Antioxidant Carbon Nanoparticles Inhibit Fibroblast-Like Synoviocyte Invasiveness and Reduce Disease Severity in a Rat Model of Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. PEG-HCCs
2.2. Animals and Induction and Monitoring of Pristane-Induced Arthritis
2.3. Cells
2.4. Internalization of PEG-HCCs by RA-FLS
2.5. Quantification of Intracellular Superoxide
2.6. Cytotoxicity Assays
2.7. Cell Proliferation Assays
2.8. Invasion Assays
2.9. Gelatin Gel Zymographies
2.10. Bead Array-Based Quantification of MMPs and Cytokines
2.11. Detection of PEG-HCCs by Immunohistochemistry
2.12. Quantification of Circulating Rheumatoid Factor
2.13. X-rays, Histology, and Immunohistochemistry on Hind Limbs of Rats with PIA
2.14. Phenotyping of T Lymphocyte Populations
2.15. Statistics
3. Results
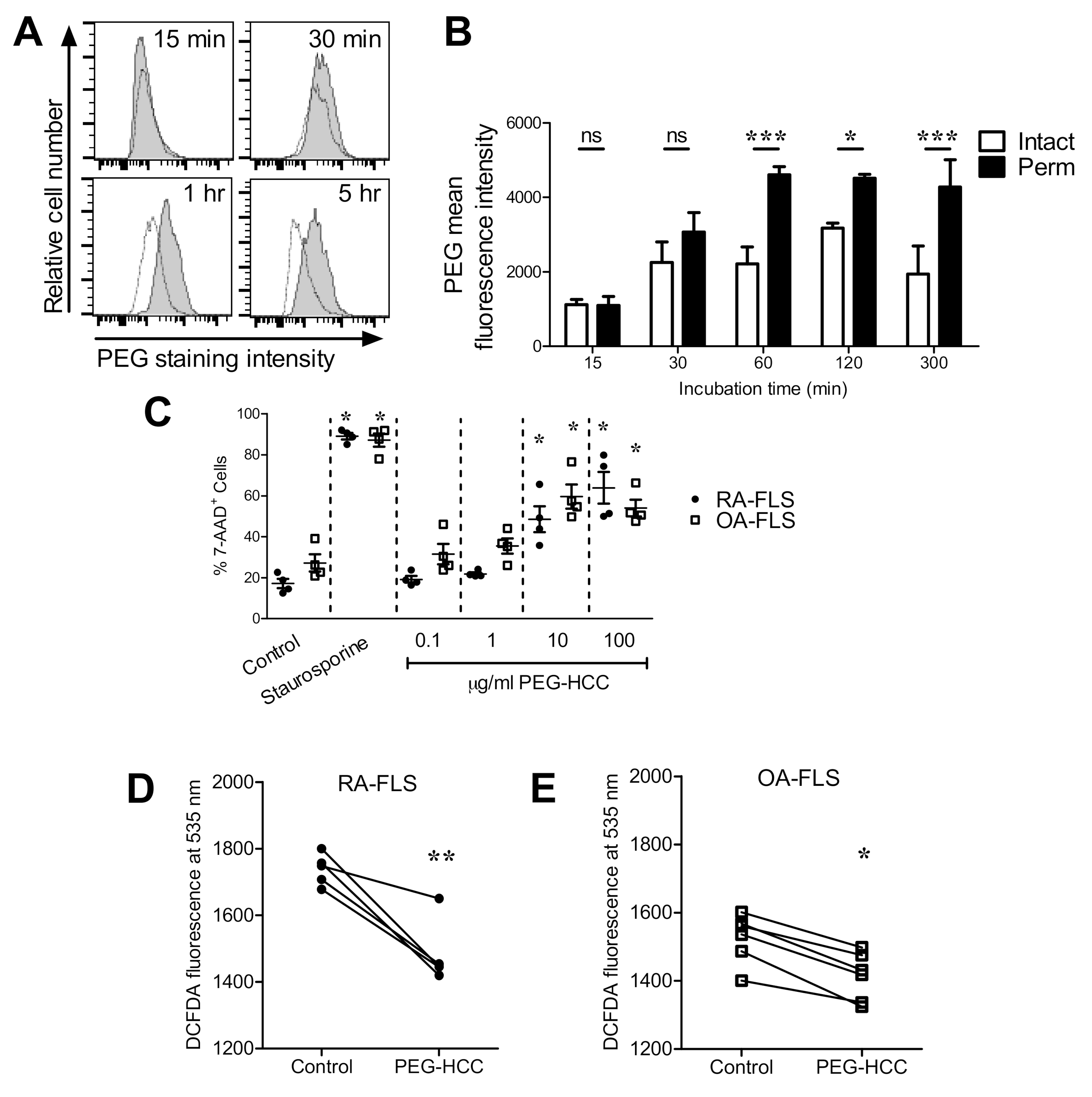
3.1. PEG-HCCs Are Internalized by RA-FLS and Reduce the Cells’ Intracellular O2•− Levels
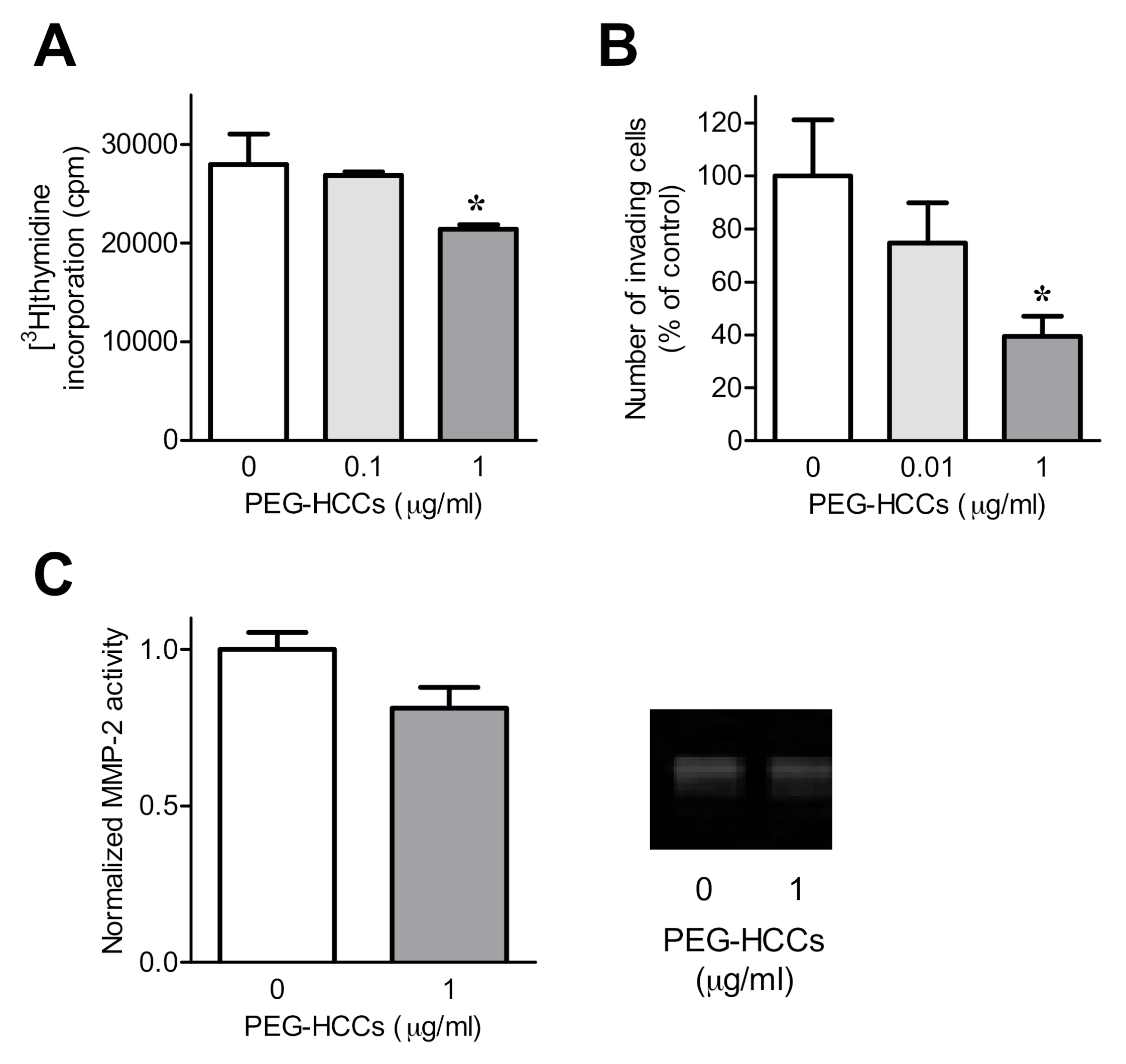
3.2. PEG-HCCs Alter FLS Phenotypes In Vitro
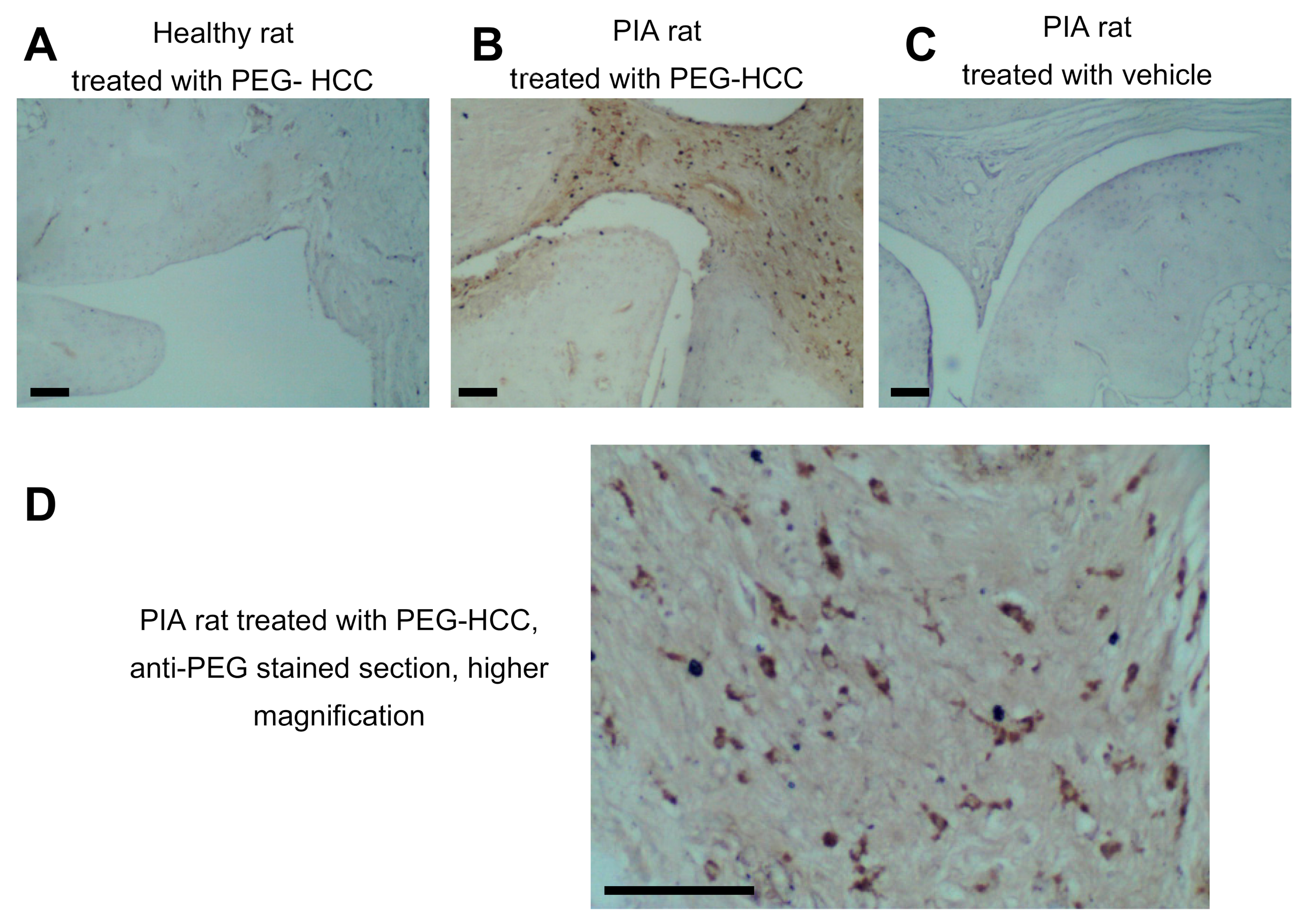
3.3. PEG-HCCs Are Found in Synovial Cells during PIA
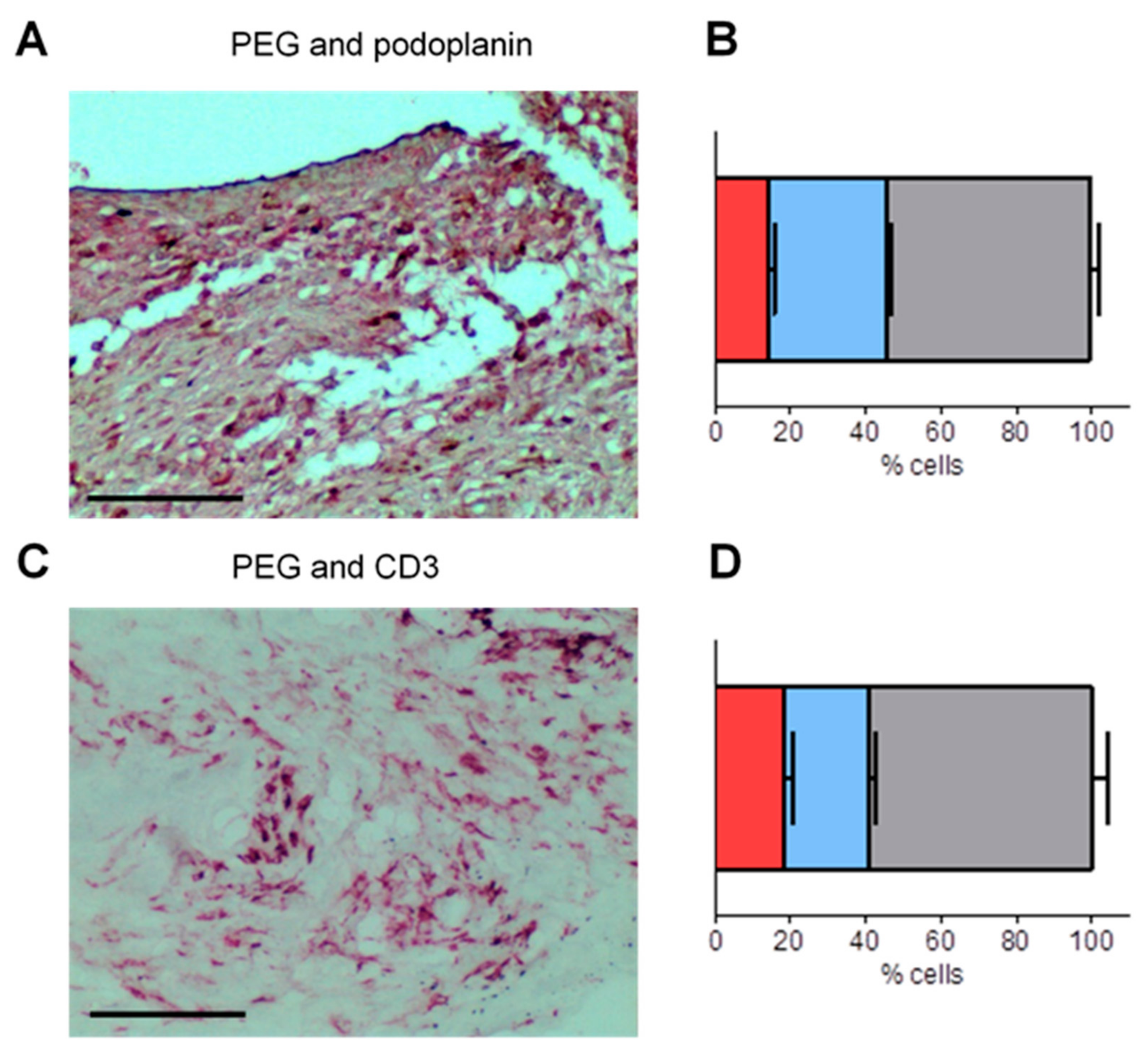
3.4. PEG-HCCs Co-Localize with Podoplanin and CD3 in the Synovium of Rats with PIA
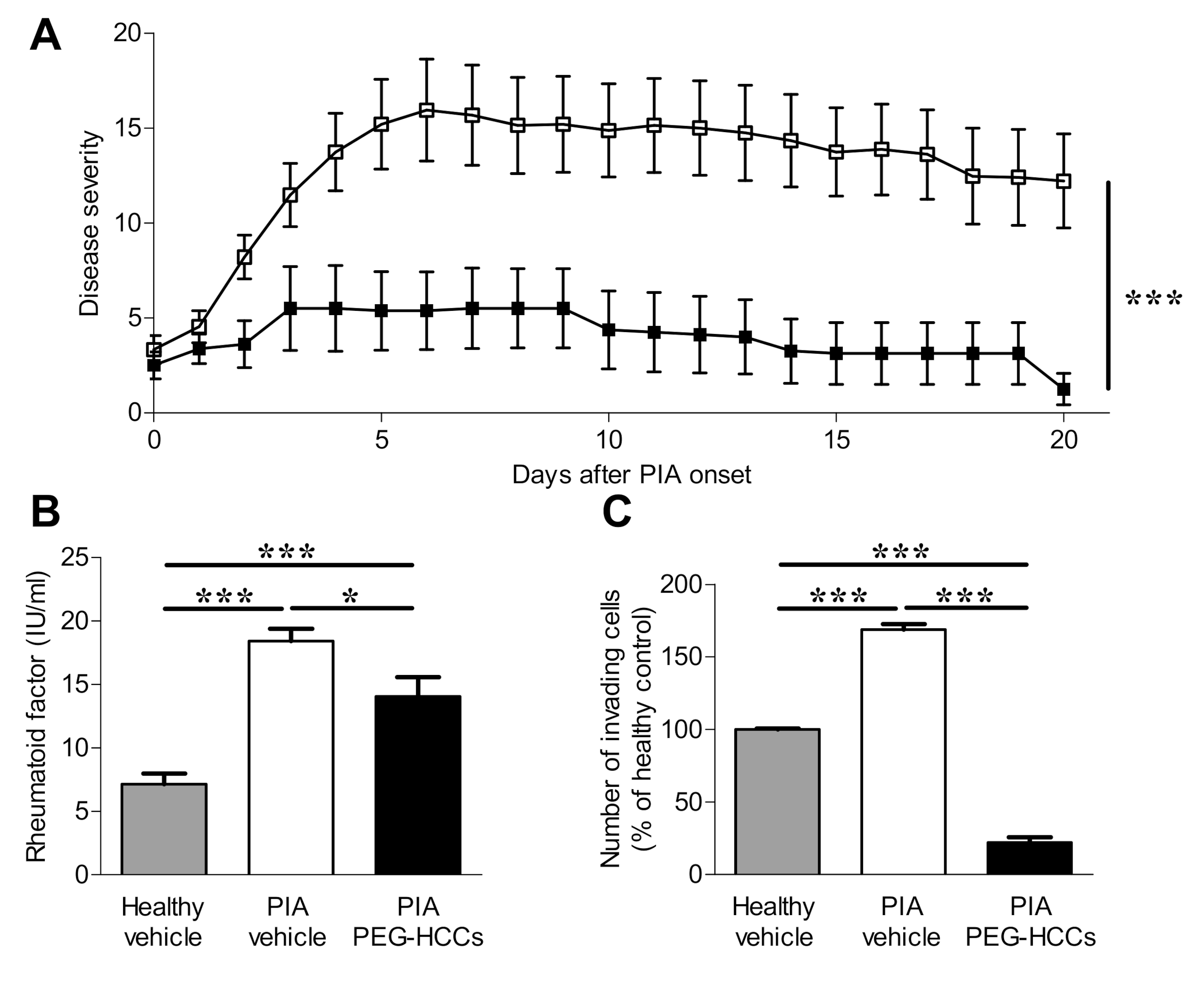
3.5. PEG-HCCs Reduce Disease Severity in PIA
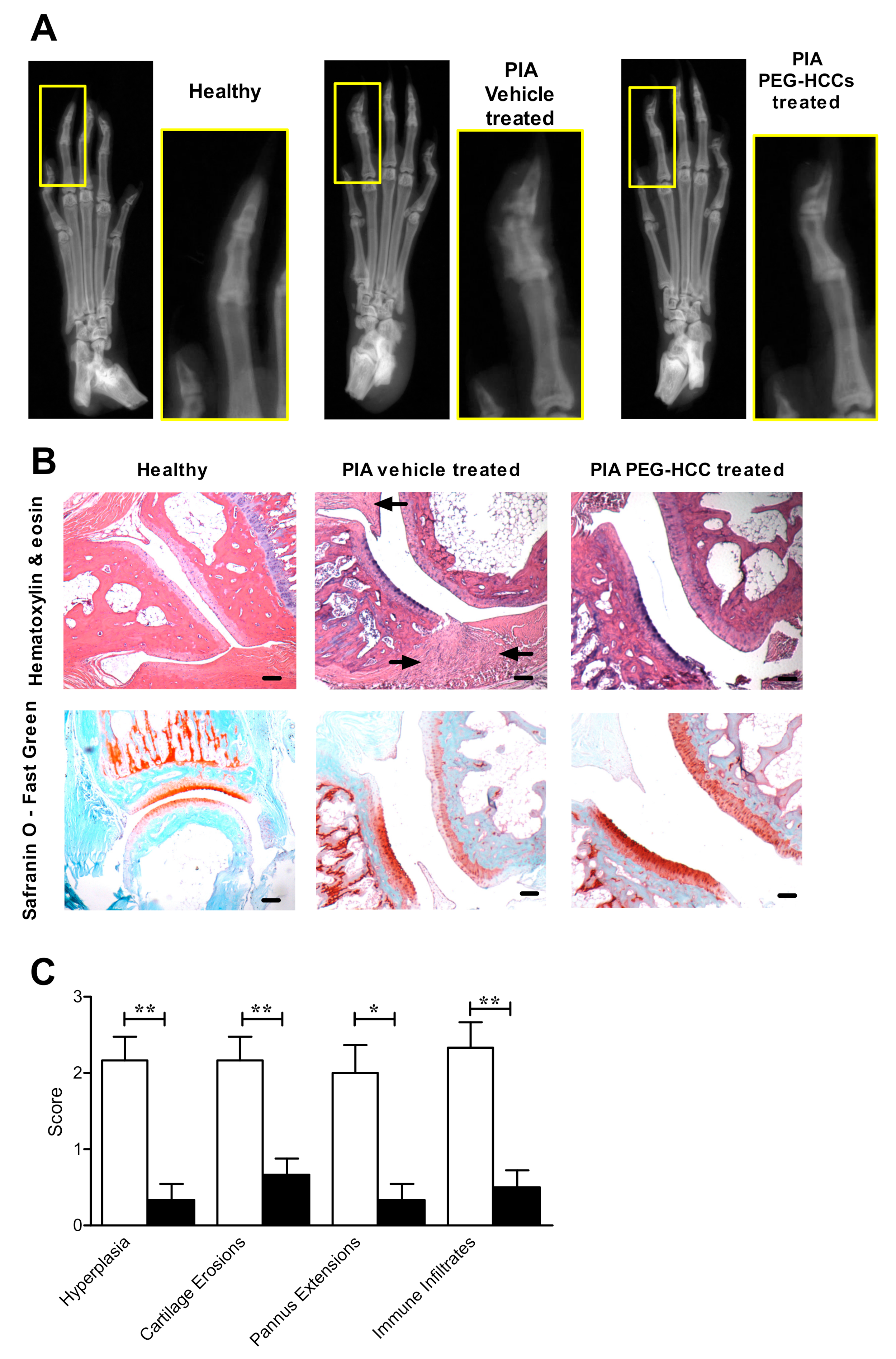
3.6. PEG-HCCs Reduce Bone and Joint Damage in PIA
3.7. PEG-HCCs Do Not Induce Lymphopenia during PIA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abeles, A.M.; Pillinger, M.H. The role of the synovial fibroblast in rheumatoid arthritis: Cartilage destruction and the regulation of matrix metalloproteinases. Bull. NYU Hosp. Jt. Dis. 2006, 64, 20–24. [Google Scholar] [PubMed]
- Mor, A.; Abramson, S.B.; Pillinger, M.H. The fibroblast-like synovial cell in rheumatoid arthritis: A key player in inflammation and joint destruction. Clin. Immunol. 2005, 115, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.C.; Distler, O.; Tarner, I.; Gay, R.E.; Gay, S.; Pap, T. Synovial fibroblasts: Key players in rheumatoid arthritis. Rheumatology 2006, 45, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Bottini, N.; Firestein, G. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted agressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.D.; Filer, A. The role of the synovial fibroblast in rheumatoid arthritis pathogenesis. Curr. Opin. Rheumatol. 2015, 27, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Ghosh, P.; Datta, S.; Ghosh, A.; Chattopadhyay, S.; Chatterjee, M. Oxidative stress as a potential biomarker for determining disease activity in patients with Rheumatoid Arthritis. Free Radic. Res. 2012, 46, 1482–1489. [Google Scholar] [CrossRef]
- Gamal, R.M.; Hammam, N.; Zakary, M.M.; Abdelaziz, M.M.; Razek, M.R.A.; Mohamed, M.S.E.; Emad, Y.; Elnaggar, M.G.; Furst, D.E. Telomere dysfunction-related serological markers and oxidative stress markers in rheumatoid arthritis patients: Correlation with diseases activity. Clin. Rheumatol. 2018, 37, 3239–3246. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Kardeş, S.; Karagülle, M.; Durak, I.; Avcı, A.; Karagülle, M.Z. Association of oxidative stress with clinical characteristics in patients with rheumatoid arthritis. Eur. J. Clin. Investig. 2017, 48, e12858. [Google Scholar] [CrossRef]
- Al-Azab, M.; Qaed, E.; Ouyang, X.; Elkhider, A.; Walana, W.; Li, H.; Li, W.; Tang, Y.; Adlat, S.; Wei, J.; et al. TL1A/TNFR2-mediated mitochondrial dysfunction of fibroblast-like synoviocytes increases inflammatory response in patients with rheumatoid arthritis via reactive oxygen species generation. FEBS J. 2020. [Google Scholar] [CrossRef]
- Chenevier-Gobeaux, C.; Lemarechal, H.; Bonnefont-Rousselot, D.; Poiraudeau, S.; Ekindjian, O.G.; Borderie, D. Superoxide production and NADPH oxidase expression in human rheumatoid synovial cells: Regulation by interleukin-1β and tumour necrosis factor-α. Inflamm. Res. 2006, 55, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.; Appel, L.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Jalilov, A.S.; Zhang, C.; Samuel, E.L.; Sikkema, W.K.A.; Wu, G.; Berka, V.; Kent, T.A.; Tsai, A.-L.; Tour, J.M. Mechanistic Study of the Conversion of Superoxide to Oxygen and Hydrogen Peroxide in Carbon Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 15086–15092. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.L.G.; Marcano, D.C.; Berka, V.; Bitner, B.R.; Wu, G.; Potter, A.; Fabian, R.H.; Pautler, R.G.; Kent, T.A.; Tsai, A.-L.; et al. Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. Proc. Natl. Acad. Sci. USA 2015, 112, 2343–2348. [Google Scholar] [CrossRef]
