Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
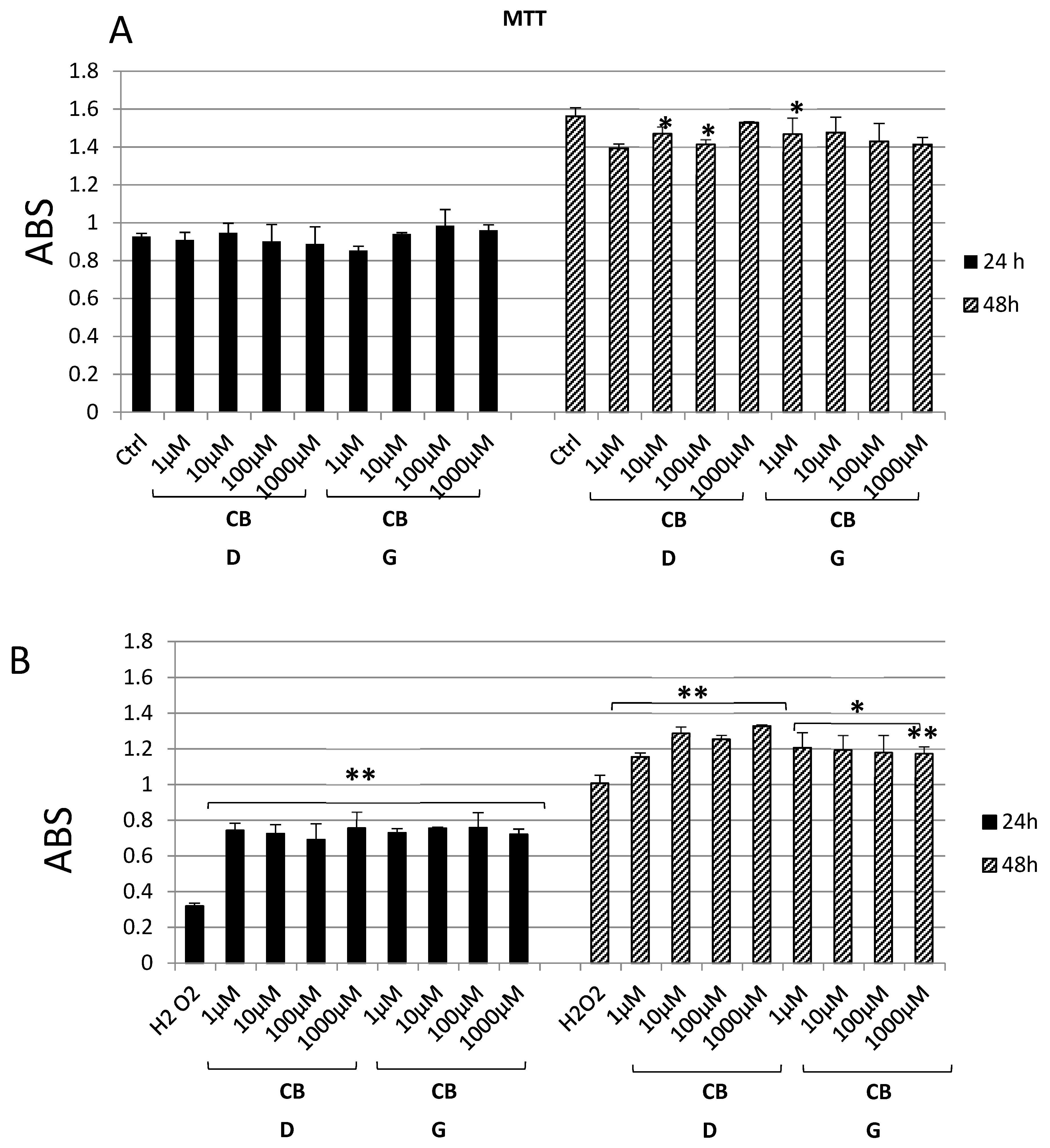
2.2. In Vitro Studies
2.3. Ex Vivo Studies
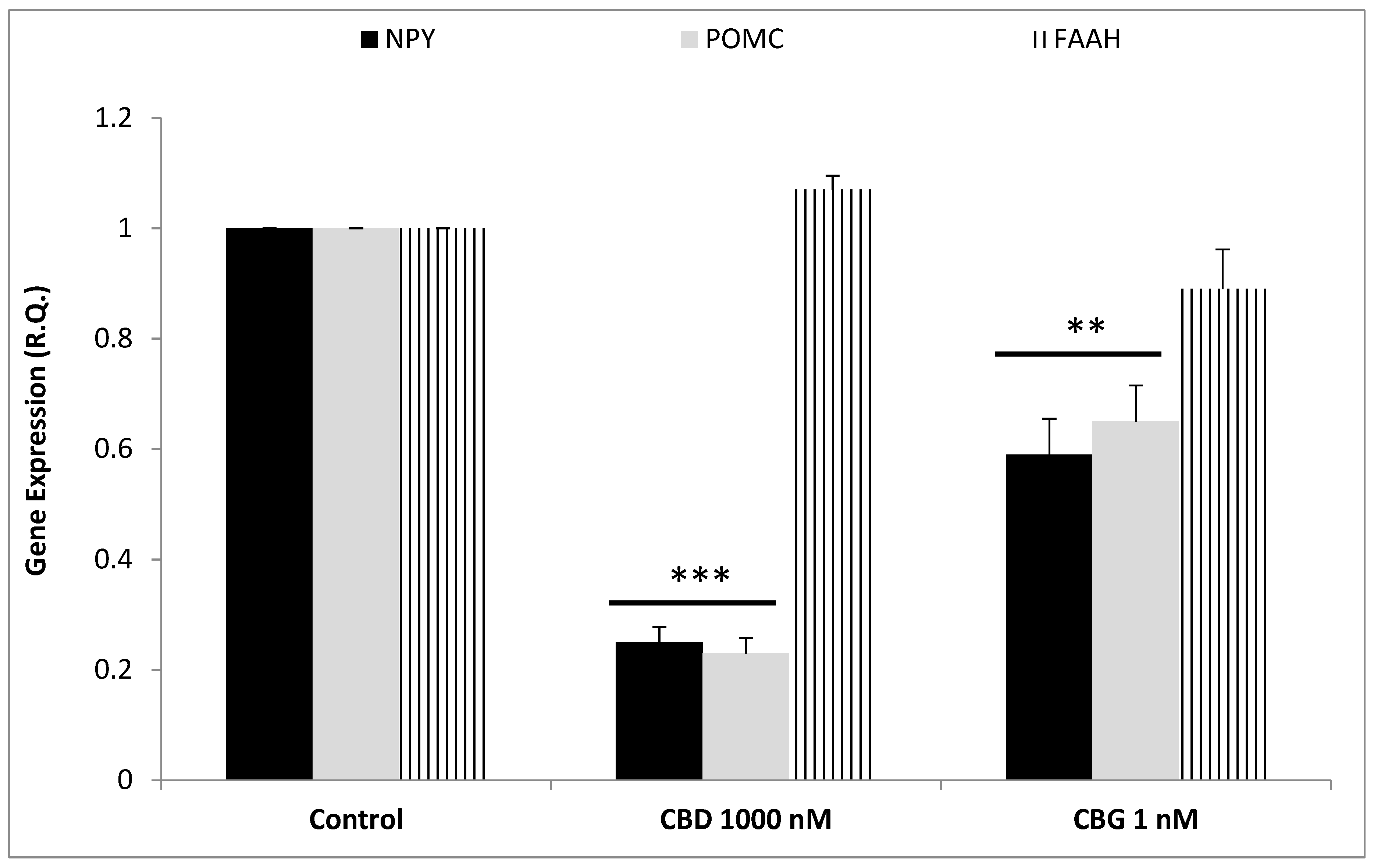
2.4. RNA Extraction, Reverse Transcription and Real-Time Reverse Transcription Polymerase Chain Reaction (Real-Time RT PCR)
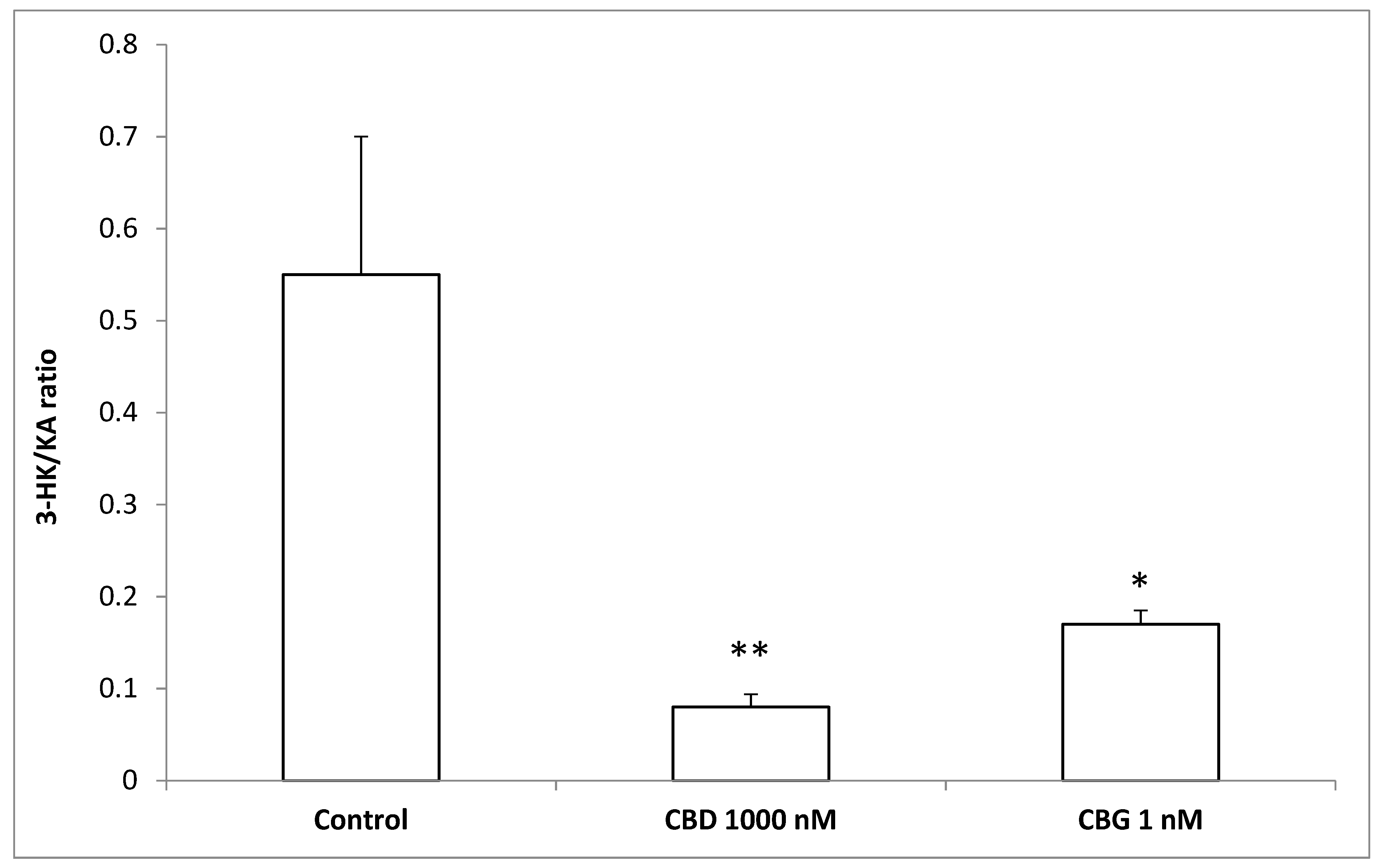
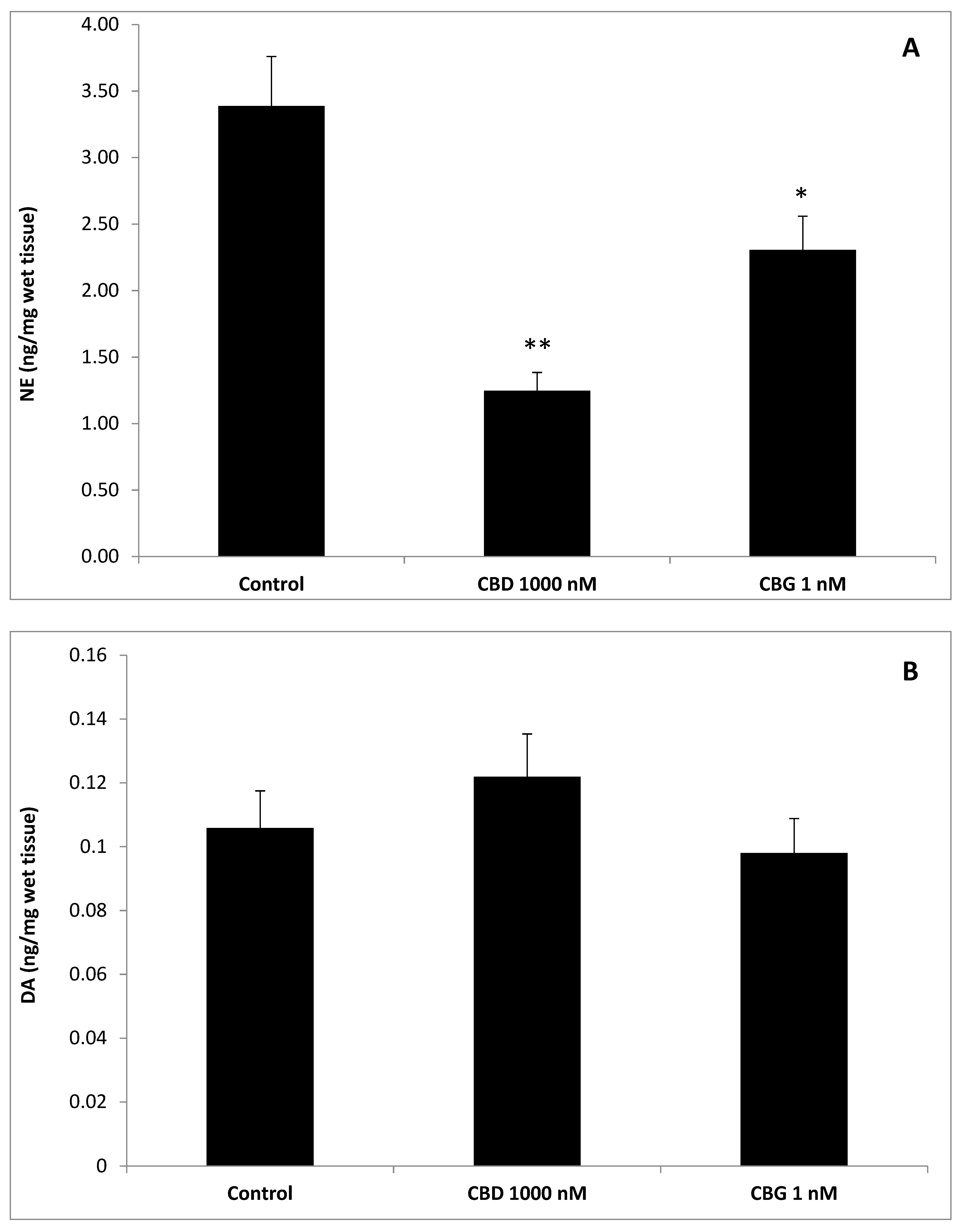
2.5. High Performance Liquid Chromatography (HPLC) Determination of Dopamine (DA), Norepinephrine (NE),Serotonin (5-HT), and 3-Hydroxykinurenine (3-HK)
2.6. HPLC-Fluorimetric Determination of Kinurenic Acid (KA)
2.7. Statistical Analysis
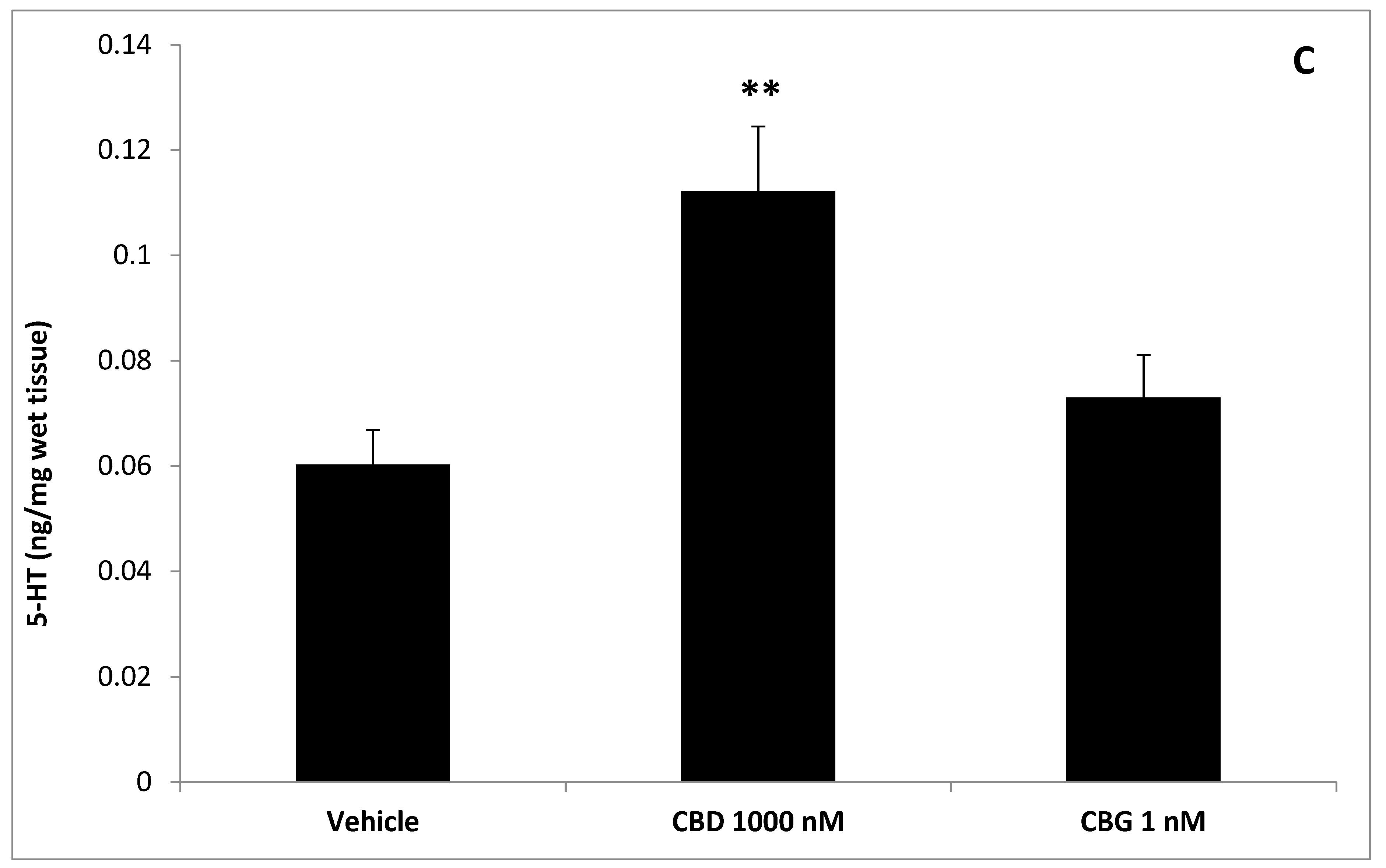
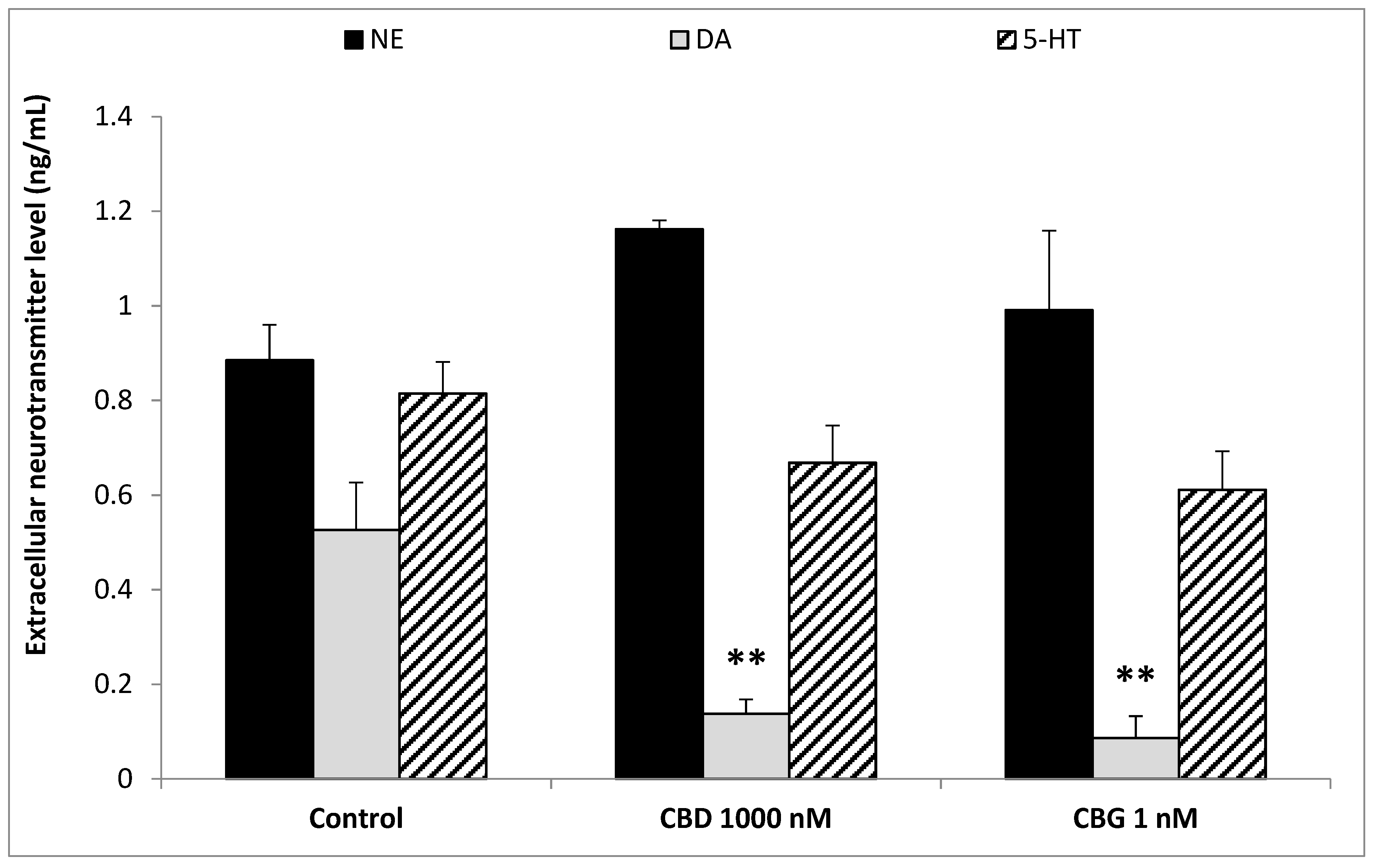
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fasinu, P.S.; Phillips, S.; ElSohly, M.A.; Walker, L.A. Current status and prospects for cannabidiol preparations as new therapeutic agents. Pharmacotherapy 2016, 36, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Di Marzo, V. Non-psychotropic analgesic drugs from the endocannabinoid system: “magic bullet” or “multiple-target” strategies? Eur. J. Pharmacol. 2013, 716, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Marchalant, Y.; Brothers, H.M.; Norman, G.J.; Karelina, K.; DeVries, A.C.; Wenk, G.L. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol. Dis. 2009, 34, 300–307. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. Cannabinoids for treatment of Alzheimer’s disease: Moving toward the clinic. Front. Pharmacol. 2014, 5, 37. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef]
- Bi, G.H.; Galaj, E.; He, Y.; Xi, Z.X. Cannabidiol inhibits sucrose self-administration by CB1 and CB2 receptor mechanisms in rodents. Addict. Biol. 2019, 12783, 1–11. [Google Scholar] [CrossRef]
- Jager, G.; Witkamp, R.F. The endocannabinoid system and appetite: Relevance for food reward. Nutr. Res. Rev. 2014, 27, 172–185. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Sexton, P.M. Emerging paradigms in GPCR allostery: Implications for drug discovery. Nat. Rev. Drug Discov. 2013, 12, 630–644. [Google Scholar] [CrossRef]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef]
- Lee, J.L.C.; Bertoglio, L.J.; Guimarães, F.S.; Stevenson, C.W. Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety-related and substance abuse disorders. Br. J. Pharmacol. 2017, 174, 3242–3256. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A. Allosterism and cannabinoid CB(1) receptors: The shape of things to come. Trends Pharmacol. Sci. 2007, 28, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Goparaju, S.K.; Wang, L.; Liu, J.; Bátkai, S.; Járai, Z.; Fezza, F.; Miura, G.I.; Palmiter, R.D.; Sugiura, T.; et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001, 410, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Ignatowska-Jankowska, B.; Jankowski, M.M.; Swiergiel, A.H. Cannabidiol decreases body weight gain in rats: Involvement of CB2 receptors. Neurosci. Lett. 2011, 490, 82–84. [Google Scholar] [CrossRef]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. A cannabigerol-rich Cannabis sativa extract, devoid of 9-tetrahydrocannabinol, elicits hyperphagia in rats. Behav. Pharmacol. 2017, 28, 280–284. [Google Scholar] [CrossRef]
- Brunetti, L.; Di Nisio, C.; Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Leone, S.; Di Michele, P.; Shohreh, R.; Vacca, M. Obestatin inhibits dopamine release in rat hypothalamus. Eur. J. Pharmacol. 2010, 641, 142–147. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of kisspeptin-10 on hypothalamic neuropeptides and neurotransmitters involved in appetite control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Wu, H.Q.; Elmer, G.I.; Bruno, J.P.; Schwarcz, R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur. J. Neurosci. 