Abstract
Polyphenolic compounds, plant secondary metabolites essential for plant survival, are known for their high antioxidant and anti-inflammatory activity. In addition, several polyphenols, such as phloretin, also have potential antiviral effects, making these compounds potential ingredients of biofunctional foods. A promising source for the extraction of phloretin is a by-product of apple production—apple tree leaves. Focusing on green technologies, the first aim of the present study was to optimize the direct ultrasound-assisted extraction conditions to gain the maximum yield of phloretin from air-dried apple leaves. For the optimization of process parameters, we applied the response surface method with Box–Behnken design. The optimal extraction conditions were extraction time 14.4 min, sonication amplitude 10% and 10 g of sample per 100 mL solvent (70% ethanol, w/w). Using these conditions, we assessed the content of individual and total polyphenolic compounds along with antioxidant activity in the leaves of different autumn and winter apple cultivars grown in Estonia. The analyses were carried out with chromatographic (HPLC-DAD-MS/MS) and spectrophotometric methods. The phloretin concentration ranged from 292 to 726 µg/g and antioxidant activity from 6.06 to 11.42 mg GA eq./g, these being the highest in the local winter cultivars ‘Paide taliõun’ and ‘Tellissaare’, respectively.
1. Introduction
There are over 8000 polyphenolic compounds found in plant-derived material [1]. The polyphenolic compounds are known for their high antioxidant and anti-inflammatory activity, which favorably affects health risks for metabolic disorders [2]. Several polyphenols of plant origin, such as phloretin, quercetin, etc., are reported to have remarkable antiviral effects, preventing the attachment and replication of viruses and stimulating immune responses [3]. The presence of phenolic compounds in the diet has also been related to positive effects on cognitive decline, asthma and pulmonary function, bone health, and weight control [4]. In herbal therapy, natural polyphenols have been used in the form of infusions and extracts for treating ailments of digestive, vascular, urinary, and respiratory systems, in dermatology and for anticancer therapy [5]. Their main role is considered to consist in counteracting oxidative stress at the cellular level [6]. The health effects of these compounds are dependent on their amount in daily intake and their bio-availability [2].
The effects of polyphenols on human health make these compounds prospective components in the production of functional foods [7]. Besides the enrichment of food products, these compounds are also used in practical healthcare for the production of dietary supplements and cosmetic preparations [8], e.g., phloretin as a natural skin whitening agent due to its ability to inhibit tyrosinase activity [9]. In experimental cell models, the antioxidant effect of phloretin towards different stressors has been reported already at concentrations of 0.27 μg/mL [10].
Apple trees (Malus domestica Borkh.) are grown widely in different climate zones throughout the world, thus placing apples among the major fruits on the market [11], but they are also a potential source of antioxidant polyphenolic compounds. The most abundant phenolic compounds in apples are chlorogenic acid, phloretin, phloridzin, epicatechin, quercetin, and procyanidin B2 [12,13]. Levels of total phenolics and individual phenolic compounds with diverse antioxidant properties vary in different parts of apple trees (and fruits), but also between apple cultivars [13,14]. It has been established that various polyphenols, and in particular phloridzin and phloretin, are most abundant in apple leaves [12,15] and twigs [16], while procyanidins predominate in apple fruits [12,15]. The polyphenolic concentration in fruits peaks early in the season, and decreases during fruit development [1]. Large amounts of apple tree leaves, as well as unripe fruits, are harvested during summer pruning season to improve the apple production quality. This generates large-scale by-products that are a valuable source of polyphenolic compounds with high antioxidant potential [1,12,17].
Phenolic compounds are plant secondary metabolites required for plant survival [18]. Following the attacks of pathogenic bacteria and fungi, changes in the content of phenolics, particularly phloridzin and phloretin, have been detected both in resistant and susceptible apple cultivars [1,19]. It is also interesting to note that after inoculation of Erwinia amylovora, the phloretin content increased at a higher rate in resistant cultivars, although the initial concentrations of phloridzin and phloretin in the leaves were similar [1].
Dihydrochalcone phloretin and its glucoside phloridzin are found to be the major phenolic components in apple leaves. Depending on the cultivar and leaf maturation stage (terminal, medium or basal stage), phloridzin represented from 5.4% to 14% of leaf dry weight [15]. Greater variations were found for phloretin concentrations among different cultivars and leaf ages when compared to phloridzin [15]. In young apple leaves and twigs, phloridzin can account for up to 10% of dry weight [16]. Phloretin and phloridzin have been also found in apple seeds [20]. For the industrial production of dihydrochalcones, root bark of the apple tree is commonly used as a raw material. However, apple leaves contain similar concentrations of dihydrochalcones and have the advantage of being more abundant and renewable compared to the root bark, which can be harvested once in the tree’s life cycle [12,17].
