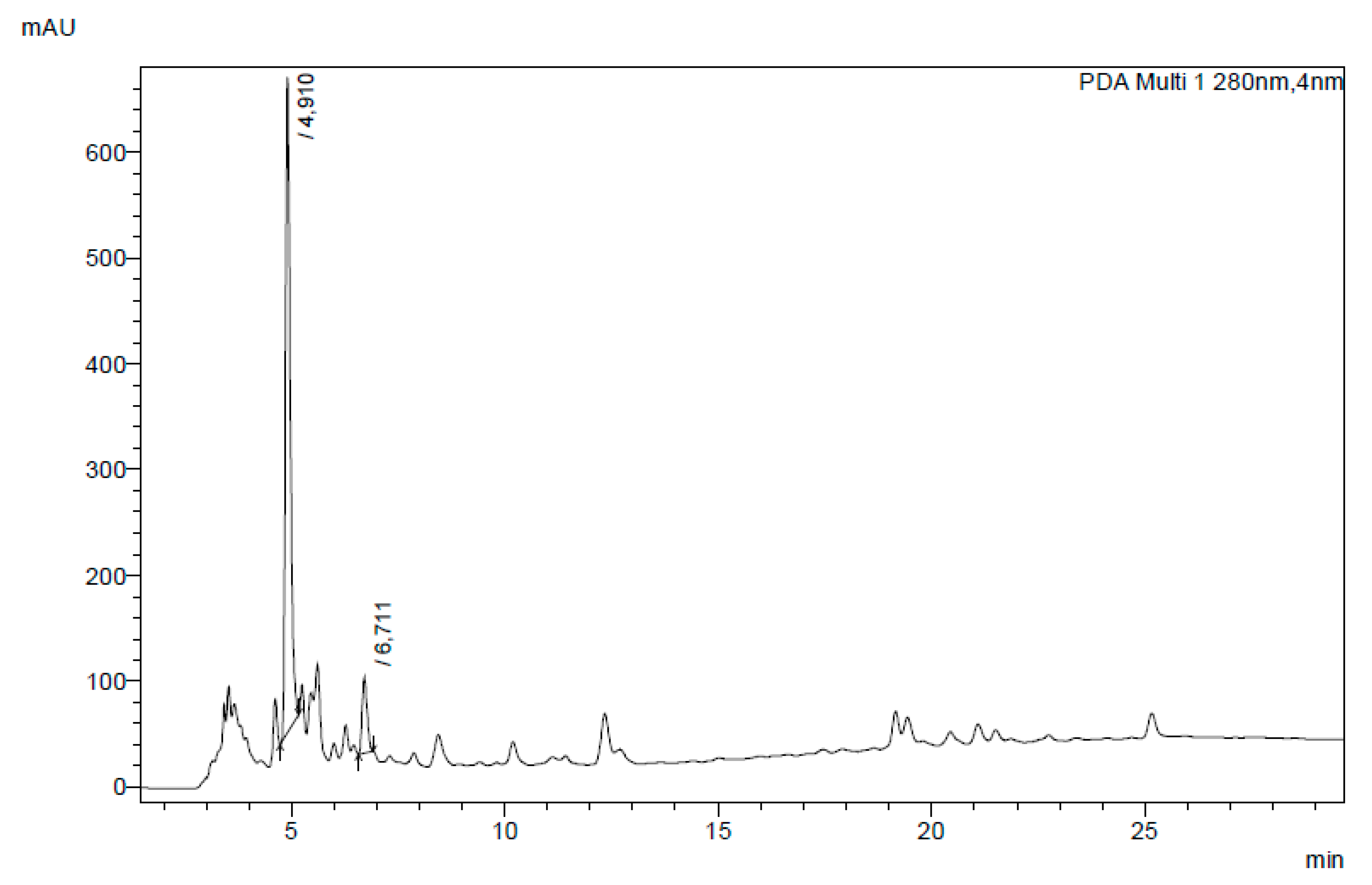
2.5.3. HPLC Analysis and Quantification
2.5.4. Antimicrobial Activity
3. Results and Discussion
3.1. EOP Composition
3.2. Effect of Solvent Extraction
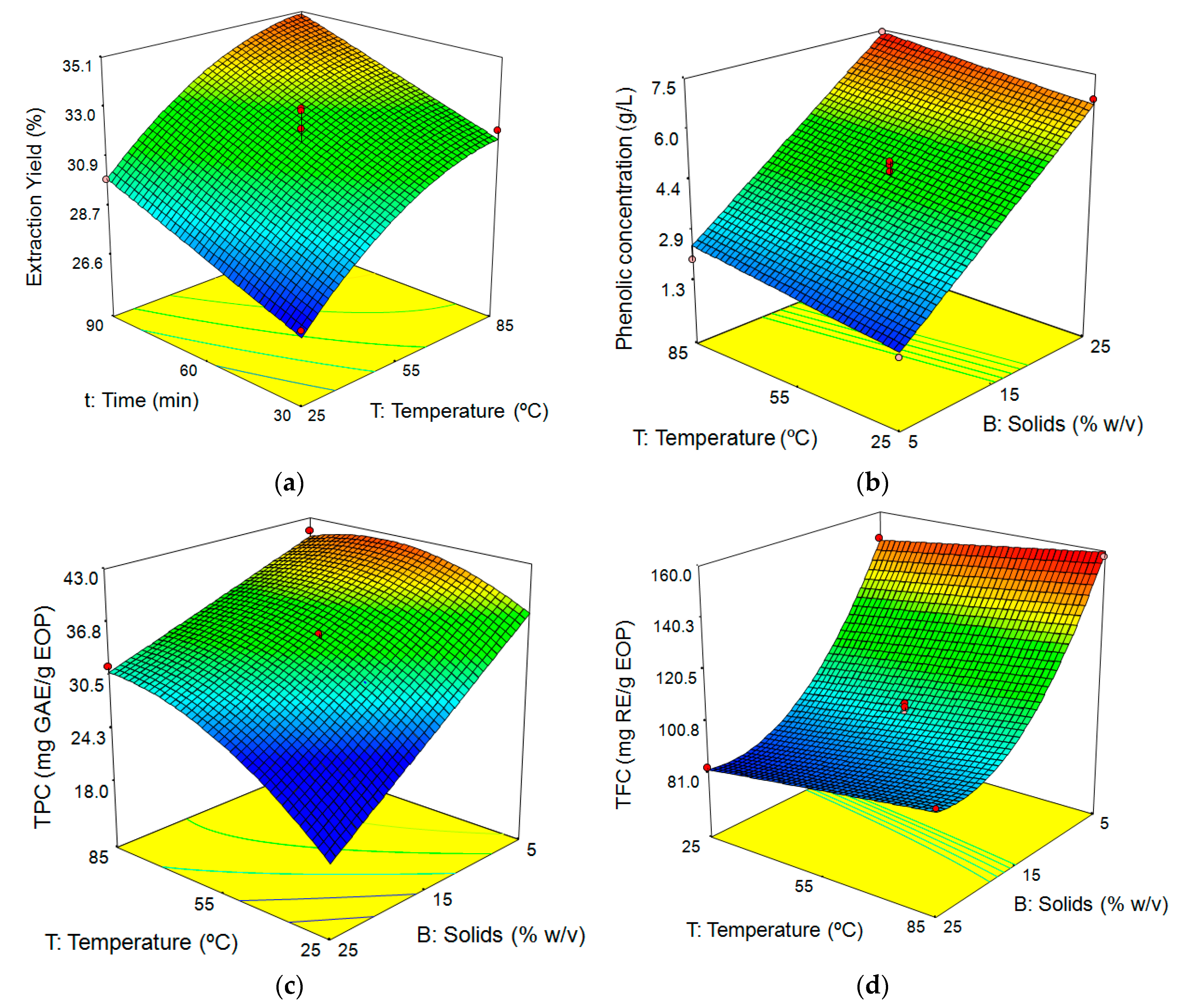
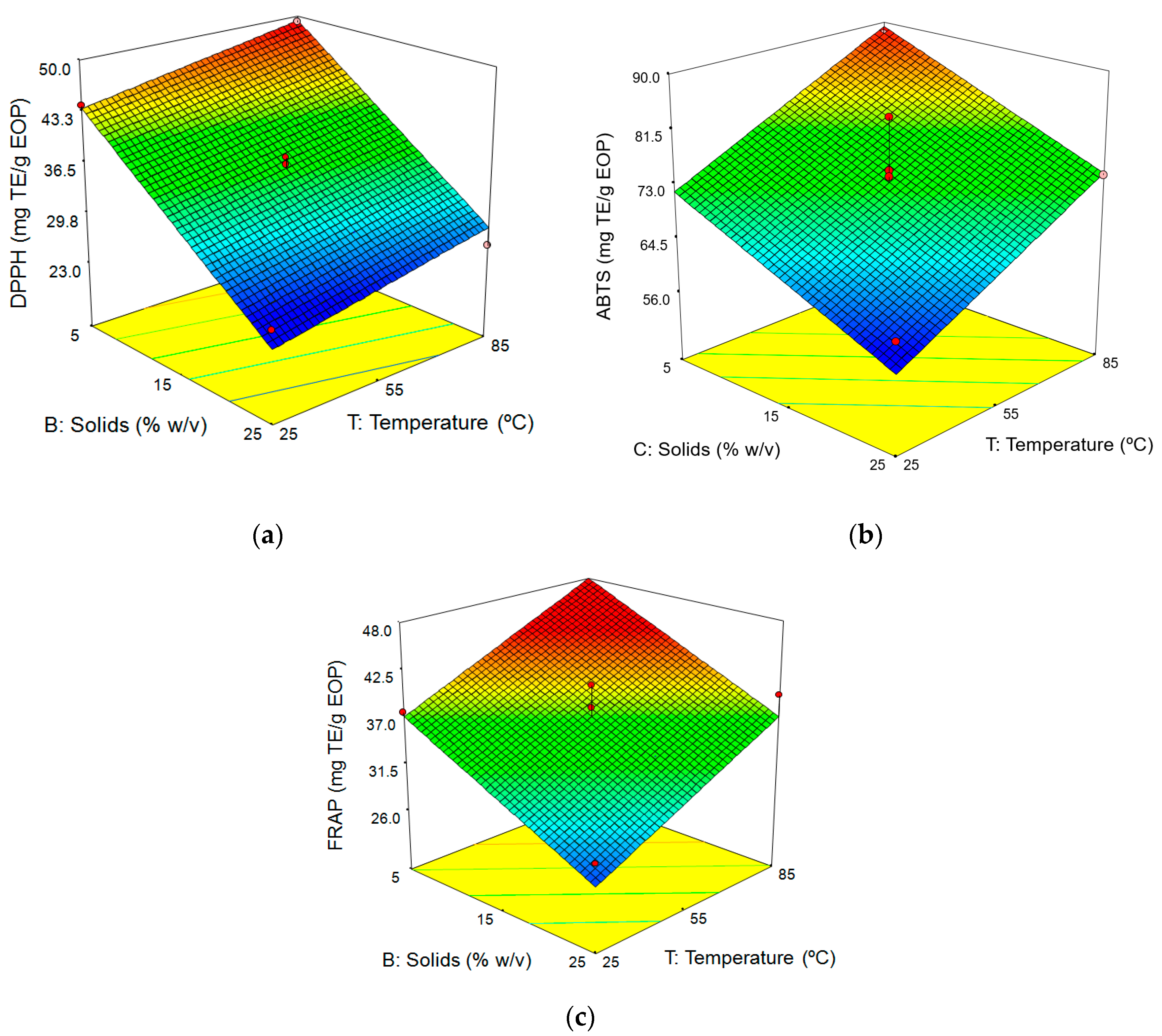
3.3. Influence of the Factors on the Aqueous Extraction of EOP
3.3.1. Fitting the Model
3.3.2. Response Surface Analysis
Influence of Extraction Conditions on Extraction Yield
Influence of Extraction Conditions on Phenolic Concentration, TPC, and TFC
Influence of Extraction Conditions on Antioxidant Activity
3.4. Optimisation of Water Extraction for EOP and Model Validation
3.5. Bioactive Compounds in Aqueous Extracts
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Manzanares, P.; Ruiz, E.; Ballesteros, M.; Negro, M.J.; Gallego, F.J.; López-Linares, J.C.; Castro, E. Residual biomass potential in olive tree cultivation and olive oil industry in Spain: Valorization proposal in a biorefinery context. Span. J. Agric. Res. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Ollero, P.; Serrera, A.; Arjona, R.; Alcantarilla, S. The CO2 gasification kinetics of olive residue. Biomass Bioenergy 2003, 24, 151–161. [Google Scholar] [CrossRef]
- Moral, P.S.; Méndez, M.V.R. Production of pomace olive oil. Grasas Aceites 2006, 57, 47–55. [Google Scholar]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Miranda, T.; Nogales, S.; Román, S.; Montero, I.; Arranz, J.I.; Sepúlveda, F.J. Control of several emissions during olive pomace thermal degradation. Int. J. Mol. Sci. 2014, 15, 18349–18361. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gómez-Cruz, I.; Romero, I.; Gullón, B.; Ruiz, E.; Brnčićc, M.; Castro, E. Ultrasound-assisted extraction as a first step in a biorefinery strategy for valorisation of extracted olive pomace. Energies 2019, 12, 2679. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Eibes, G.; Cara, C.; De Torres, A.; López-Linares, J.C.; Ruiz, E.; Castro, E. Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci. Total Environ. 2018, 645, 533–542. [Google Scholar] [CrossRef]
- Paini, M.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Binello, A.; Cravotto, G. Original article Influence of ethanol/water ratio in ultrasound and high-pressure/high-temperature phenolic compound extraction from agri-food waste. Int. J. Food Sci. Technol. 2016, 51, 349–358. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Valorization of olive oil solid waste using high pressure-high temperature reactor. Food Chem. 2011, 128, 704–710. [Google Scholar] [CrossRef]