- Marcano, D.C.; Bitner, B.R.; Berlin, J.M.; Jarjour, J.; Lee, J.M.; Jacob, A.; Fabian, R.H.; Kent, T.A.; Tour, J.M. Design of Poly(ethylene Glycol)-Functionalized Hydrophilic Carbon Clusters for Targeted Therapy of Cerebrovascular Dysfunction in Mild Traumatic Brain Injury. J. Neurotrauma 2013, 30, 789–796. [Google Scholar] [CrossRef]
- Huq, R.; Samuel, E.L.G.; Sikkema, W.K.A.; Nilewski, L.G.; Lee, T.; Tanner, M.R.; Khan, F.S.; Porter, P.C.; Tajhya, R.B.; Patel, R.S.; et al. Preferential uptake of antioxidant carbon nanoparticles by T lymphocytes for immunomodulation. Sci. Rep. 2016, 6, srep33808. [Google Scholar] [CrossRef]
- Inoue, T.; Griffin, D.M.; Huq, R.; Samuel, E.L.; Ruano, S.H.; Stinnett, G.; Majid, T.J.; Beeton, C.; Tour, J.M.; Pautler, R.G. Characterization of a novel MR-detectable nanoantioxidant that mitigates the recall immune response. NMR Biomed. 2016, 29, 1436–1444. [Google Scholar] [CrossRef]
- Bustamante, M.F.; Garcia-Carbonell, R.; Whisenant, K.D.; Guma, M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 110. [Google Scholar] [CrossRef]
- Lucente-Schultz, R.M.; Moore, V.C.; Leonard, A.D.; Price, B.K.; Kosynkin, D.V.; Lu, M.; Partha, R.; Conyers, J.L.; Tour, J.M. Antioxidant Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2009, 131, 3934–3941. [Google Scholar] [CrossRef]
- Berlin, J.M.; Leonard, A.D.; Pham, T.T.; Sano, D.; Marcano, D.C.; Yan, S.; Fiorentino, S.; Milas, Z.L.; Kosynkin, D.V.; Price, B.K.; et al. Effective Drug Delivery, In Vitro and In Vivo, by Carbon-Based Nanovectors Noncovalently Loaded with Unmodified Paclitaxel. ACS Nano 2010, 4, 4621–4636. [Google Scholar] [CrossRef]