Phloridzin plays an important role as a dietary polyphenol, with its capacity to reduce intestinal glucose uptake. In a recent study, Niederberger et al. [21] reported that the estimated average dietary phloridzin intake in Europe was low compared to levels used in human studies, in which phloridzin had a positive effect on glucose uptake. The author suggested that an increased dietary intake of phloridzin could have positive effects on the development and progression of some diet-related chronic diseases. Thus, apple leaves extracts have an interesting potential use for the enrichment of food products with phloridzin.
Today, studies focusing on the recovery of polyphenol extracts from apple leaves and information about the optimal extraction methods and conditions are still scarce. In Table 1, we have summarized different solvents and experimental procedures used for the extraction of dihydrochalcones from apple tree leaves and wood, available from recent literature reports. The collected data indicate that the yields of these compounds recovered through extraction are significantly dependent on the extraction procedure, but also vary between different cultivars.

Table 1.
Polyphenols extraction from apple leaves and wood.
A well-recognized green extraction technique allowing the full extraction of bioactive compounds in a short time with high reproducibility, reduced solvent consumption, and lower energy consumption is ultrasound-assisted extraction (UAE) [27]. The optimization of UAE allows one to increase the extraction yield while preserving the extract’s biological activity, and to prevent the wastage of raw material and solvent [28]. Indirect sonication in ultrasound (US) water baths, wherein the extraction medium is not in direct contact with the US source, has been used in earlier studies (Table 1) [8,12,22]. However, direct contact between the US source and the extraction medium allows one to intensify the cavitation effect and improve the extraction yield of bioactive compounds from the plant cell matrix [28].
The objective of the present study was to investigate the potential of apple tree leaves of different Estonian autumn and winter apple cultivars as an under-utilized source for the recovery of polyphenolic compounds. To gain the maximal phloretin yield and retain the maximum antioxidant activity of polyphenolic compounds during extraction, we used a US probe, exposing samples to direct sonication for the extraction of the targeted compounds from air-dried leaves. For the extraction, we used environmental friendly ethanol:water solutions. First, we optimized the UAE parameters, such as sonication amplitude, extraction time, and sample to solvent ratio, by applying the response surface methodology (RSM). After determination of the optimum extraction conditions, we compared extracts from different local apple cultivars in terms of antioxidant activity, total phenolic content and concentration of different individual phenolic compounds.
2. Materials and Methods
2.1. Selection of Apple Tree Cultivars
Two different autumn apple cultivars ‘Tiina’ and ‘Liivi kuldrenett’, and five winter apple cultivars ‘Tellissaare’, ‘Karksi renett’, ‘Paide taliõun’, ‘Talvenauding’ (all bred in Estonia), and “Cortland” (bred in US) were chosen for the study. These cultivars are all appreciated for the taste of fruits, and are widely grown in the region. Most of them are also recommended for commercial production orchards. In addition, all these cultivars have medium or high susceptibility to apple scab [29]. In the case of ‘Tiina’ and ‘Liivi kuldrenett’, the leaves are more susceptible to apple scab than the fruit.
2.2. Collection of Apple Tree Leaves and Preparation of Samples
The apple tree lateral shoots with leaves were collected from a private orchard in Tartu County, South Estonia (58°23′ N, 26°84′ E), during the summer pruning season in July 2020. The material was air-dried, and the leaves were removed from the shoots and stored at room temperature. The dried samples were ground to a fine powder using a cutting mill SM 300 (Retsch, Haan, Germany) at 1500 rpm with a 2 mm bottom sieve, reaching a final particle size of <1 mm.
2.3. Extraction Procedure
The ultrasound-assisted extraction of polyphenolic compounds from dried apple tree leaves was performed using a Digital Sonifier® S450 CE equipped with a 13 mm diameter disruptor horn (400 W Power, 20 kHz Frequency; Branson Ultrasonics Co., Danburry, CT, USA). Dried leaf powder (1–10 g) was mixed with 100 mL ethanol–water solution (70:30, w/w) in a double-wall glass tempering beaker connected to a circulating tap water to avoid heating during the extraction. The obtained extract was separated from the residual plant material by vacuum filtration through paper filter (12–15 µm retention rate, grade 1288; Sartorius AG, Göttingen, Germany). Recovered extracts were then analyzed for individual phenolic compounds and total phenolic content, and DPPH free radical scavenging activity.
2.4. RSM Design for the Optimization of Extraction
For the analysis of the influence of three major input variables of the UAE process on the extraction efficiency of total phenolic content (TPC), antioxidant activity, and selected phenolic compounds (incl. phloretin, phloridzin quercetin and others), we used the response surface methodology (RSM) coupled with a Box–Behnken design. The selected variables for optimization were extraction time (A, min), sonicator amplitude (B, %), and sample to solvent ratio (C, g sample/100 mL solvent). The variation ranges of input factors were 5–30 min, 10–100% and 1–10 g/100 mL solvent, and these were coded for the design and analyses. The complete design included 17 runs with 5 runs for the central point (Table 2).

