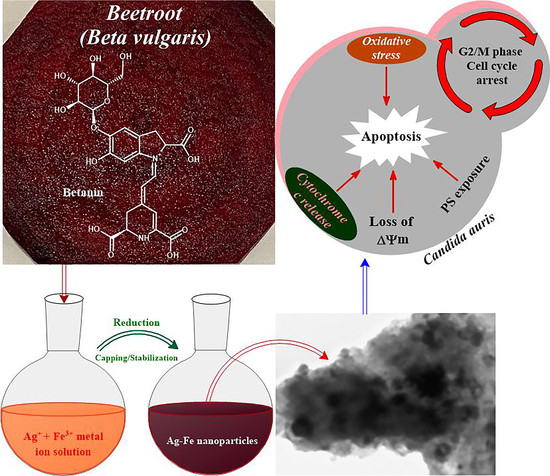
Phytogenic Fabrication of Ag–Fe Bimetallic Nanoparticles for Cell Cycle Arrest and Apoptosis Signaling Pathways in Candida auris by Generating Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection and Preparation of the Beta vulgaris L. Extract
2.3. Preparation of Ag–Fe NPs
2.4. Characterization of Ag–Fe Bimetallic NPs
2.5. Biological Assays
2.5.1. Antifungal Activity of Ag–Fe Nanoparticles (Ag–Fe NPs)
2.5.2. Agar Diffusion Assay
2.5.3. Cell Count and Viability
2.6. Apoptotic Studies
2.6.1. Protoplast Preparation
2.6.2. Effect on C. auris Mitochondrial Membrane Potential (Δψm)
2.6.3. Effect on Cytochrome c Discharge
2.6.4. Phosphatidylserine Externalization
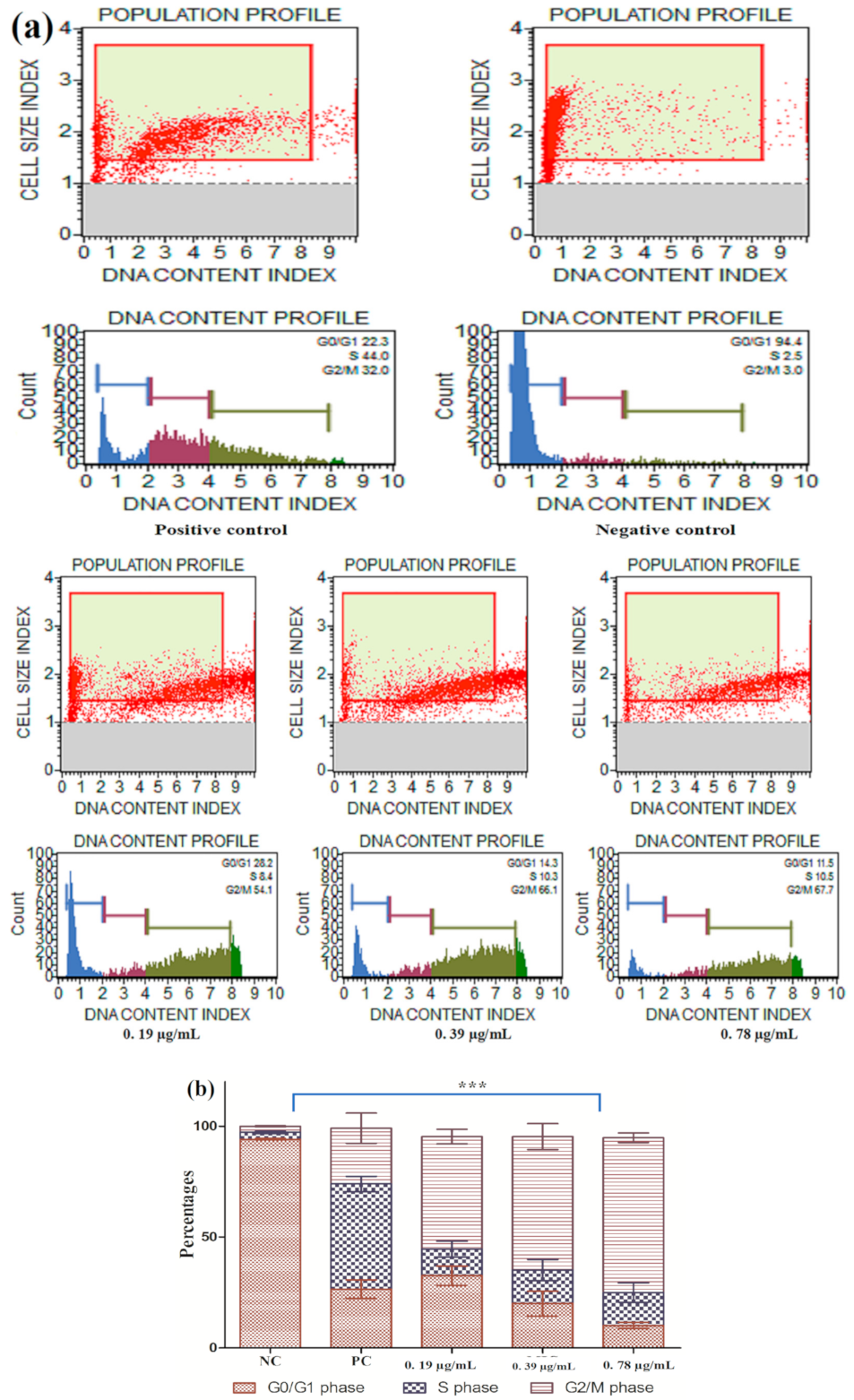
2.7. Cell Cycle Arrest
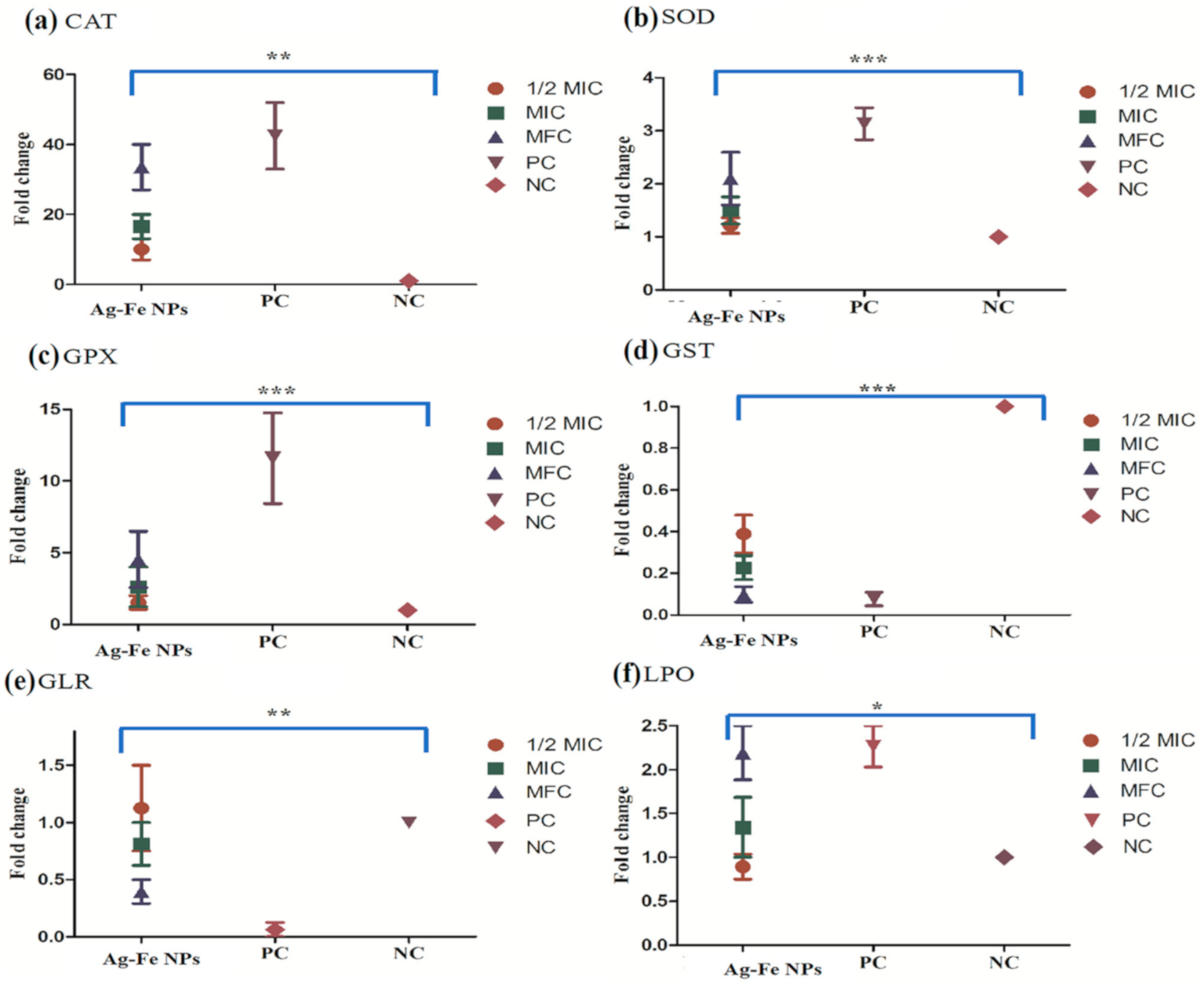
2.8. Antioxidant Enzymes Assays
2.8.1. Catalase (CAT)
2.8.2. Superoxide Dismutase (SOD)
2.8.3. Glutathione Peroxidase (GPx)
2.8.4. Glutathione Reductase (GLR)
2.8.5. Glutathione Transferase (GST)
2.9. Lipid Peroxidation (LPO)
2.10. Cytotoxicity Assay
2.11. Statistics
3. Results and Discussion
3.1. Chemistry
3.1.1. UV–Visible and FTIR Spectroscopic Analysis of Ag–Fe NPs
3.1.2. XRD Analysis of Biosynthesized Ag–Fe Nanoparticles from Beta vulgaris L. Extract
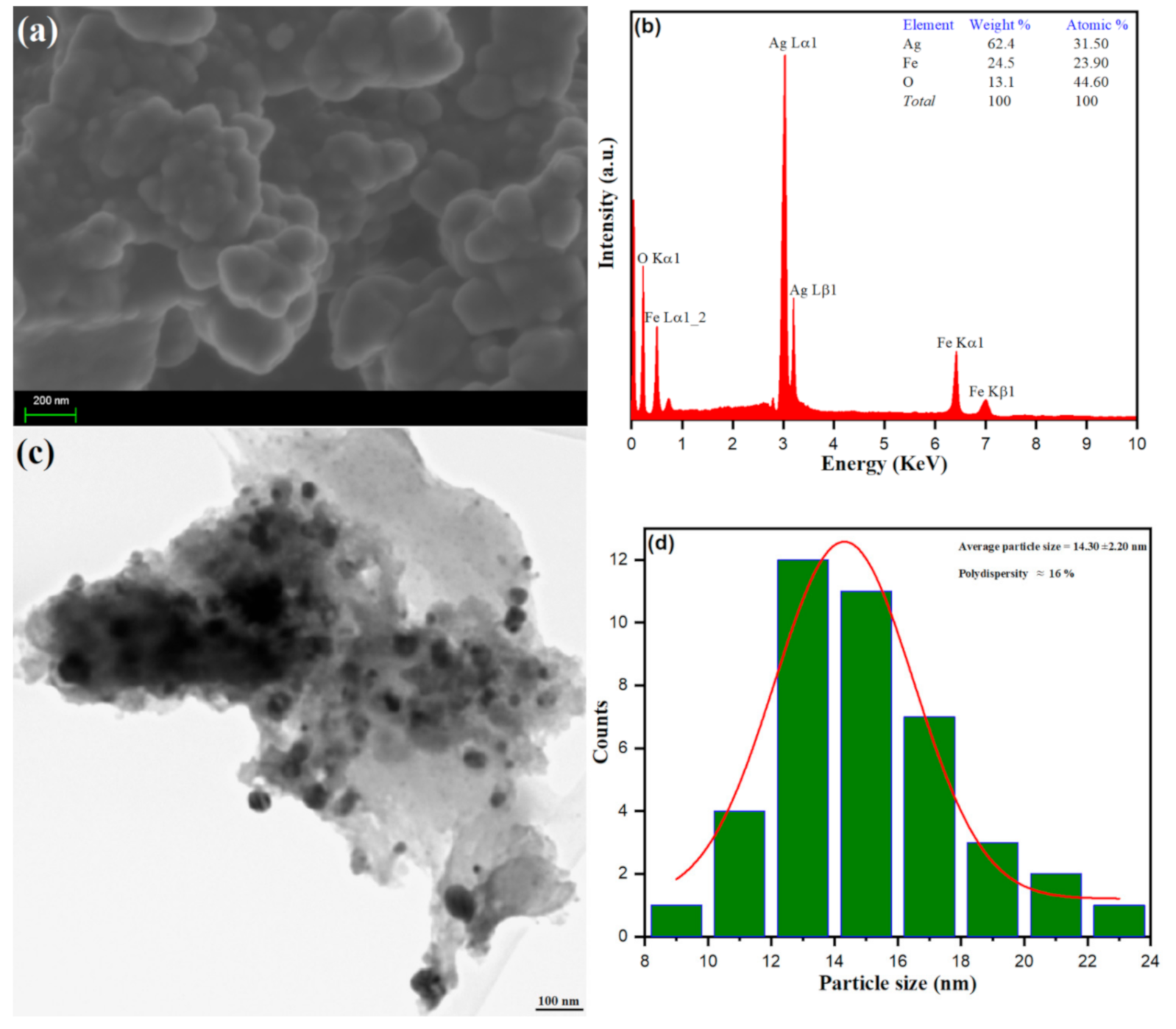
3.1.3. Morphological Analysis of Ag–Fe NPs
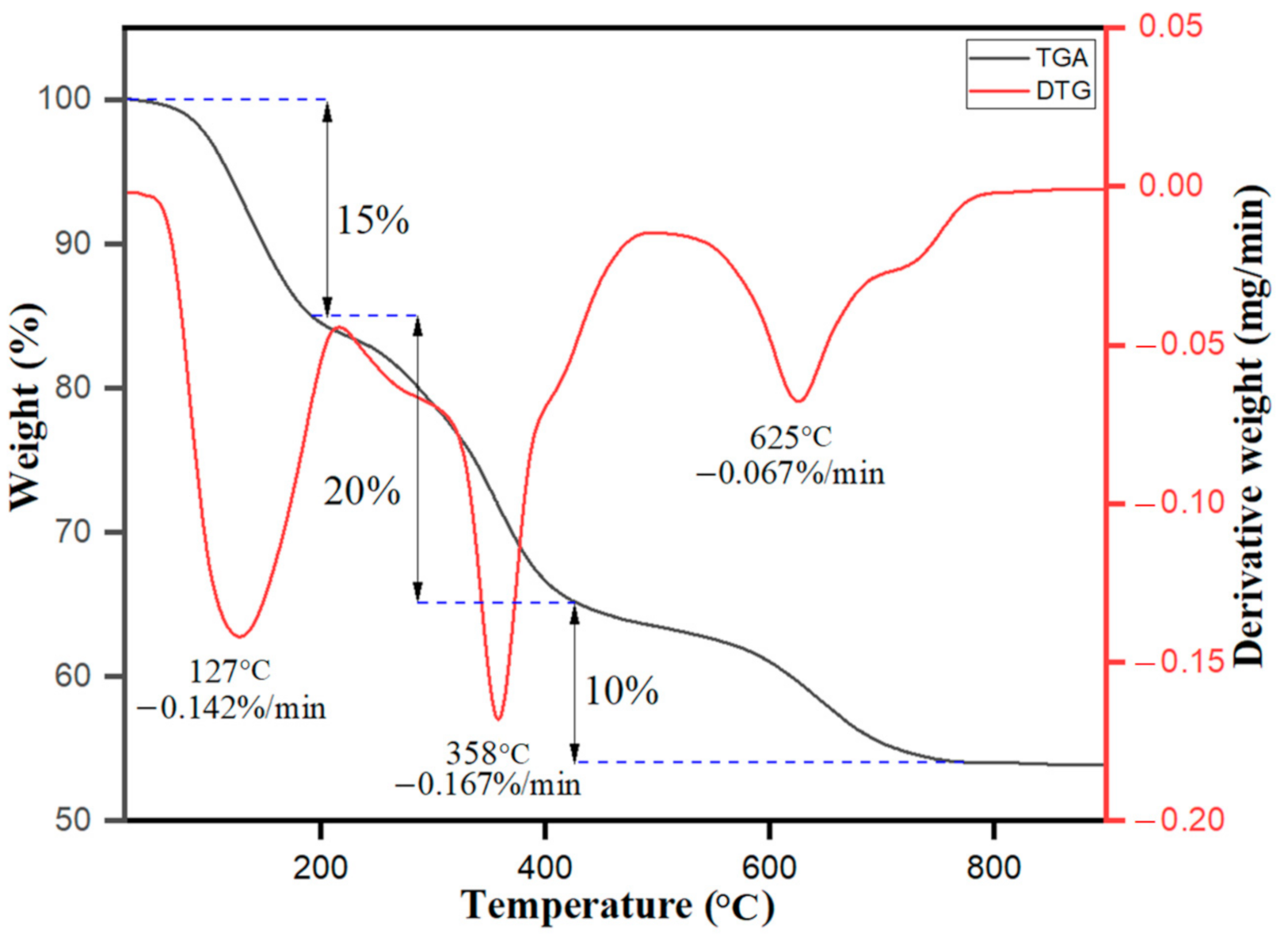
3.1.4. TGA/DTG Analysis of Ag–Fe NPs
3.2. Biology
3.2.1. Anti-Candida Activity of Ag–Fe NPs
3.2.2. Agar Diffusion Assay
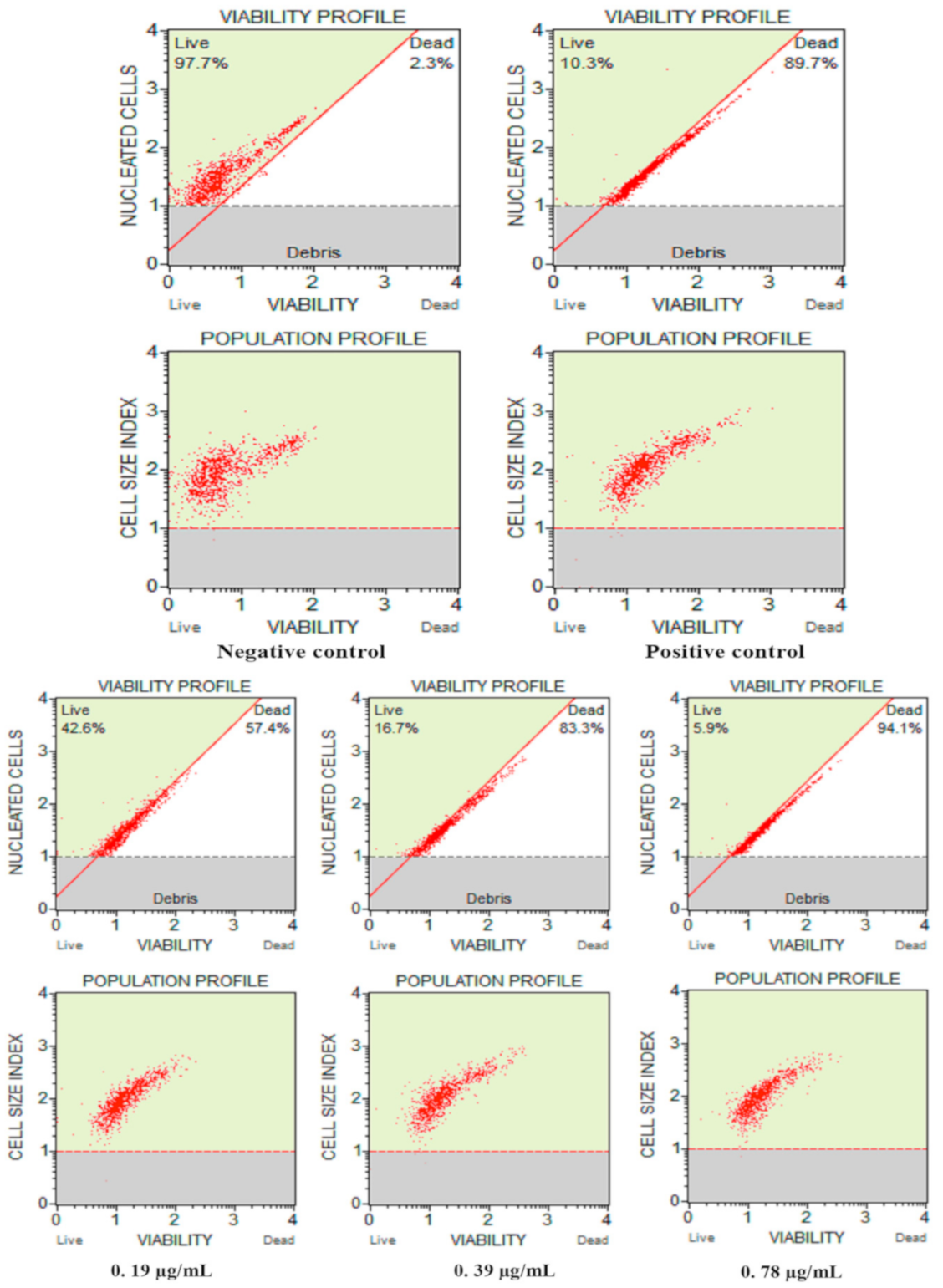
3.2.3. Cell Count and Viability Assay
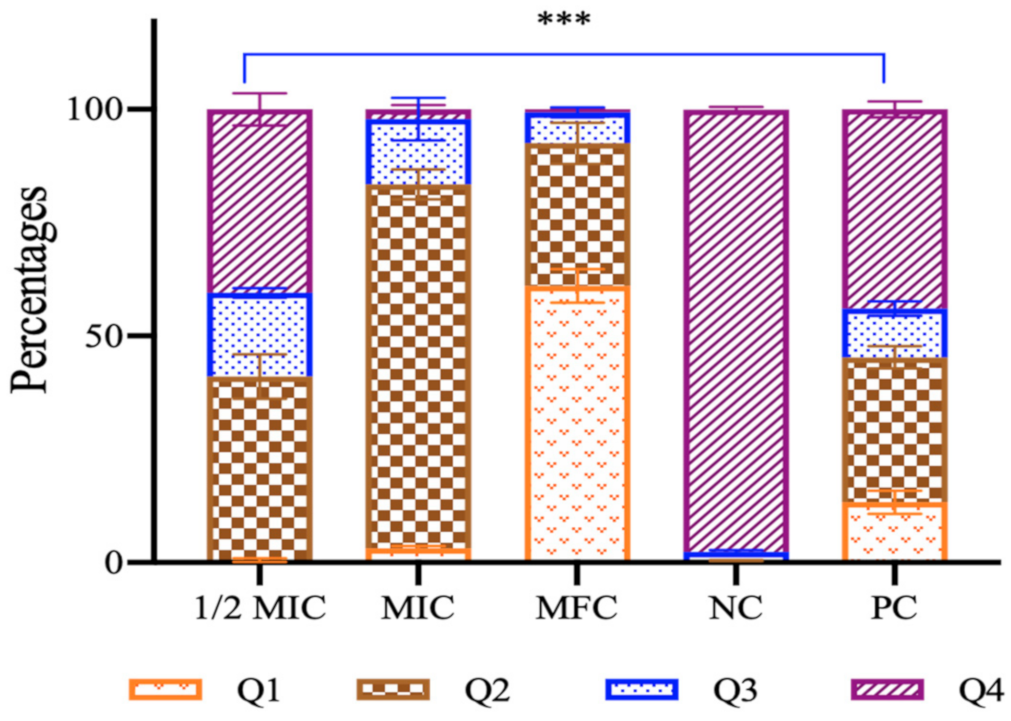
3.2.4. Apoptotic Studies
- Mitochondrial membrane potential
- Cytochrome c release
3.2.5. Cell Cycle Arrest
3.2.6. Enzyme Assays
3.2.7. Hemolytic Activity of Ag–Fe NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kordalewska, M.; Perlin, D.S. Identification of drug resistant Candida auris. Front. Microbiol. 2019, 10, 1918. [Google Scholar] [CrossRef] [PubMed]
- Zamith-Miranda, D.; Heyman, H.M.; Cleare, L.G.; Couvillion, S.P.; Clair, G.C.; Bredeweg, E.L.; Gacser, A.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Multi-omics signature of Candida auris, an emerging and multidrug-resistant pathogen. mSystems 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Ramkumar, V.S.; Archunan, G.; Chaiyasut, C.; Suganthy, N. Biogenic synthesis of silver palladium bimetallic nanoparticles from fruit extract of Terminalia chebula—In vitro evaluation of anticancer and antimicrobial activity. J. Drug Deliv. Sci. Technol. 2019, 51, 139–151. [Google Scholar] [CrossRef]