- Holmdahl, R.; Lorentzen, J.C.; Lu, S.; Olofsson, P.; Wester, L.; Holmberg, J.; Pettersson, U. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol. Rev. 2001, 184, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.; Hu, X.; Huq, R.; Tajhya, R.B.; Sun, L.; Khan, F.S.; Laragione, T.; Horrigan, F.T.; Gulko, P.S.; Beeton, C. KCa1.1 inhibition attenuates fibroblast-like synoviocyte invasiveness and ameliorates rat models of rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Tarcha, E.J.; Chi, V.; Muñoz-Elías, E.J.; Bailey, D.; Londono, L.M.; Upadhyay, S.K.; Norton, K.; Banks, A.; Tjong, I.; Nguyen, H.; et al. Durable Pharmacological Responses from the Peptide ShK-186, a Specific Kv1.3 Channel Inhibitor That Suppresses T Cell Mediators of Autoimmune Disease. J. Pharmacol. Exp. Ther. 2012, 342, 642–653. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.B., III; Birnbaum, N.S.; Burmester, G.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Chan, A.; Akhtar, M.; Brenner, M.; Zheng, Y.; Gulko, P.S.; Symons, M. The GTPase Rac Regulates the Proliferation and Invasion of Fibroblast-Like Synoviocytes from Rheumatoid Arthritis Patients. Mol. Med. 2007, 13, 297–304. [Google Scholar] [CrossRef]
- Laragione, T.; Gulko, P.S. mTOR Regulates the Invasive Properties of Synovial Fibroblasts in Rheumatoid Arthritis. Mol. Med. 2010, 16, 352–358. [Google Scholar] [CrossRef]
- Laragione, T.; Brenner, M.; Mello, A.; Symons, M.; Gulko, P.S. The arthritis severity locus Cia5d is a novel genetic regulator of the invasive properties of synovial fibroblasts. Arthritis Rheum. 2008, 58, 2296–2306. [Google Scholar] [CrossRef]
- Hu, X.; Laragione, T.; Sun, L.; Koshy, S.; Jones, K.R.; Ismailov, I.I.; Yotnda, P.; Horrigan, F.T.; Gulko, P.S.; Beeton, C. KCa1.1 potassium channels regulate key pro-inflammatory and invasive properties of fibroblast-like synoviocytes in rheumatoid arthritis. J. Biol. Chem. 2012, 287, 4014–4022. [Google Scholar] [CrossRef]
- Tajhya, R.B.; Hu, X.; Tanner, M.; Huq, R.; Kongchan, N.; Neilson, J.R.; Rodney, G.G.; Horrigan, F.T.; Timchenko, L.T.; Beeton, C. Functional KCa1.1 channels are crucial for regulating the proliferation, migration and differentiation of human primary skeletal myoblasts. Cell Death Dis. 2016, 7, e2426. [Google Scholar] [CrossRef][Green Version]