Table 2.
Input factors for the Box–Behnken design.
The experimental design and analyses of the results were carried out with the Design-Expert® software (ver.12, Stat-Ease Inc., Minneapolis, MN, USA).
2.5. Determination of Total Phenolic Content
Total polyphenols content (TPC) was measured using the modified Folin–Ciocalteau (FC) method [30]. In brief, the gallic acid (GA) standards were prepared with the following concentrations: 50, 150, 250, 350, and 400 µg/mL. For the calibration, 0.4 mL of each standard was injected into a 4 mL spectrophotometer cuvette, to which 2.0 mL of FC reagent (0.2 N) was added, and after 5 min 1.6 mL of Na2CO3 (75 g/L) was added and the samples were incubated for 60 min in the dark at room temperature. Prior to the analyses of apple leaf extracts, the crude samples were diluted 40 times (250 µL of crude sample + 9750 µL of 70% w/w aqueous ethanol). The measurement procedure was the same as for the standard calibration described previously. The absorbance values of the samples were measured at 760 nm using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The results were expressed in mg of gallic acid equivalents per g of dry weight (mg GA eq./g dw). All chemicals used were of laboratory grade and purchased from Sigma (Steinheim, Germany).
2.6. DPPH Free Radical Scavenging Activity
Free radical scavenging activity measurements were performed in duplicate using a 2.2-diphenyl-picrylhydrazyl (DPPH) assay with slight modifications [31]. Briefly, the gallic acid calibration for the analysis was prepared as follows: 0.125, 0.100, 0.0625, 0.050, 0.025 and 0.010 mg/mL. For the measurement, 0.1 mL of each standard was pipetted into a 4 mL spectrophotometer cuvette, to which 3.7 mL of DPPH radical (63.5 µM) was added. The samples were incubated for 60 min in the dark at room temperature. The analytical procedure of the previously diluted apple leaf extracts was the same as for the standard calibration described previously. The absorbance values of the samples were measured at 515 nm using a spectrophotometer (UV-1800, Shimadzu, Japan). The results were expressed in mg of gallic acid equivalent per g of dry weight (mg GA eq./g dw). All chemicals used were of laboratory grade and purchased from Sigma (Steinheim, Germany).
2.7. Identification and Quantification of Polyphenols by LC-MS Method
Qualitative and quantitative analyses were performed on a Shimadzu Nexera X2 UHPLC with mass spectrometer LCMS 8040 (Shimadzu Scientific Instruments, Kyoto, Japan). The UHPLC system was equipped with a binary solvent delivery pump LC-30AD, an autosampler Sil-30AC, column oven CTO-20AC and diode array detector SPD-M20A. A reverse phase column ACE Excel 3 (C18, PFP, 100 × 2.1 mm; from ACE® Advanced Chromatography Technologies Ltd., Aberdeen, Scotland) and pre-column (SecurityGuard ULTRA, C18; from Phenomenex, Torrance, CA, USA) were used at 40 °C for the separation of individual polyphenols. The flow rate of the mobile phase was 0.25 mL/min, and the injected sample size was 1 µL or 0.2 µL depending on the concentration of the sample. Mobile phases consisted of 1% formic acid in Milli-Q water (mobile phase A) and 1% formic acid in methanol (mobile phase B). Separation was carried out for 40 min under the following conditions: gradient 0–27 min, 15–80% B; 27–29 min, 80–90% B; 29–35 min, isocratic 90% B, and re-equilibration of the system with 15% B 8 min prior to the next injection. All samples were kept at 4 °C during the analysis.
The total polyphenol content, expressed as mg chlorogenic acid equivalent per g of dry weight (mg ChlA eq./g dw), and total flavonols expressed as mg quercetin equivalent per g of dry weight (mg Q eq./g dw) were quantified at the wavelengths of 280 nm and 360 nm, respectively [32].
Individual phenolic compounds were identified by comparing the retention times, UV spectra, and parent and daughter ion masses with those of the standard compounds. MS data acquisitions were performed on LCMS 8040 with the ESI source operating in both positive and negative modes. The interface voltage was set to 4.5 kV (both ESI+ and ESI−). Nitrogen was used as the nebulizing gas (3 L/min) and drying gas (15 L/min). The heat block temperature was 350 °C and the desolvation line (DL) temperature was 250 °C. All samples were analyzed in triplicate, and the results were expressed as mg per g of dry weight (mg/g dw). Retention times and mass spectral data of standard phenolic compounds are summarized in Table 3.

Table 3.
Transition list and MS parameters used for the analysis of phenolic compounds.
All standards (chlorogenic acid, p-coumaric acid, caffeic acid, epicatechin, phloridzin dihydrate, phloretin, quercetin-3-d-galactoside, quercetin-3-d-glucoside, kaempferol-3-glucoside, quercitrin hydrate, rutin) and chemicals (formic acid, methanol) used were of analytical grade and purchased from Sigma (Steinheim, Germany).