- Kamli, M.R.; Srivastava, V.; Hajrah, N.H.; Sabir, J.S.M.; Hakeem, K.R.; Ahmad, A.; Malik, M.A. Facile Bio-Fabrication of Ag-Cu-Co Trimetallic Nanoparticles and Its Fungicidal Activity against Candida auris. J. Fungi 2021, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- George, J.M.; Priyanka, R.N.; Mathew, B. Bimetallic Ag–Au nanoparticles as pH dependent dual sensing probe for Mn (II) ion and ciprofloxacin. Microchem. J. 2020, 155, 104686. [Google Scholar] [CrossRef]
- Song, Y.; Lin, Y.; Chu, X.; Tang, J.; Xu, S. Facile synthesis of supported AuNi and PtNi bimetallic nanomaterials and their enhanced catalytic properties. J. Mater. Res. Technol. 2020, 9, 2237–2246. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theoryto applications of alloy clusters and nanoparticles. Chem Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Wang, D.S.; Li, Y.D. Bimetallic nanocrystals: Liquid-phasesynthesis and catalytic applications. Adv. Mater. 2011, 23, 1044–1060. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P.D. Synergisticgeometric and electronic effects for electrochemicalreduction of carbon dioxide using gold-copper bimetallicnanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef]
- Ataee-Esfahani, H.; Wang, L.; Nemoto, Y.; Yamauchi, Y. Synthesisof bimetallic Au@Pt nanoparticles with Au core andnanostructured Pt shell toward highly activeelectrocatalysts. Chem. Mater. 2010, 22, 6310–6318. [Google Scholar] [CrossRef]
- Cui, Y.; Ren, B.; Yao, J.L.; Gu, R.A.; Tian, Z.Q. Synthesis of Ag core Aushell bimetallic nanoparticles for immunoassay based onsurface-enhanced Raman spectroscopy. J. Phys. Chem. B 2006, 110, 4002–4006. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.J.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.M.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygenreduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.Z.; Yuan, W.T.; Meng, Y.; Zou, S.H.; Zhou, Y.H.; Hong, W.; Che, J.; Hao, M.; Ye, B.; Xiao, L.; et al. Arational solid-state synthesis of supported Au-Ni bimetallicnanoparticles with enhanced activity for gas-phase selectiveoxidation of alcohols. ACS Appl. Mater. Interfaces 2017, 9, 31853–31860. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, B.; Burrows, A.; Watanabe, M.; Kiely, C.J.; Liz-Marzan, L.M. Multishell bimetallic AuAg nanoparticles:synthesis, structure and optical properties. J. Mater. Chem. 2005, 15, 1755–1759. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of stable Au, Ag and Au-Ag alloy nanoparticles using J. sambac leaves extract, and their enhanced antimicrobial activity in presence of organic antimicrobials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 236–243. [Google Scholar] [CrossRef]