- Rincón, B.; Rodríguez-Gutiérrez, G.; Bujalance, L.; Fernández-Bolaños, J.; Borja, R. Influence of a steam-explosion pre-treatment on the methane yield and kinetics of anaerobic digestion of two-phase olive mil solid waste or alperujo. Process Saf. Environ. Prot. 2016, 102, 361–369. [Google Scholar] [CrossRef]
- Caballero, A.S.; Romero-García, J.M.; Castro, E.; Cardona, C.A. Supercritical fluid extraction for enhancing polyphenolic compounds production from olive waste extracts. J. Chem. Technol. Biotechnol. 2019, 95, 356–362. [Google Scholar] [CrossRef]
- Seçmeler, Ö.; Güçlü, Ö.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Effect of subcritical water and steam explosion pretreatments on the recovery of sterols, phenols and oil from olive pomace. Food Chem. 2018, 265, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314, 126218. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT—Food Sci. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
- Manzanares, P.; Ballesteros, I.; Negro, M.J.; González, A.; Oliva, J.M.; Ballesteros, M. Processing of extracted olive oil pomace residue by hydrothermal or dilute acid pretreatment and enzymatic hydrolysis in a biorefinery context. Renew. Energy 2020, 145, 1235–1245. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Gómez-Cruz, I.; Ruiz, E.; Romero, I.; Castro, E. Production of ethanol from hemicellulosic sugars of exhausted olive pomace by Escherichia coli. Processes 2020, 8, 533. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Ruiz, E.; Romero, I.; Castro, E.; Manzanares, P. Xylitol production from exhausted olive pomace by Candida boidinii. Appl. Sci. 2020, 10, 6966. [Google Scholar] [CrossRef]
- Albahari, P.; Jug, M.; Radi, K.; Jurmanovi, S.; Brn, M.; Rimac, S.; Vitali, D. Characterization of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT—Food Sci. Technol. 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef]
- Hartonen, K.; Riekkola, M.L. Water as the First Choice Green Solvent. The Application of Green Solvents in Separation Processes; Pena-Pereira, F., Tobiszewski, M., Eds.; Elvesier: Helsinki, Finland, 2017; pp. 19–55. [Google Scholar]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Herrera, R.; Labidi, J.; Gullón, P. Yerba mate waste: A sustainable resource of antioxidant compounds. Ind. Crops Prod. 2018, 113, 398–405. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, S.A. Colorimetric of total phenolics with phosphomolibic Phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.D.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Optimization of oleuropein and luteolin-7-O-glucoside extraction from olive leaves by ultrasound-assisted technology. Energies 2019, 12, 2486. [Google Scholar] [CrossRef]
- Pérez, J.A.; González, A.; Oliva, J.M.; Ballesteros, I.; Manzanares, P. Effect of process variables on liquid hot water pretreatment of wheat straw for bioconversion to fuel-ethanol in a batch reactor. J. Chem. Technol. Biotechnol. 2007, 82, 929–938. [Google Scholar] [CrossRef]
- Krishnan, C.; Sousa, L.D.C.; Jin, M.; Chang, L.; Dale, B.E.; Balan, V. Alkali-Based AFEX Pretreatment for the conversion of sugarcane bagasse and cane leaf residues to ethanol. Biotechonol. Bioeng. 2010, 107, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Romaní, A.; Garrote, G.; Parajó, J.C. Bioethanol production from autohydrolyzed Eucalyptus globulus by simultaneous saccharification and fermentation operating at high solids loading. Fuel 2012, 94, 305–312. [Google Scholar] [CrossRef]
- Hernández, V.; Romero-García, J.M.; Dávila, J.A.; Castro, E.; Cardona, C.A. Techno-economic and environmental assessment of an olive stone based biorefinery. Resour. Conserv. Recycl. 2014, 92, 145–150. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Moya, M.; Ruiz, E.; Fernández-Bolaños, J.; Castro, E. Obtaining sugars and natural antioxidants from olive leaves by steam-explosion. Food Chem. 2016, 210, 457–465. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Ruiz, E.; Romero, I.; Cara, C.; López-Linares, J.C.; Castro, E. Combined acid/alkaline-peroxide pretreatment of olive tree biomass for bioethanol production. Bioresour. Technol. 2017, 239, 326–335. [Google Scholar] [CrossRef]
- Díaz-Blanco, D.I.; De La Cruz, J.; López-Linares, J.C.; Morales-Martínez, T.K.; Ruiz, E.; Rios-González, L.J.; Romero, I.; Castro, E. Optimization of dilute acid pretreatment of Agave lechuguilla and ethanol production by co-fermentation with Escherichia coli MM160. Ind. Crops Prod. 2018, 114, 154–163. [Google Scholar] [CrossRef]
- Cara, C.; Ruiz, E.; Carvalheiro, F.; Moura, P.; Ballesteros, I.; Castro, E.; Gírio, F. Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis. Ind Crops Prod. 2012, 40, 225–231. [Google Scholar] [CrossRef]