2.8. Statistical Analysis
The statistical analysis was conducted using Prism version 5 (GraphPad Software, San Diego, CA, USA). Data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s test. The correlation was evaluated by Pearson analysis. Differences at p < 0.05 were considered to be significant.
3. Results and Discussion
3.1. Optimization of Ultrasound-Assisted Extraction of Phloretin
Ultrasound-assisted extraction (UAE) was selected for the extraction of phloretin from apple leaves, as this technique combined with the cooling of the sample allows one to conduct extractions at relatively low temperatures, which is favorable to enhance the extraction yield of heat-sensitive components. There are several independent process parameters which affect UAE, such as extraction time, temperature, solvent composition, power capacity, sample to solvent ratio, issues of sample grinding and mixing, shape of the vessel, and others. Three independent commonly modified factors (extraction time, amplitude of the sonicator, and the sample to solvent ratio) were selected for the optimization of the process. The minimum and maximum levels of these factors for the extraction of phenolic compounds from leaves were established during the preliminary experiments, so we did not expand the design space and used the Box–Behnken design for the optimization of these factors for the UAE process. Considering temperature, we kept this factor constantly below 25 °C with a cooling system to avoid the degradation of the phenolic compounds during the extraction process. Further, as the present study was focused on achieving maximum phloretin yields, requiring maximum interface between the solid and liquid phases as a precondition, we did not optimize the degree of sample grinding and the mixing rate in the extraction vessel, and kept these factors at the maximum possible levels. The choice of 70% ethanol:water solution as an extraction solvent was based on earlier data indicating the highest yields of polyphenols from apple leaves at this particular ethanol:water ratio [8,24,33].
The analysis of experimental results was based on the yield of the targeted individual phenolic compounds, total phenolic content, and the antioxidant activity, with the main focus on maximizing the output of phloretin. Phloretin fulfils the criteria of Lipinski’s rule of five, which would make it a likely orally active drug-like compound in humans, and its bioavailability is 1. The phloretin molecule is lipophilic (log p value is 2.2–3.9), and it is practically insoluble in water (0.13 g/L) [34].
Based on the summary statistics from model fitting, the best model to maximize the phloretin output was the reduced two-factor interaction (2FI) model (p = 0.0116). The 2FI model’s F-value of 5.50 and p-value of 0.0116 imply that the model was significant. There was no influence of time-related lurking variables in the background, as the plot of the residuals versus runs showed a random scatter (data not shown). The ANOVA test indicated that the extraction time and its interactions with other studied factors were statistically not significant, so these terms were excluded from the model. The final equation in terms of actual factors was the following:
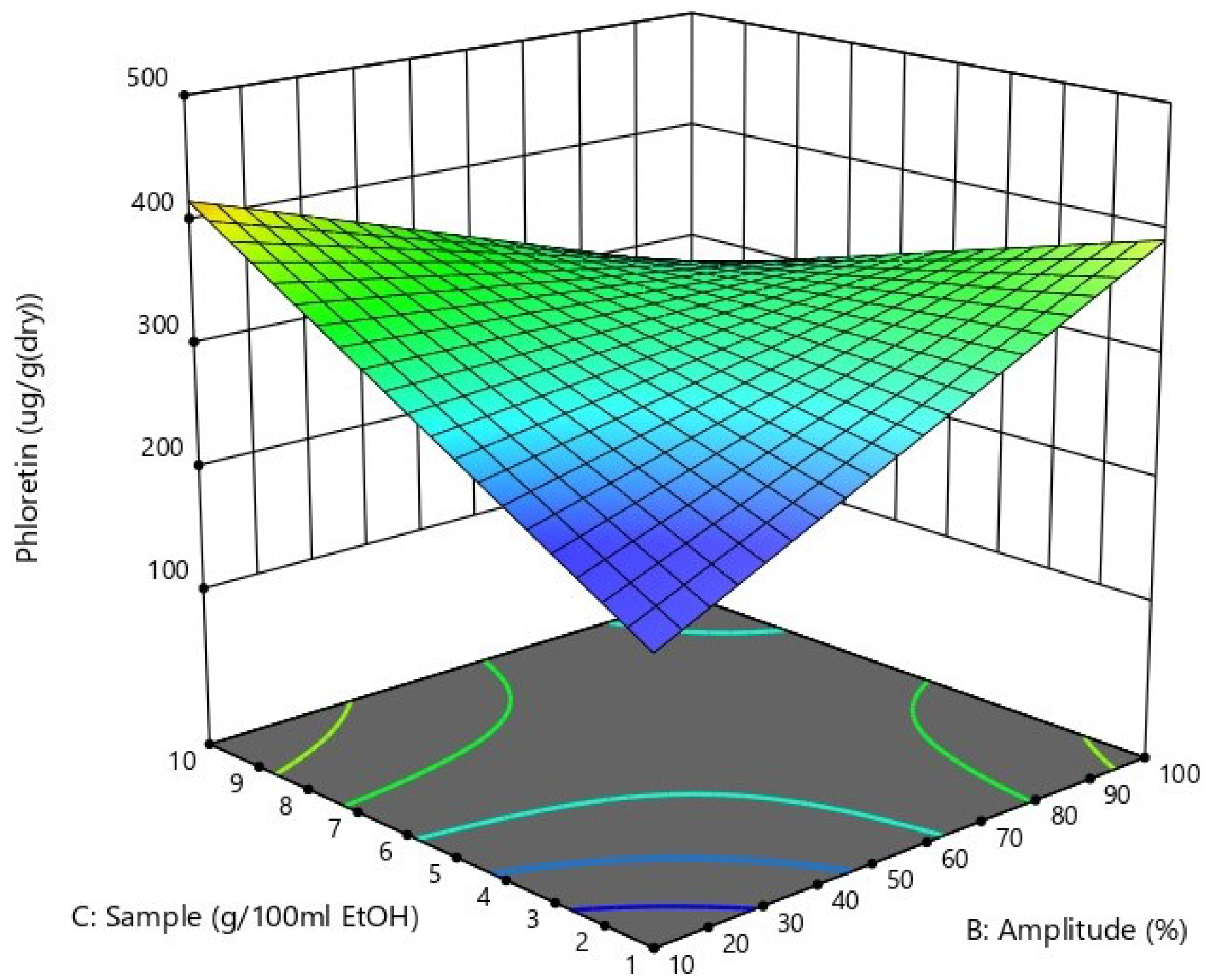