- Zhang, G.; Du, M.; Li, Q.; Li, X.; Huang, J.; Jiang, X.; Sun, D. Green synthesis of Au–Ag alloy nanoparticles using Cacumen platycladi extract. RSC Adv. 2013, 3, 1878–1884. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. Synth. Inorg. Nanomater. 2018, 121–139. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. Rsc Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Malik, M.A.; Khan, M.N.; Khan, T.A. Cobalt@ silver bimetallic nanoparticles: Solution based seedless surfactant assisted synthesis, optical properties, and morphology. J. Mol. Liq. 2016, 222, 272–278. [Google Scholar] [CrossRef]
- Alzahrani, S.A.; Al-Thabaiti, S.A.; Shamsan Al-Arjan, W.; Malik, M.A.; Khan, Z. Preparation of ultra-long α-MnO2 and Ag@MnO2 nanoparticles by seedless approach and their photocatalytic performance. J. Mol. Str. 2017, 1137, 495–505. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Deniz Yilmaz, M. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Malik, M.A. Facile One-pot biogenic synthesis of Cu-Co-Ni trimetallic nanoparticles for enhanced photocatalytic dye degradation. Catalysts 2020, 10, 1138. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J. Nanostr. Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef]
- AbdelHamid, A.A.; Al-Ghobashy, M.A.; Fawzy, M.; Mohamed, M.B.; Abdel-Mottaleb, M.M. Phytosynthesis of Au, Ag, and Au–Ag bimetallic nanoparticles using aqueous extract of sago pondweed (Potamogeton pectinatus L.). ACS Sustain. Chem. Eng. 2013, 1, 1520–1529. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Loganathan, B.; Raghu, K. Green synthesis of Au–Ag bimetallic nanocomposites using Silybum marianum seed extract and their application as a catalyst. RSC Adv. 2015, 5, 31691–31699. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Biosynthesis of Au and Au/Ag alloy nanoparticles using Coleus aromaticus essential oil and evaluation of their catalytic, antibacterial and antiradical activities. J. Mol. Liq. 2016, 221, 179–189. [Google Scholar] [CrossRef]
- Sharma, C.; Ansari, S.; Ansari, M.S.; Satsangee, S.P.; Srivastava, M.M. Single-step green route synthesis of Au/Ag bimetallic nanoparticles using clove buds extract: Enhancement in antioxidant bio-efficacy and catalytic activity. Mater. Sci. Eng. C 2020, 116, 111153. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Guo, M.; Luo, F.; Chen, Z. One-step green synthesis of bimetallic Fe/Ni nanoparticles by eucalyptus leaf extract: Biomolecules identification, characterization and catalytic activity. Chem. Eng. J. 2017, 308, 904–911. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79. [Google Scholar] [CrossRef]