- Gullón, P.; Eibes, G.; Lorenzo, J.M.; Pérez-Rodríguez, N.; Lú-Chau, T.A.; Gullón, B. Green sustainable process to revalorize purple corn cobs within a biore fi nery frame: Co-production of bioactive extracts. Sci. Total Environ. 2020, 709, 136236. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Andary, J.; Maalouly, J.; Ouaini, R.; Chebib, H.; Beyrouthy, M.; Rutledge, D.N.; Ouaini, N. Phenolic Compounds from Diluted Acid Hydrolysates of Olive Stones: Effect of Overliming. Adv. Crop Sci. Tech. 2013, 1, 1–7. [Google Scholar]
- Alexovič, M.; Dotsikas, Y.; Bober, P.; Sabo, J. Achievements in robotic automation of solvent extraction and related approaches for bioanalysis of pharmaceuticals. J. Chromatogr. B 2018, 1092, 402–421. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Filho, R.M. Recent advances in lipid extraction using green solvents. Renew. Sust. Energ. Rev. 2020, 133. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Analyt. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wen, I.; Wibisono, R.; Melton, L.D.; Wadhwa, S. Evaluation of the extraction efficiency for polyphenol extracts from by-products of green kiwifruit juicing. Int. J. Food Sci. Technol. 2009, 44, 2644–2652. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; De Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. Int. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Agulló, A.; Freire, M.S.; González-Álvarez, J. Effect of the extraction technique on the recovery of bioactive compounds from eucalyptus (Eucalyptus globulus) wood industrial wastes. Ind. Crops Prod. 2015, 64, 105–113. [Google Scholar] [CrossRef]
- Vázquez, G.; Fernández-Agulló, A.; Gómez-Castro, C.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. Response surface optimization of antioxidants extraction from chestnut (Castanea sativa) bur. Ind. Crops Prod. 2012, 35, 126–134. [Google Scholar] [CrossRef]
- Mello, B.C.B.S.; Hubinger, M.D. Antioxidant activity and polyphenol contents in Brazilian green propolis extracts prepared with the use of ethanol and water as solvents in different pH values. Int. J. Food Sci. Technol. 2012, 47, 2510–2518. [Google Scholar] [CrossRef]
- Almanasrah, M.; Roseiro, L.B.; Bogel-Lukasik, R.; Carvalheiro, F.; Brazinha, C.; Crespo, J.; Kallioinen, M.; Mänttäri, M.; Duarte, L.C. Selective recovery of phenolic compounds and carbohydrates from carob kibbles using water-based extraction. Ind. Crops Prod. 2015, 70, 443–450. [Google Scholar] [CrossRef]