- Pethő, Z.; Tanner, M.; Tajhya, R.B.; Huq, R.; Laragione, T.; Panyi, G.; Gulko, P.S.; Beeton, C. Different expression of β subunits of the KCa1.1 channel by invasive and non-invasive human fibroblast-like synoviocytes. Arthritis Res. Ther. 2016, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Beeton, C. Detection of functional matrix metalloproteinases by zymography. J. Vis. Exp. 2010, 45, e2445. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.R.; Pennington, M.W.; Chamberlain, B.H.; Huq, R.; Gehrmann, E.J.; Laragione, T.; Gulko, P.S.; Beeton, C. Targeting KCa1.1 Channels with a Scorpion Venom Peptide for the Therapy of Rat Models of Rheumatoid Arthritis. J. Pharmacol. Exp. Ther. 2018, 365, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.; Chandy, K.G. Drawing blood from the saphenous vein of rats and by cardiac puncture. J. Visualized. Exp. 2007, 7, 266. [Google Scholar] [CrossRef]
- Beeton, C.; Chandy, K.G. Isolation of Mononuclear Cells from the Central Nervous System of Rats with EAE. J. Vis. Exp. 2007, 10, 527. [Google Scholar] [CrossRef]
- Brenner, M.; Meng, H.-C.; Yarlett, N.C.; Griffiths, M.M.; Remmers, E.F.; Wilder, R.L. The non-major histocompatibility complex quantitative trait locusCia10 contains a major arthritis gene and regulates disease severity, pannus formation, and joint damage. Arthritis Rheum. 2005, 52, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.; Pennington, M.W.; Chauhan, S.S.; Laragione, T.; Gulko, P.S.; Beeton, C. KCa1.1 and Kv1.3 channels regulate the interactions between fibroblast-like synoviocytes and T lymphocytes during rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 6. [Google Scholar] [CrossRef]
- Cai, W.-W.; Yu, Y.; Zong, S.-Y.; Wei, F. Metabolic reprogramming as a key regulator in the pathogenesis of rheumatoid arthritis. Inflamm. Res. 2020, 69, 1087–1101. [Google Scholar] [CrossRef]
- Svensson, M.N.D.; Zoccheddu, M.; Yang, S.; Nygaard, G.; Secchi, C.; Doody, K.M.; Slowikowski, K.; Mizoguchi, F.; Humby, F.; Hands, R.; et al. Synoviocyte-targeted therapy synergizes with TNF inhibition in arthritis reversal. Sci. Adv. 2020, 6, eaba4353. [Google Scholar] [CrossRef]