- Al-Asfar, A.; Zaheer, Z.; Aazam, E.S. Eco-friendly green synthesis of Ag-Fe bimetallic nanoparticles: Antioxidant, antimicrobial and photocatalytic degradation of bromothymol blue. J. Photochem. Photobiol. B 2018, 185, 143–152. [Google Scholar] [CrossRef]
- Venugopal, K.; Ahmad, H.; Manikandan, E.; Thanigai, A.K.; Kavitha, K.; Moodley, M.K.; Rajagopal, K.; Balabhaskar, R.; Bhaskar, M. The impact of anticancer activity upon Beta vulgaris extract mediated biosynthesized silver nanoparticles (Ag-NPs) against human breast (MCF-7), lung (A549) and pharynx (Hep-2) cancer cell lines. J. Photochem. Photobiol. B 2017, 173, 99–107. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Kalaiselvam, S.; Jayavel, R. Green synthesis of silver nanoparticles using Beta vulgaris: Role of process conditions on size distribution and surface structure. Mater. Chem. Phys. 2013, 140, 135–147. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2012, 46, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87. [Google Scholar] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chen, H.F.; Yeh, Y.C.; Xue, Y.P.; Lan, C.Y. The transcription factor Sfp1 regulates the oxidative stress response in Candida albicans. Microorganisms 2019, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, Approved Standard M27-A3; Clinical and Laboratory Standards Institute Standards: Annapolis Junction, MD, USA, 2008; Volume 28, pp. 6–12. [Google Scholar]
- Gutiérrez, J.A.; Caballero, S.; Diaz, L.A.; Guerrero, M.A.; Ruiz, J.; Ortiz, C.C. High antifungal activity against candida species of monometallic and bimetallic nanoparticles synthesized in nanoreactors. ACS Biomater. Sci. Eng. 2018, 4, 647–653. [Google Scholar] [CrossRef]
- Markova, Z.; Siskova, K.M.; Filip, J.; Cuda, J.; Kolar, M.; Safarova, K.; Medrik, I.; Zboril, R. Air stable magnetic bimetallic fe–ag nanoparticles for advanced antimicrobial treatment and phosphorus removal. Environ. Sci. Technol. 2013, 47, 5285–5293. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Lerner, M.I.; Pervikov, A.V.; Kazantsev, S.O.; Fomenko, A.N. Development of Fe/Cu and Fe/Ag Bimetallic nanoparticles for promising biodegradable materials with antimicrobial effect. Nanotechnol. Russ. 2018, 13, 18–25. [Google Scholar] [CrossRef]