- Rico, X.; Gullón, B.; Alonso, J.L.; Parajó, J.C.; Yáñez, R. Valorization of peanut shells: Manufacture of bioactive oligosaccharides. Carbohydr. Polym. 2018, 183, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, I.; Ahmad-Qasem, M.H.; Barrajón, E.; Micol, V.; García-Pérez, J.V.; Ayadi, M.A.; Bouaziz, M. Kinetic improvement of olive leave’s bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrason. Sonochem. 2017, 34, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Živković, J.; Janković, T.; Menković, N.; Šavikin, K. Optimization of ultrasound-assisted extraction of isogentisin, gentiopicroside and total polyphenols from gentian root using response-surface methodology. Ind. Crops. Prod. 2019, 139. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Wagner, A.; Busso, C.; Wouk, J.; Iurckevicz, G.; Fernandes, P.; Yamashita, F.; Ricardo, C.; Malfatti, M. Influence of time, temperature and solvent on the extraction of bioactive compounds of Baccharis dracunculifolia: In vitro antioxidant activity, antimicrobial potential, and phenolic compound quantification. Ind. Crops Prod. 2018, 125, 207–219. [Google Scholar] [CrossRef]
- Kamarudin, A.A.; Esa, M.N.; Saad, N.; Sayuti, H.N.; Razak, N.A.A. Heat assisted extraction of phenolic compounds from Eleutherine bulbosa (Mill) bulb and its bioactive profiles using response surface methodology. Ind. Crops Prod. 2020, 144, 112064. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimizing a sustainable ultrasound-assisted extraction method for the recovery of polyphenols from lemon by-products: Comparison with hot water and organic solvent extractions. Eur. Food Res. Technol. 2018, 244, 1353–1365. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Tavares, C.S.; Roseiro, J.C.; Rauter, A.P. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind. Crops Prod. 2013, 44, 119–126. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutíerrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New phenolic compounds hydrothermally extracted from the olive oil byproduct alperujo and their antioxidative activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Guyot, S.; Marnet, N.; Lopes-da-Silva, J.A.; Renard, C.M.G.C.; Coimbra, M.A. Characterisation of phenolic extracts from olive pulp and olive pomace by electrospray mass spectrometry. J. Sci. Food Agric. 2005, 32, 21–32. [Google Scholar] [CrossRef]