- Mizoguchi, F.; Slowikowski, K.; Wei, K.; Marshall, J.L.; Rao, D.A.; Chang, S.K.; Nguyen, H.N.; Noss, E.H.; Turner, J.D.; Earp, B.E.; et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Tanner, M.R.; Pennington, M.W.; Laragione, T.; Gulko, P.S.; Beeton, C. KCa1.1 channels regulate β 1-integrin function and cell adhesion in rheumatoid arthritis fibroblast-like synoviocytes. FASEB J. 2017, 31, 3309–3320. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, L.; Cheng, C.; Shan, W.; Ma, R.; Yin, Z.; Zhu, C. Parallel comparison of fibroblast-like synoviocytes from the surgically removed hyperplastic synovial tissues of rheumatoid arthritis and osteoarthritis patients. BMC Musculoskelet. Disord. 2019, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pap, T.; Dankbar, B.; Wehmeyer, C.; Korb-Pap, A.; Sherwood, J. Synovial fibroblasts and articular tissue remodelling: Role and mechanisms. Semin. Cell Dev. Biol. 2020, 101, 140–145. [Google Scholar] [CrossRef]
- Ekwall, A.-K.H.; Eisler, T.; Anderberg, C.; Jin, C.; Karlsson, N.G.; Brisslert, M.; Bokarewa, M.I. The tumour-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R40. [Google Scholar] [CrossRef] [PubMed]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nat. Cell Biol. 2019, 570, 246–251. [Google Scholar] [CrossRef]
- Treumer, F.; Zhu, K.; Gläser, R.; Mrowietz, U. Dimethylfumarate Is a Potent Inducer of Apoptosis in Human T Cells. J. Investig. Dermatol. 2003, 121, 1383–1388. [Google Scholar] [CrossRef]
- Matheu, M.P.; Beeton, C.; Garcia, A.; Chi, V.; Rangaraju, S.; Safrina, O.; Monaghan, K.; Uemura, M.; Li, D.; Pal, S.; et al. Imaging of Effector Memory T Cells during a Delayed-Type Hypersensitivity Reaction and Suppression by Kv1.3 Channel Block. Immunity 2008, 29, 602–614. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.; Zhang, Y.; Zhang, J.; Cao, W.; Chen, X. Effect of lentivirus-mediated overexpression or silencing of MnSOD on apoptosis of resveratrol-treated fibroblast-like synoviocytes in rheumatoid arthritis. Eur. J. Pharmacol. 2019, 844, 65–72. [Google Scholar] [CrossRef]
- You, S.; Yoo, S.-A.; Choi, S.; Kim, J.-Y.; Park, S.-J.; Ji, J.D.; Kim, T.-H.; Kim, K.-J.; Cho, C.-S.; Hwang, D.; et al. Identification of key regulators for the migration and invasion of rheumatoid synoviocytes through a systems approach. Proc. Natl. Acad. Sci. USA 2014, 111, 550–555. [Google Scholar] [CrossRef]