- Lone, S.A.; Wani, M.Y.; Fru, P.; Ahmad, A. Cellular apoptosis and necrosis as therapeutic targets for novel eugenol tosylate congeners against Candida albicans. Sci. Rep. 2020, 10, 1191. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Manzoor, N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm. 2013, 48, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.G.; Lee, D.G. Silibinin triggers yeast apoptosis related to mitochondrial Ca2+ influx in Candida albicans. Int. J. Biochem. Cell. Biol. 2016, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Ahmad Khan, L.; Padoa, C.J.; Van Vuuren, S.; Manzoor, N. Effect of two monoterpene phenols on antioxidant defense system in Candida albicans. Microb. Pathog. 2015, 80, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Effect of diallyldisulphide on an antioxidant enzyme system in Candida species. Can. J. Microbiol. 2010, 56, 816–821. [Google Scholar] [CrossRef]
- Maras, B.; Angiolella, L.; Mignogna, G.; Vavala, E.; Macone, A.; Colone, M.; Pitari, G.; Stringaro, A.; Dupré, S.; Palamara, A.T. Glutathione metabolism in Candida albicans resistant strains to fluconazole and micafungin. PLoS ONE 2014, 9, e98387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lanier, S.M.; Downing, J.A.; Avent, J.L.; Lum, J.; McHale, J.L. Betalain pigments for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2008, 195, 72–80. [Google Scholar] [CrossRef]
- Lankoff, A.; Sandberg, W.J.; Wegierek-Ciuk, A.; Lisowska, H.; Refsnes, M.; Sartowska, B.; Schwarze, P.E.; Meczynska-Wielgosz, S.; Wojewodzka, M.; Kruszewski, M. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol. Lett. 2012, 208, 197–213. [Google Scholar] [CrossRef]
- Argentiere, S.; Cella, C.; Cesaria, M.; Milani, P.; Lenardi, C. Silver nanoparticles in complex biological media: Assessment of colloidal stability and protein corona formation. J. Nanopart. Res. 2016, 18, 253. [Google Scholar] [CrossRef]
- Bae, E.; Lee, B.-C.; Kim, Y.; Choi, K.; Yi, J. Effect of agglomeration of silver nanoparticle on nanotoxicity depression. Korean J. Chem. Eng. 2013, 30, 364–368. [Google Scholar] [CrossRef]
- Peña-González, C.E.; Pedziwiatr-Werbicka, E.; Martín-Pérez, T.; Szewczyk, E.M.; Copa-Patiño, J.L.; Soliveri, J.; Pérez-Serrano, J.; Gómez, R.; Bryszewska, M.; Sánchez-Nieves, J.; et al. Antibacterial and antifungal properties of dendronized silver and gold nanoparticles with cationic carbosilane dendrons. Int. J. Pharm. 2017, 528, 55–61. [Google Scholar] [CrossRef]