- Habibi, H.; Mohammadi, A.; Farhoodi, M.; Sahar, J. Application and optimization of microwave-assisted extraction and dispersive liquid–liquid microextraction followed by high-performance liquid chromatography for the determination of oleuropein and hydroxytyrosol in olive pomace. Food Anal. Methods 2018, 11, 3078–3088. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Japon-Lujan, R.; de Castro, M.L. Simultaneous microwave-assisted solid–liquid extraction of polar and nonpolar compounds from alperujo. Anal. Chim. Acta 2007, 602, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Barroso, M.F.; Vasconcellos, J.; Ramalhosa, M.J.; Jaroslava, Š.-G.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-ahmad, A. High-Level Antimicrobial efficacy of representative mediterranean natural plant extracts against oral microorganisms. BioMed Res. Int. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Samli, R.; Tan Birteksoz, A.S.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef]
- Harkat-Madouri, L.; Asma, B.; Madani, K.; Si, Z.B.; Rigou, P.; Grenier, D.; Allalou, H.; Remini, H.; Adjaoud, A.; Boulekbache-Makhlouf, L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crops Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Moreira, D.; Gullón, B.; Gullón, P.; Gomes, A.; Tavaria, F. Function the prevention of microbial food-spoilage. J. Funct. Foods 2016, 7, 3273–3282. [Google Scholar] [CrossRef]
- Liu, Y.; Mckeever, L.C.; Malik, N.S.A. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H.; Azaizeh, H. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid. Based Complement. Altern. Med. 2011, 2011, 9. [Google Scholar] [CrossRef]
- Eilamiet, O.; Oliverio, M.; Hosseinian, S.; Motlagh, A.H.; Naghmachi, M. Antimicrobial effects of hydroxytyrosol extracted from olive leaves, on Propionibacterium. World Family. Med. 2017, 15, 187–191. [Google Scholar] [CrossRef]
- Ghalandari, M.; Naghmachi, M.; Oliverio, M.; Nardi, M.; Reza, H.; Shirazi, G.; Eilami, O. Antimicrobial effect of hydroxytyrosol, hydroxytyrosol acetate and hydroxytyrosol oleate on Staphylococcus Aureus and Staphylococcus Epidermidis. Electron. J. Gen. Med. 2018, 15, em46. [Google Scholar] [CrossRef]
| Component | % |
|---|---|
| Extractives | 41.78 ± 1.85 |
| Aqueous extractives | 37.94 ± 1.89 |
| Glucose | 1.77± 0.06 |
| Mannitol | 4.49 ± 0.10 |
| Phenolics | 5.15 ±1.07 |
| Ethanol extractives | 3.83 ± 0.16 |
| Cellulose | 9.67 ± 0.84 |
| Hemicellulose | 10.94 ± 0.53 |
| Xylan | 9.79 ± 0.53 |
| Galactan | 0.31 ± 0.01 |
| Arabinan | 1.82 ± 0.03 |