2012, 35, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.M.; Larson, S.A.; Dominko, T. Tryptophan metabolites kynurenine and serotonin regulate fibroblast activation and fibrosis. Cell. Mol. Life Sci. 2018, 75, 3663–3681. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Barnett, M.P.; Roy, N.C.; Joyce, N.I.; Zhu, S.; Armstrong, K.; Helsby, N.A.; Ferguson, L.R.; Rowan, D.D. Metabolomic analysis identifies inflammatory and noninflammatory metabolic effects of genetic modification in a mouse model of Crohn’s disease. J. Proteome Res. 2010, 9, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.; Wnorowski, A.; Smolińska, K.; Walczyna, B.; Turski, W.; Kocki, T.; Paluszkiewicz, P.; Parada-Turska, J. Kynurenic Acid Protects against Thioacetamide-Induced Liver Injury in Rats. Anal. Cell. Pathol. 2018, 2018, 1270483. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, M.; Zang, X.; Fan, Q.; Liu, Y.; Che, Y.; Guan, X.; Hou, Y.; Wang, G.; Hao, H. Kynurenic acid/GPR35 axis restricts NLRP3 inflammasome activation and exacerbates colitis in mice with social stress. Brain Behav. Immun. 2019, 79, 244–255. [Google Scholar] [CrossRef]
- Parrott, J.M.; Redus, L.; O’Connor, J.C. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J. Neuroinflamm. 2016, 13, 124. [Google Scholar] [CrossRef]