- Selvaraj, M.; Pandurangan, P.; Ramasami, N.; Rajendran, S.B.; Sangilimuthu, S.N.; Perumal, P. Highly potential antifungal activity of quantum-sized silver nanoparticles against Candida albicans. Appl. Biochem. Biotechnol. 2014, 173, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Wang, S.; Li, C.; Xue, B.; Yang, L.; Shen, Z.; Jin, M.; Wang, J.; Qiu, Z. The detailed bactericidal process of ferric oxide nanoparticles on E. coli. Molecules 2018, 23, 606. [Google Scholar] [CrossRef] [PubMed]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef]
- Dlugaszewska, J.; Dobrucka, R. Effectiveness of biosynthesized trimetallic Au/Pt/Ag nanoparticles on planktonic and biofilm Enterococcus faecalis and Enterococcus faecium forms. J. Clust. Sci. 2019, 30, 1091–1101. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Hwang, I.S.; Lee, J.; Hwang, J.H.; Kim, K.J.; Lee, D.G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef]
- Zhu, M.T.; Wang, Y.; Feng, W.Y.; Wang, B.; Wang, M.; Ouyang, H.; Chai, Z.F. Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J. Nanosci. Nanotechnol. 2010, 10, 8584–8590. [Google Scholar] [CrossRef]
- Huttemann, M.; Pecina, P.; Rainbolt, M.; Sanderson, T.H.; Kagan, V.E.; Samavati, L.; Doan, J.W.; Lee, I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 2011, 11, 369–381. [Google Scholar] [CrossRef]
- Adrain, C.; Martin, S.J. The mitochondrial apoptosome: A killer unleashed by the cytochrome seas. Trends Biochem. Sci. 2001, 26, 390–397. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, J.; Yu, L.; Wang, C.; Yang, Y.; Rong, X.; Xu, K.; Chu, M. Antifungal activity of coumarin against Candida albicans is related to apoptosis. Front. Cell Infect. Microbiol. 2019, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Panácek, A.; Kolár, M.; Vecerová, R.; Prucek, R.; Soukupová, J.; Krystof, V.; Hamal, P.; Zboril, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef]
- Wady, A.F.; Machado, A.L.; Zucolotto, V.; Zamperini, C.A.; Berni, E.; Vergani, C.E. Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J. Appl. Microbiol. 2012, 112, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jimenez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, 2–12. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Takamiya, A.S.; Feresin, L.P.; Gorup, L.F.; De Camargo, E.R.; Delbem, A.C.; Henriques, M.; Barbosa, D.B. Susceptibility of Candida albicans and Candida glabrata biofilms to silver nanoparticles in intermediate and mature development phases. J. Prosthodont. Res. 2015, 59, 42–48. [Google Scholar] [CrossRef]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nanoparticles on Candida albicans. Biometals 2009, 22, 235–242. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS ONE 2014, 9, e108876. [Google Scholar] [CrossRef]