| Mannan | 0.42 ± 0.02 |
| Acetyl groups | 1.51 ± 0.17 |
| Lignin | 21.82 ± 0.89 |
| Acid insoluble lignin | 20.29 ± 0.68 |
| Acid soluble lignin | 1.54 ± 0.47 |
| Ash | 6.41 ± 0.21 |
| Solvent | Extraction Yield (%) | TPC (mg GAE) | TFC (mg RE) | DPPH (mg TE) | ABTS (mg TE) | FRAP (mg TE) |
|---|---|---|---|---|---|---|
| Water | 37.5± 0.21 | 38.1 ± 1.30 | 71.4 ± 2.92 | 22.4 ± 0.82 | 70.7 ± 3.90 | 39.9 ± 1.42 |
| Acidified water | 40.3± 1.51 | 29.7 ± 0.95 | 63.3 ± 3.40 | 16.3 ± 1.29 | 57.1 ± 7.49 | 33.9 ± 1.77 |
| 50% EtOH | 39.3± 0.51 | 39.5 ± 2.36 | 76.3 ± 2.25 | 27.9 ± 0.98 | 62.9 ± 5.44 | 41.5 ± 1.51 |
| 20% EtOH | 35.0± 1.03 | 34.6 ± 1.93 | 67.1 ± 5.13 | 22.4 ± 0.91 | 64.2 ± 4.70 | 38.1 ± 1.01 |
| 50% Acetone | 41.0 ± 0.25 | 41.6 ± 1.75 | 76.0 ± 3.14 | 35.1 ± 2.36 | 63.5 ± 4.14 | 46.2 ± 1.79 |
| Run | T (°C) | t (min) | B (%w/v) | Yield (%) | Phenolic Concentration (g GAE/L) | TPC (mg GAE) | TFC (mg RE) | DPPH (mg TE) | ABTS (mg TE) | FRAP (mg TE) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 (−1) | 60 (0) | 25 (1) | 28.7 | 6.7 | 28.8 | 82.7 | 25.8 | 61.63 | 28.7 |
| 2 | 55 (0) | 60 (0) | 15 (0) | 30.6 | 4.6 | 32.9 | 100.1 | 34.5 | 66.19 | 34.8 |
| 3 | 55 (0) | 30 (−1) | 25 (1) | 28.7 | 6.0 | 25.8 | 78.5 | 26.3 | 58.40 | 29.8 |
| 4 | 85 (1) | 90 (1) | 15 (0) | 34.5 | 5.2 | 37.0 | 106.6 | 42.1 | 89.40 | 43.7 |
| 5 | 55 (0) | 60 (0) | 15 (0) | 32.1 | 4.8 | 34.3 | 97.7 | 35.7 | 71.44 | 38.1 |
| 6 | 85 (1) | 60 (0) | 25 (1) | 33.6 | 7.4 | 31.7 | 94.6 | 26.6 | 73.88 | 39.4 |
| 7 | 85 (1) | 30 (−1) | 15 (0) | 32.0 | 4.7 | 33.4 | 104.5 | 39.7 | 77.87 | 40.9 |
| 8 | 55 (0) | 60 (0) | 15 (0) | 32.9 | 4.9 | 35.2 | 113.4 | 39.7 | 73.88 | 40.7 |
| 9 | 25 (−1) | 60 (0) | 5 (−1) | 27.7 | 1.4 | 38.0 | 148.7 | 44.1 | 72.44 | 37.6 |
| 10 | 55 (0) | 90 (1) | 25 (1) | 31.9 | 7.1 | 30.6 | 86.5 | 26.4 | 65.69 | 29.1 |
| 11 | 25 (−1) | 30 (−1) | 15 (0) | 26.9 | 3.6 | 25.8 | 90.7 | 31.4 | 58.44 | 25.1 |
| 12 | 55 (0) | 90 (1) | 5 (−1) | 35.6 | 2.0 | 43.6 | 155.6 | 46.4 | 81.31 | 41.6 |
| 13 | 55 (0) | 30 (−1) | 5 (−1) | 30.6 | 1.9 | 41.2 | 153.4 | 46.5 | 78.38 | 43.7 |
| 14 | 55 (0) | 60 (0) | 15 (0) | 32.1 | 4.9 | 34.9 | 104.2 | 36.9 | 74.81 | 35.1 |
| 15 | 85 (1) | 60 (0) | 5 (−1) | 33.2 | 1.9 | 41.2 | 157.6 | 49.2 | 87.81 | 42.3 |
| 16 | 25 (−1) | 90 (1) | 15 (0) | 29.9 | 4.2 | 29.7 | 95.6 | 33.6 | 67.69 | 30.9 |
| 17 | 55 (0) | 60 (0) | 15 (0) | 33.0 | 4.9 | 35.0 | 102.7 | 37.8 | 83.06 | 36.5 |