- Brunetti, L.; Di Nisio, C.; Orlando, G.; Ferrante, C.; Vacca, M. The regulation of feeding: A cross talk between peripheral and central signalling. Int. J. Immunopathol. Pharmacol. 2005, 18, 201–212. [Google Scholar] [CrossRef]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar] [CrossRef]
- Koch, M.; Varela, L.; Kim, J.G.; Kim, J.D.; Hernández-Nuño, F.; Simonds, S.E.; Castorena, C.M.; Vianna, C.R.; Elmquist, J.K.; Morozov, Y.M.; et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 2015, 519, 45–50. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Martinotti, S.; Mollica, A.; Macedonio, G.; Stefanucci, A.; Dvorácskó, S.; et al. Anorexigenic effects induced by RVD-hemopressin(α) administration. Pharmacol. Rep. 2017, 69, 1402–1407. [Google Scholar] [CrossRef]
- Morozov, Y.M.; Koch, M.; Rakic, P.; Horvath, T.L. Cannabinoid type 1 receptor-containing axons innervate NPY/AgRP neurons in the mouse arcuate nucleus. Mol. Metab. 2017, 6, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Suglia, A.; Chianese, R.; Migliaccio, M.; Ambrosino, C.; Fasano, S.; Pierantoni, R.; Cobellis, G.; Chioccarelli, T. Bisphenol A induces hypothalamic down-regulation of the cannabinoid receptor 1 and anorexigenic effects in male mice. Pharmacol. Res. 2016, 113, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Merroun, I.; El