- Neto, J.B.A.; Da Silva, C.R.; Neta, M.A.S.; Campos, R.S.; Siebra, J.T.; Silva, R.A.C.; Gaspar, D.M.; Magalhaes, H.I.; De Moraes, M.O.; Lobo, M.D.; et al. Antifungal activity of Naphthoquinoidal compounds in vitro against fluconazole-resistant strains of different Candida species: A special emphasis on mechanisms of action on Candida tropicalis. PLoS ONE 2014, 9, e93698. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K.; et al. Carvacrol induces Candida albicans apoptosis associated with Ca2+/calcineurin pathway. Front. Cell Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Seyedjavadi, S.S.; Khani, S.; Eslamifar, A.; Ajdary, S.; Goudarzi, M.; Halabian, R.; Akbari, R.; Zare-Zardini, H.; Imani Fooladi, A.A.; Amani, J.; et al. The antifungal peptide MCh-AMP1 derived from Matricaria chamomilla inhibits Candida albicans growth via inducing ROS generation and altering fungal cell membrane permeability. Front. Microbiol. 2020, 10, 3150. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.; Sudbery, I.; Ramsdale, M. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. USA 2003, 100, 14327–14332. [Google Scholar] [CrossRef]
- Rubiolo, J.A.; Ternon, E.; López-Alonso, H.; Thomas, O.P.; Vega, F.V.; Vieytes, M.R.; Botana, L.M. Crambescidin-816 acts as a fungicidal with more potency than crambescidin-800 and -830, inducing cell cycle arrest, increased cell size and apoptosis in Saccharomyces cerevisiae. Mar. Drugs 2013, 11, 4419–4434. [Google Scholar] [CrossRef]
- Stefanini, I.; Rizzetto, L.; Rivero, D.; Carbonell, S.; Gut, M.; Heath, S.; Gut, I.G.; Trabocchi, A.; Guarna, A.; Ghazzi, N.B.; et al. Deciphering the mechanism of action of 089, a compound impairing the fungal cell cycle. Sci. Rep. 2018, 8, 5964. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Erwin, L.; Holmes, A.R.; Niimi, K.; Koichi, T.; Niimi, M.; Monk, B.C. Candida albicans drug resistance e another way to cope with stress. Microbiology 2007, 153, 3211–3217. [Google Scholar] [CrossRef]
- Covarrubias, L.; Hernandez-Garcia, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregon, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants—Quo vadis? Trends Pharmacol. Sci. 2011, 32, 125–130. [Google Scholar] [CrossRef]
- Ficociello, G.; De Caris, M.G.; Trillò, G.; Cavallini, D.; Sarto, M.S.; Uccelletti, D.; Mancini, P. Anti-Candidal activity and in vitro cytotoxicity assessment of graphene nanoplatelets decorated with zinc oxide nanorods. Nanomaterials 2018, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Harper, B.; Sinche, F.; Ho Wu, R.; Gowrishankar, M.; Marquart, G.; Mackiewicz, M.; Harper, S.L. The Impact impact of surface ligands and synthesis method on the toxicity of Glutathione-coated gold nanoparticles. Nanomaterials 2014, 4, 355–371. [Google Scholar] [CrossRef]