- Frank-Bertoncelj, M.; Trenkmann, M.; Klein, K.; Karouzakis, E.; Rehrauer, H.; Bratus, A.; Kolling, C.; Armaka, M.; Filer, A.; Michel, B.A.; et al. Epigenetically-driven anatomical diversity of synovial fibroblasts guides joint-specific fibroblast functions. Nat. Commun. 2017, 8, 14852. [Google Scholar] [CrossRef]
- Wunder, A.; Müller-Ladner, U.; Stelzer, E.H.K.; Funk, J.; Neumann, E.; Stehle, G.; Pap, T.; Sinn, H.; Gay, S.; Fiehn, C. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis. J. Immunol. 2003, 170, 4793–4801. [Google Scholar] [CrossRef] [PubMed]
- Levick, J.R. Permeability of Rheumatoid and Normal Human Synovium to Specific Plasma Proteins. Arthritis Rheum. 1981, 24, 1550–1560. [Google Scholar] [CrossRef]
- Burger, J.A.; Zvaifler, N.J.; Tsukada, N.; Firestein, G.S.; Kipps, T.J. Fibroblast-like synoviocytes support B-cell pseudoemperipolesis via a stromal cell–derived factor-1– and CD106 (VCAM-1)–dependent mechanism. J. Clin. Investig. 2001, 107, 305–315. [Google Scholar] [CrossRef]

| Donor | Diagnosis | Sex | Ethnicity | Age | Disease Duration (Years) | RF | Medication(s) | Origin |
|---|---|---|---|---|---|---|---|---|
| OA-1 | OA | Female | Caucasian | UN | UN | N/A | None | PSG |
| OA-2 | OA | Female | African American | 56 | 2 | N/A | None | PSG |
| OA-3 | OA | Female | Caucasian | 56 | 5 | N/A | None | PSG |
| OA-4 | OA | Male | Caucasian | 56 | 25 | N/A | None | PSG |
| RA-1 | RA | Female | Hispanic | 64 | >10 | + | Prednisone, Methotrexate | PSG |
| RA-2 | RA | Female | Caucasian | 66 | 20 | + | Prednisone, Etanercept | PSG |
| RA-3 | RA | Male | Hispanic | 48 | <1 | + | DMARD, Prednisone | Asterand |
| RA-4 | RA | Female | African American | 49 | 11 | + | Prednisone, Plaquenil | PSG |
| RA-5 | RA | Female | Caucasian | 71 | 3 | + | Methotrexate, Prednisone | PSG |
| RA-6 | RA | Male | Caucasian | 54 | >10 | + | Etanercept | PSG |
| RA-7 | RA | Female | Caucasian | 39 | 3 | + | NSAID | Asterand |
| Target | Host | Vendor (Location) | Catalog Number | Conjugation |
|---|---|---|---|---|
| Primary Antibodies | ||||
| PEG | Rabbit | Abcam | Ab51257 | N/A |
| Rat CD3 | Mouse | Becton Dickinson | 557030 | Allophycocyanin |
| Rat CD3 | Mouse | Becton Dickinson | 550295 | N/A |