Mlili, N.; Martinez, R.; Porres, J.M.; Llopis, J.; Ahabrach, H.; Aranda, P.; Sanchez Gonzalez, C.; Errami, M.; Lopez-Jurado, M. Interaction between orexin A and cannabinoid system in the lateral hypothalamus of rats and effects of subchronic intraperitoneal administration of cannabinoid receptor inverse agonist on food intake and the nutritive utilization of protein. J. Physiol. Pharmacol. 2015, 66, 181–190. [Google Scholar] [PubMed]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef]
- Wellman, P.J.; Davies, B.T.; Morien, A.; McMahon, L. Modulation of feeding by hypothalamic paraventricular nucleus alpha 1- and alpha 2-adrenergic receptors. Life Sci. 1993, 53, 669–679. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Gillard, E.R.; Dang, D.Q.; Stanley, B.G. Evidence that neuropeptide Y and dopamine in the perifornical hypothalamus interact antagonistically in the control of food intake. Brain Res. 1993, 628, 128–136. [Google Scholar] [CrossRef]
- Pothos, E.N.; Creese, I.; Hoebel, B.G. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J. Neurosci. 1995, 15, 6640–6650. [Google Scholar] [CrossRef]
- Brunetti, L.; Michelotto, B.; Orlando, G.; Vacca, M. Leptin inhibits norepinephrine and dopamine release from rat hypothalamic neuronal endings. Eur. J. Pharmacol. 1999, 372, 237–240. [Google Scholar] [CrossRef]