The 3D response surface, indicating the effects of sonication amplitude (factor B) and sample amount per 100 mL solvent (factor C) on phloretin yield, is shown in Figure 1. According to the process model (Equation (1)), the impacts of factors B and C on phloretin yield are quite similar in actual terms (as the amplitude range is 10 times bigger than the range of sample amount), and the increase in phloretin yield can be achieved both by increasing the sample amount and by decreasing the amplitude, or decreasing the sample amount and increasing the amplitude. High amounts of sample combined with high sonication amplitude led also to a decrease in the yield of phloretin (Figure 1), which is probably caused by the clotting of the sample and the ineffectiveness of stirring in such a thick mixture.

Figure 1.
Three-dimensional response surface plot showing the effects of the amount of sample and sonication amplitude on phloretin yield.
As the phloretin yield was not significantly dependent on extraction time, we also considered the TPC and DPPH values to maximize the efficiency of the extraction of all phenolic compounds. As the phenolic compounds can be degraded at high sonication amplitudes (high energy), the optimal extraction conditions were as follows: extraction time 14.4 min, extraction amplitude 10%, and the amount of sample per 100 mL solvent was 10 g. To assess the effect of sonication on the extraction yield, the optimized protocol was repeated also in silent conditions without sonication. Comparing the yields of polyphenols extracted from the air-dried apple leaves, the extraction yields in silent conditions were from 4 to 7.5 times lower depending on the particular phenolic compound. For phloretin, the sonication increased the yield 6.5-fold.
3.2. Polyphenols Content and Radical Scavenging Activity of Apple Leaves Extracts from Different Cultivars
The TPC, flavonols, and DPPH radical scavenging activity in apple leaf extracts from the seven locally grown cultivars are presented in Table 4. The TPC in the recovered extracts ranged from 35.67 to 57.74 mg GA eq./g dw. When determined by UPLC-DAD analysis, the total polyphenols content was slightly higher, ranging between 37.28 and 71.06 mg ChlA eq./g dw. Nevertheless, both methods showed that the highest polyphenol content was obtained from the leaf powder of cultivar ‘Tellissaare’, followed by cultivars ‘Karksi renett’ > ‘Paide taliõun’ > ‘Cortland’ > ‘Liivi kuldrenett’ > ‘Talvenauding’ > ‘Tiina’. Statistical analysis showed that the TPC in extracts from the ‘Tellissaare’ cultivar was significantly higher than that in all other six cultivars, whereas the TPC was not significantly different between the extracts from cultivars that showed the lowest content (i.e., ‘Liivi kuldrenett’, ‘Talvenauding’, and ‘Tiina’). Previous studies on leaf extracts from different apple cultivars have reported a significant difference in TPC [8,12,22,35]. Parvaneh et al. [35] showed that the rootstock, the cultivar genotype, and their interactions had significant effects on the polyphenol contents of apple leaves, and this in part was related to the activity of enzymes responsible for the biosynthesis of phenolic compounds and flavonoids.

Table 4.
Polyphenol content and antioxidant activity in the extracts from air-dried leaves of different apple cultivars.