| Dependent Variables | Models | CV (%) | R2 | Adjusted R2 | F-Value | Lack of Fit (p-Values) |
|---|---|---|---|---|---|---|
| Extraction yield (%) | 31.9 + 2.52∙T + 1.67∙t − 1.07∙T2 (Equation (1)) | 2.63 | 0.902 | 0.875 | 33.71 | 0.762 |
| Phenolic concentration (g GAE/L) | 4.50 + 0.41∙T + 0.28∙t + 2.50∙B (Equation (2)) | 7.74 | 0.970 | 0.964 | 143.30 | 0.020 |
| TPC(mg GAE/g EOP) | 34.83 + 4.03∙T + 1.83∙t − 7.12∙B + 2.35∙T∙B − 3.03∙T2 (Equation (3)) | 2.76 | 0.979 | 0.967 | 83.44 | 0.486 |
| TFC (mg RE/g EOP) | 100.26 + 5.69∙T − 32.95∙B + 20.61∙B2 (Equation (4)) | 2.04 | 0.994 | 0.993 | 644.61 | 0.815 |
| DPPH (mg TE/g EOP) | 36.45 + 2.82∙T − 10.13∙B (Equation (5)) | 4.27 | 0.966 | 0.960 | 182.27 | 0.497 |
| ABTS (mg TE/g EOP) | 73.15 + 8.67∙T + 3.87∙t −
7.49∙B (Equation (6)) | 3.22 | 0.833 | 0.791 | 19.90 | 0.9413 |
| FRAP (mg TE/g EOP) | 37.08 + 5.40∙T − 5.50∙B (Equation (7)) | 5.69 | 0.861 | 0.838 | 37.27 | 0.743 |
| Predicted Values | Experimental Values | |
|---|---|---|
| Extraction yield (%) | 35.0 | 40.9 ± 0.54 |
| Phenolic concentration (g GAE/L) | 3.7 | 4.5 ± 0.03 |
| TPC (mg GAE/g EOP) | 40.5 | 44.5 ± 0.25 |
| TFC (mg RE/g EOP) | 132.4 | 114.9 ± 0.39 |
| DPPH (mg TE/g EOP) | 45.2 | 36.1 ± 0.36 |
| ABTS (mg TE/g EOP) | 89.5 | 95.4 ± 0.72 |
| FRAP (mg TE/g EOP) | 45.7 | 47.6 ± 0.24 |
| Microorganism | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| E. coli | 45 | 55 |
| Salmonella sp | 40 | 50 |
| S. aureus | 30 | 35 |
| L. innocua | 25 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of Exhausted Olive Pomace by an Eco-Friendly Solvent Extraction Process of Natural Antioxidants. Antioxidants 2020, 9, 1010. https://doi.org/10.3390/antiox9101010
Gómez-Cruz I, Cara C, Romero I, Castro E, Gullón B. Valorisation of Exhausted Olive Pomace by an Eco-Friendly Solvent Extraction Process of Natural Antioxidants. Antioxidants. 2020; 9(10):1010. https://doi.org/10.3390/antiox9101010
Chicago/Turabian StyleGómez-Cruz, Irene, Cristóbal Cara, Inmaculada Romero, Eulogio Castro, and Beatriz Gullón. 2020. "Valorisation of Exhausted Olive Pomace by an Eco-Friendly Solvent Extraction Process of Natural Antioxidants" Antioxidants 9, no. 10: 1010. https://doi.org/10.3390/antiox9101010
APA StyleGómez-Cruz, I., Cara, C., Romero, I., Castro, E., & Gullón, B. (2020). Valorisation of Exhausted Olive Pomace by an Eco-Friendly Solvent Extraction Process of Natural Antioxidants. Antioxidants, 9(10), 1010. https://doi.org/10.3390/antiox9101010