- Brunetti, L.; Orlando, G.; Recinella, L.; Michelotto, B.; Ferrante, C.; Vacca, M. Resistin, but not adiponectin, inhibits dopamine and norepinephrine release in the hypothalamus. Eur. J. Pharmacol. 2004, 493, 41–44. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Recinella, L.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Lazzarin, F.; Vacca, M. Glucagon-like peptide 1 (7–36) amide (GLP-1) and exendin-4 stimulate serotonin release in rat hypothalamus. Peptides 2008, 29, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Chicca, A.; Tamborrini, M.; Eisen, D.; Lerner, R.; Lutz, B.; Poetz, O.; Pluschke, G.; Gertsch, J. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 2012, 287, 36944–36967. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides 2014, 51, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Orexigenic effects of omentin-1 related to decreased CART and CRH gene expression and increased norepinephrine synthesis and release in the hypothalamus. Peptides 2013, 44, 66–74. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, L.; Tang, L.; Liu, F.; Chen, Z.; Zhang, J.; Chen, L.; Pang, C.; Yu, X. Role of the indoleamine-2,3-dioxygenase/kynurenine pathway of tryptophan metabolism in behavioral alterations in a hepatic encephalopathy rat model. J. Neuroinflamm. 2018, 15, 3. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Giacomo, V.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Leone, S.; Brunetti, L.; Menghini, L.; Recinella, L.; et al. Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus. Antioxidants 2020, 9, 71. https://doi.org/10.3390/antiox9010071
di Giacomo V, Chiavaroli A, Orlando G, Cataldi A, Rapino M, Di Valerio V, Leone S, Brunetti L, Menghini L, Recinella L, et al. Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus. Antioxidants. 2020; 9(1):71. https://doi.org/10.3390/antiox9010071
Chicago/Turabian Styledi Giacomo, Viviana, Annalisa Chiavaroli, Giustino Orlando, Amelia Cataldi, Monica Rapino, Valentina Di Valerio, Sheila Leone, Luigi Brunetti, Luigi Menghini, Lucia Recinella, and et al. 2020. "Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus" Antioxidants 9, no. 1: 71. https://doi.org/10.3390/antiox9010071
APA Styledi Giacomo, V., Chiavaroli, A., Orlando, G., Cataldi, A., Rapino, M., Di Valerio, V., Leone, S., Brunetti, L., Menghini, L., Recinella, L., & Ferrante, C. (2020). Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus. Antioxidants, 9(1), 71. https://doi.org/10.3390/antiox9010071