Comparison of the results from previous studies on extracts from apple leaves (Table 1) shows that TPC largely varies according to the preparation method of the raw material, as well as the extraction conditions. The TPC in extracts obtained from lyophilized leaf powder ranged between 56.74 and 163.35 mg GA eq./g dw, while TPC was relatively lower in extracts recovered from dried apple leaf powder, ranging between 24.10 and 37.10 mg GA eq./g dw. In the present study, we used air-dried leaf powder, and, on one hand, the recovered polyphenols content was relatively low compared to extracts recovered from lyophilized leaves. On the other hand, the polyphenol content was higher than in the previously reported results for extracts of air-dried leaves as well as apple tree wood. Lyophilization is widely used for dehydrating and improving the stability of pharmaceutical products. However, due to the high cost of this process, its application is still limited in the food industry [36]. Thus, air-drying seems to be a more suitable treatment for apple leaves prior to extraction, since lyophilization is an expensive process and is more energy-consuming.
The total flavonols content in apple leaf extracts (Table 4) ranged between 7.47 and 12.23 mg Q eq./g dw, accounting for approximately 16% to 23% of the TPC. Liaudanskas et al. [8] reported a comparable proportion of total flavonoids in the ethanol extracts of apple leaves, ranging between 21% and 27% of the total polyphenol content. Similar to the total polyphenol content, extracts of cultivar ‘Tellissaare’ displayed significantly higher flavonols contents, whereas extracts from the leaves of ‘Paide taliõun’ had the lowest flavonols contents.
The antioxidant activity of the recovered extracts was determined by evaluating their DPPH free radical scavenging activity, and was expressed as mg GA eq./g dw, as presented in Table 4. Leaf extracts of cultivar ‘Tellissaare’, which showed the highest TPC and flavonols, exhibited the strongest antioxidant capacity, with 48.4% radical scavenging activity equivalent to 11.42 mg GA eq./g dw. The radical scavenging activity of extracts from other cultivars ranged between 40.1% and 28.9% (6.06 and 9.19 mg GA eq./g dw). The correlation between the antioxidant activities of apple leaf extracts and their total polyphenol and flavonols contents was confirmed by Pearson correlation analysis. There was a significant correlation between DPPH radical scavenging activity and the total polyphenols content (r = 0.9533, p = 0.0009), while the correlation between DPPH radical scavenging activity and flavonols content was not significant (r = 0.6758, p = 0.0957). A previous study investigating the antioxidant activity of apple leaf extracts reported a strong positive correlation between the total polyphenols and flavonoids contents of the extracts and their antioxidant activities [8]. Teleszko and Wojdyło [22] investigated the antioxidant activity of leaf extracts from different fruit trees and bushes, including apple, quince, chokeberry, cranberry, etc. Their results showed that apple leaf extract exhibited the third-highest content of total polyphenols, whereas it had one of the lowest antioxidant activities, which was explained by the differences in the polyphenols profiles of different plant species.
3.3. Identification and Quantification of Individual Phenolic Compounds in Apple Leaves Extracts
Samples were analyzed by UHPLC-MS for the qualitative and quantitative analysis of the individual phenolic compounds present in apple leaf extracts from different cultivars. The method was optimized, and the calibration ranges of standards were adjusted considering the estimated concentrations of polyphenolic compounds in apple leaf extracts. The limit of detection (LOD) and limit of quantification (LOQ) values for the targeted phenolic compounds were far below the actual measured concentrations in the extracts, and ranged from 0.018 to 0.081 μg/mL and 0.060 to 0.271 μg/mL, respectively. The LOD and LOQ values were the highest for kaempferol-3-glucoside and the lowest for quercetin-3-glucoside. For phloretin, the value of LOD was 0.039 μg/mL, and the LOQ was 0.130 μg/mL.
The distributions of the individual polyphenols in the leaf extracts from apple cultivars under study are presented in Table 5. The results show that apple leaf extracts contained compounds from four polyphenolic groups: phenolic acids, dihydrochalcones, flavonols and catechins. The latter were only present in the form of epicatechin in low concentrations, which were below the detection limit of the quantification method.

Table 5.
Individual phenolic compounds contents in extracts of air-dried leaves from different apple cultivars.
In Section 3.1, the UAE conditions were optimized to maximize the extraction yield of phloretin. Under these optimal extraction conditions, the concentrations of phloretin obtained ranged between 292 and 726 µg/g dw. The phloretin concentration was the highest in the leaves of winter cultivars ‘Paide Taliõun’ and ‘Tellissaare’, with 726 and 505 µg/g, respectively. The concentrations of phloretin recovered in this study were higher than that reported by Rana and Bhushan [24] in extracts from dried apple leaves, which was 150 µg/g. In addition, the recovered phloretin concentrations are comparable to those obtained from apple tree roots (399 µg/g), which are more commonly used as a raw material for the industrial production of phloretin [26].