| Rat CD4 | Mouse | Biolegend | 201516 | Phycoerythrin-cyanine-7 |
| Rat CD8 | Mouse | Becton Dickinson | 554857 | Phycoerythrin |
| Rat CD25 | Mouse | Becton Dickinson | 554865 | Fluorescein isothyocyanate |
| Rat CD45RC | Mouse | Invitrogen | MA5-17458 | Biotin |
| Rat/human podoplanin | Mouse | Abcam | Ab10288 | N/A |
| Secondary Antibodies | ||||
| Rabbit IgG | Goat | Life Technologies | P-10994 | Pacific Blue |
| Rabbit IgG | Horse | Vector Labs | MP-7401-50 | Horseradish peroxidase |
| Mouse IgG | Horse | Vector Labs | AP-2000-1 | Alkaline phosphatase |
| Streptavidin | N/A | Invitrogen | S32351 | Alexa Fluor 405 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanner, M.R.; Huq, R.; Sikkema, W.K.A.; Nilewski, L.G.; Yosef, N.; Schmitt, C.; Flores-Suarez, C.P.; Raugh, A.; Laragione, T.; Gulko, P.S.; et al. Antioxidant Carbon Nanoparticles Inhibit Fibroblast-Like Synoviocyte Invasiveness and Reduce Disease Severity in a Rat Model of Rheumatoid Arthritis. Antioxidants 2020, 9, 1005. https://doi.org/10.3390/antiox9101005
Tanner MR, Huq R, Sikkema WKA, Nilewski LG, Yosef N, Schmitt C, Flores-Suarez CP, Raugh A, Laragione T, Gulko PS, et al. Antioxidant Carbon Nanoparticles Inhibit Fibroblast-Like Synoviocyte Invasiveness and Reduce Disease Severity in a Rat Model of Rheumatoid Arthritis. Antioxidants. 2020; 9(10):1005. https://doi.org/10.3390/antiox9101005
Chicago/Turabian StyleTanner, Mark R., Redwan Huq, William K. A. Sikkema, Lizanne G. Nilewski, Nejla Yosef, Cody Schmitt, Carlos P. Flores-Suarez, Arielle Raugh, Teresina Laragione, Pércio S. Gulko, and et al. 2020. "Antioxidant Carbon Nanoparticles Inhibit Fibroblast-Like Synoviocyte Invasiveness and Reduce Disease Severity in a Rat Model of Rheumatoid Arthritis" Antioxidants 9, no. 10: 1005. https://doi.org/10.3390/antiox9101005
APA StyleTanner, M. R., Huq, R., Sikkema, W. K. A., Nilewski, L. G., Yosef, N., Schmitt, C., Flores-Suarez, C. P., Raugh, A., Laragione, T., Gulko, P. S., Tour, J. M., & Beeton, C. (2020). Antioxidant Carbon Nanoparticles Inhibit Fibroblast-Like Synoviocyte Invasiveness and Reduce Disease Severity in a Rat Model of Rheumatoid Arthritis. Antioxidants, 9(10), 1005. https://doi.org/10.3390/antiox9101005