| C. auris Isolates | Ag–Fe NPs (µg/mL) | Amphoterecin B (µg/mL) | ||
|---|---|---|---|---|
| MIC | MFC | MIC | MFC | |
| MRL 6326 | 0.19 | 0.39 | 0.25 | 0.5 |
| MRL 6183 | 0.19 | 0.39 | 0.25 | 0.5 |
| MRL 4888 | 0.39 | 0.78 | 1.0 | 2.0 |
| MRL 6015 | 0.19 | 0.39 | 0.25 | 0.5 |
| MRL 6333 | 0.19 | 0.39 | 0.5 | 1.0 |
| MRL 4587 | 0.19 | 0.39 | 0.5 | 0.5 |
| MRL 6334 | 0.19 | 0.39 | 0.5 | 2.0 |
| MRL 3785 | 0.19 | 0.39 | 0.125 | 0.5 |
| MRL 6059 | 0.19 | 0.39 | 0.5 | 1.0 |
| MRL 4000 | 0.39 | 0.78 | 2.0 | 4.0 |
| MRL 6065 | 0.39 | 0.78 | 1.0 | 2.0 |
| MRL 2921 | 0.39 | 0.78 | 2.0 | 4.0 |
| MRL 6125 | 0.19 | 0.39 | 0.25 | 0.5 |
| MRL 6338 | 0.19 | 0.39 | 0.25 | 0.5 |
| MRL 3499 | 0.19 | 0.39 | 0.5 | 1.0 |
| MRL 6194 | 0.19 | 0.39 | 0.25 | 0.5 |
| MRL 6005 | 0.39 | 0.78 | 1.0 | 2.0 |
| MRL 6057 | 0.39 | 0.78 | 4.0 | 8.0 |
| MRL 5762 | 0.39 | 0.78 | 2.0 | 4.0 |
| MRL 6173 | 0.19 | 0.39 | 0.25 | 0.5 |
| MRL 5765 | 0.39 | 0.78 | 2.0 | 4.0 |
| MRL 2397 | 0.39 | 0.78 | 1.0 | 2.0 |
| MRL 5418 | 0.19 | 0.39 | 0.5 | 1.0 |
| MRL 6277 | 0.19 | 0.39 | 0.5 | 1.0 |
| MRL 6339 | 0.19 | 0.39 | 0.5 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamli, M.R.; Srivastava, V.; Hajrah, N.H.; Sabir, J.S.M.; Ali, A.; Malik, M.A.; Ahmad, A. Phytogenic Fabrication of Ag–Fe Bimetallic Nanoparticles for Cell Cycle Arrest and Apoptosis Signaling Pathways in Candida auris by Generating Oxidative Stress. Antioxidants 2021, 10, 182. https://doi.org/10.3390/antiox10020182
Kamli MR, Srivastava V, Hajrah NH, Sabir JSM, Ali A, Malik MA, Ahmad A. Phytogenic Fabrication of Ag–Fe Bimetallic Nanoparticles for Cell Cycle Arrest and Apoptosis Signaling Pathways in Candida auris by Generating Oxidative Stress. Antioxidants. 2021; 10(2):182. https://doi.org/10.3390/antiox10020182
Chicago/Turabian StyleKamli, Majid Rasool, Vartika Srivastava, Nahid H. Hajrah, Jamal S. M. Sabir, Arif Ali, Maqsood Ahmad Malik, and Aijaz Ahmad. 2021. "Phytogenic Fabrication of Ag–Fe Bimetallic Nanoparticles for Cell Cycle Arrest and Apoptosis Signaling Pathways in Candida auris by Generating Oxidative Stress" Antioxidants 10, no. 2: 182. https://doi.org/10.3390/antiox10020182
APA StyleKamli, M. R., Srivastava, V., Hajrah, N. H., Sabir, J. S. M., Ali, A., Malik, M. A., & Ahmad, A. (2021). Phytogenic Fabrication of Ag–Fe Bimetallic Nanoparticles for Cell Cycle Arrest and Apoptosis Signaling Pathways in Candida auris by Generating Oxidative Stress. Antioxidants, 10(2), 182. https://doi.org/10.3390/antiox10020182