Phloridzin, a phloretin glucoside, was the major phenolic constituent of apple leaf extracts, accounting for 60% to 80% of the total phenolic compounds in different cultivars. The concentrations of phloridzin in the extracts ranged between 11,511 and 31,654 µg/g dw. Phloridzin content was the highest in the leaves of apple cultivar ‘Tellissaare’. These results are in accordance with previous studies reporting phloridzin as the major phenolic constituent of apple leaves [8,12,15,25].
Apple leaf extracts also contained flavonols, mainly quercetin glycosides. Quercitrin (quercetin-3-rhamnoside) was the major quercetin glycoside present in the extracts, and concentrations in different cultivars ranged between 1988 and 4290 µg/g dw. The kaempferol-3-glucoside concentrations in different cultivars ranged between 809 and 1680 µg/g dw. Both quercetin and kaempferol aglycones were detected in apple leaf extracts, but their concentrations were very low compared to their glycosides. Their concentrations in different cultivars ranged between 16– and 40 µg/g dw and 2 and 4 µg/g dw, for quercetin and kaempferol, respectively. Chlorogenic acid was the major phenolic acid detected in all studied cultivar extracts, with concentrations ranging between 178 and 338 µg/g dw. Previous studies on apple leaf extracts also reported quercitrin and chlorogenic acid as the major flavonol and phenolic acid in leaf extracts, respectively [8,12].
4. Conclusions
The UAE of polyphenolic compounds from apple tree leaves, with the main focus on phloretin, was optimized using the response surface methodology. The conditions resulting in the maximal yield of phloretin were 14.4 min extraction time, 10% sonication amplitude and 10 g sample per 100 mL 70% (w/w) ethanol:water solution, while keeping the extraction temperature < 25 °C and retaining a high mixing rate during the extraction process. The highest antioxidant activity was found in the leaves of a local Estonian winter cultivar, ‘Tellissaare’. The phloretin concentration was the highest in the leaves of the winter cultivar ‘Paide taliõun’, at 726 µg/g, and the lowest (292 µg/g) was found in a popular cultivar, ‘Cortland’, originating from the US. Considering the large amounts of leaves available during the summer pruning season in orchards, and the effectiveness of UAE, the local apple tree leaves can be a valuable source of plant polyphenolic compounds to be used to increase the antioxidant activity of functional food.
Author Contributions
Conceptualization, T.R., and H.K.; methodology, S.B.-O., H.K., R.R., T.R., U.B., A.A.; investigation, S.B.-O., H.K., R.R., T.R., U.B., A.A.; writing—original draft preparation, S.B.-O., T.R.; writing—review and editing, S.B.-O., H.K., R.R., T.R., U.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon 2020 research and innovation program project VALORTECH under grant agreement No. 810630, and by the European Regional Development Fund’s project “PlantValor—full-scale product development service in synergy with the traditional activities of Polli Horticultural Research Centre” 2020–2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We did not include such data in this study.
Conflicts of Interest
The authors declare no conflict of interest in this study.
References
- Skłodowska, M.; Mikiciński, A.; Wielanek, M.; Kuźniak, E.; Sobiczewski, P. Phenolic profiles in apple leaves and the efficacy of selected phenols against fire blight (Erwinia amylovora). Eur. J. Plant Pathol. 2018, 151, 213–228. [Google Scholar] [CrossRef]
- Marranzano, M.; Rosa, R.L.; Malaguarnera, M.; Palmeri, R.; Tessitori, M.; Barbera, A.C. Polyphenols: Plant Sources and Food Industry Applications. Curr. Pharm. Des. 2018, 24, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Alexander Haslberger, G.; Jacob, U.; Hippe, B.; Karlic, H. Mechanisms of selected functional foods against viral infections with a view on COVID-19: Mini review. Funct. Foods Heal. Dis. 2020, 10, 195–209. [Google Scholar] [CrossRef]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Cyboran, S.; Oszmiański, J.; Kleszczyńska, H. Extracts from apple leaves and fruits as effective antioxidants. J. Med. Plants Res. 2011, 5, 2339–2347. [Google Scholar] [CrossRef]
- Restani, P. Polyphenol-rich foods for human health. Nutrients 2020, 12, 3738. [Google Scholar] [CrossRef] [PubMed]
- Baboota, R.K.; Bishnoi, M.; Ambalam, P.; Kondepudi, K.K.; Sarma, S.M.; Boparai, R.K.; Podili, K. Functional food ingredients for the management of obesity and associated co-morbidities—A review. J. Funct. Foods 2013, 5, 997–1012. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Raudonis, R.; Kviklys, D.; Uselis, N.; Janulis, V. Phenolic composition and antioxidant activity of Malus domestica leaves. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Z.; Han, L.; Li, S.; Zhou, W. Preparation and characterization of phloretin by complexation with cyclodextrins. New J. Chem. 2020, 44, 5218–5223. [Google Scholar] [CrossRef]
- De Oliveira, M.R. Phloretin-induced cytoprotective effects on mammalian cells: A mechanistic view and future directions. BioFactors 2016, 42, 13–40. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organisation of the United Nations). Food Wastage Footprint: Impacts on Natural Resources—Summary Report. 2013. Available online: http://www.fao.org/3/i3347e/i3347e.pdf (accessed on 5 January 2021).
- Wojdyło, A.; Oszmiański, J. Antioxidant activity modulated by polyphenol contents in apple and leaves during fruit development and ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, Y.J.; Kim, D.O.; Lee, H.J.; Lee, C.Y. Major Phenolics in Apple and Their Contribution to the Total Antioxidant Capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Heinmaa, L.; Moor, U.; Põldma, P.; Raudsepp, P.; Kidmose, U.; Lo Scalzo, R. Content of health-beneficial compounds and sensory properties of organic apple juice as affected by processing technology. LWT Food Sci. Technol. 2017, 85, 372–379. [Google Scholar] [CrossRef]
- Picinelli, A.; Dapena, E.; Mangas, J.J. Polyphenolic Pattern in Apple Tree Leaves in Relation to Scab Resistance. A Preliminary Study. J. Agric. Food Chem. 1995, 43, 2273–2278. [Google Scholar] [CrossRef]
- Ridgway, T.; Tucker, G.; Wiseman, H. Novel Bioconversions for the production of designer antioxidant and colourant flavonoids using polyphenol oxidases. Biotechnol. Genet. Eng. Rev. 1997, 14, 165–190. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Kean, C.; Nichols, D.; Embree, C. Orchard waste as a valuable bio-resource: A chemical composition analysis. Acta Hortic. 2007, 737, 17–23. [Google Scholar] [CrossRef]
- Nagendran, B.; Tan, Y.A.; Ravigadevi, S.; Kalyana, S.; Samir, S. Antioxidant properties of palm fruit extracts. Asia Pac. J. Clin. Nutr. 2006, 4, 319–324. [Google Scholar]
- Mikulic Petkovšek, M.; Stampar, F.; Veberic, R. Increased phenolic content in apple leaves infected with the apple scab pathogen. J. Plant Pathol. 2008, 90, 49–55. [Google Scholar] [CrossRef]
- Schieber, A.; Hilt, P.; Endreß, H.U.; Rentschler, C.; Carle, R. A new process for the combined recovery of pectin and phenolic compounds from apple pomace. Innov. Food Sci. Emerg. Technol. 2003, 4, 99–107. [Google Scholar] [CrossRef]
- Niederberger, K.E.; Tennant, D.R.; Bellion, P. Dietary intake of phloridzin from natural occurrence in foods. Br. J. Nutr. 2020, 123, 942–950. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Lu, Y.; Du, Y.; Qin, X.; Wu, H.; Huang, Y.; Cheng, Y.; Wei, Y. Comprehensive evaluation of effective polyphenols in apple leaves and their combinatory antioxidant and neuroprotective activities. Ind. Crops Prod. 2019, 129, 242–252. [Google Scholar] [CrossRef]
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: Assessment, analysis and application. J. Food Sci. Technol. 2016, 53, 1727–1738. [Google Scholar] [CrossRef]
- Walia, M.; Kumar, S.; Agnihotri, V.K. UPLC-PDA quantification of chemical constituents of two different varieties (golden and royal) of apple leaves and their antioxidant activity. J. Sci. Food Agric. 2016, 96, 1440–1450. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Sordivaramu.emu.ee—Database for Estonian Fruit and Berry Cultivars. Available online: http://sordivaramu.emu.ee/search.php?otsi=tiina (accessed on 5 January 2021).
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H. Bin Total phenolic contents and antioxidant capacities of selected chinese medicinal plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Raudsepp, P.; Koskar, J.; Anton, D.; Meremäe, K.; Kapp, K.; Laurson, P.; Bleive, U.; Kaldmäe, H.; Roasto, M.; Püssa, T. Antibacterial and antioxidative properties of different parts of garden rhubarb, blackcurrant, chokeberry and blue honeysuckle. J. Sci. Food Agric. 2019, 99, 2311–2320. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, S.; Rana, A.; Sharma, V.; Katoch, P.; Padwad, Y.; Bhushan, S. Phenolic constituents from apple tree leaves and their in vitro biological activity. Ind. Crops Prod. 2016, 90, 118–125. [Google Scholar] [CrossRef]
- Phloretin|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB07810 (accessed on 5 January 2021).
- Parvaneh, T.; Abedi, B.; Davarynejad, G.H.; Ganji Moghadam, E. Enzyme activity, phenolic and flavonoid compounds in leaves of Iranian red flesh apple cultivars grown on different rootstocks. Sci. Hortic. 2019, 246, 862–870. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Lenart, A. Freeze-drying—Application in food processing and biotechnology—A review. Polish J. Food Nutr. Sci. 2011, 61, 165–171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).