Melatonin Prevents Transforming Growth Factor-β1-Stimulated Transdifferentiation of Renal Interstitial Fibroblasts to Myofibroblasts by Suppressing Reactive Oxygen Species-Dependent Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
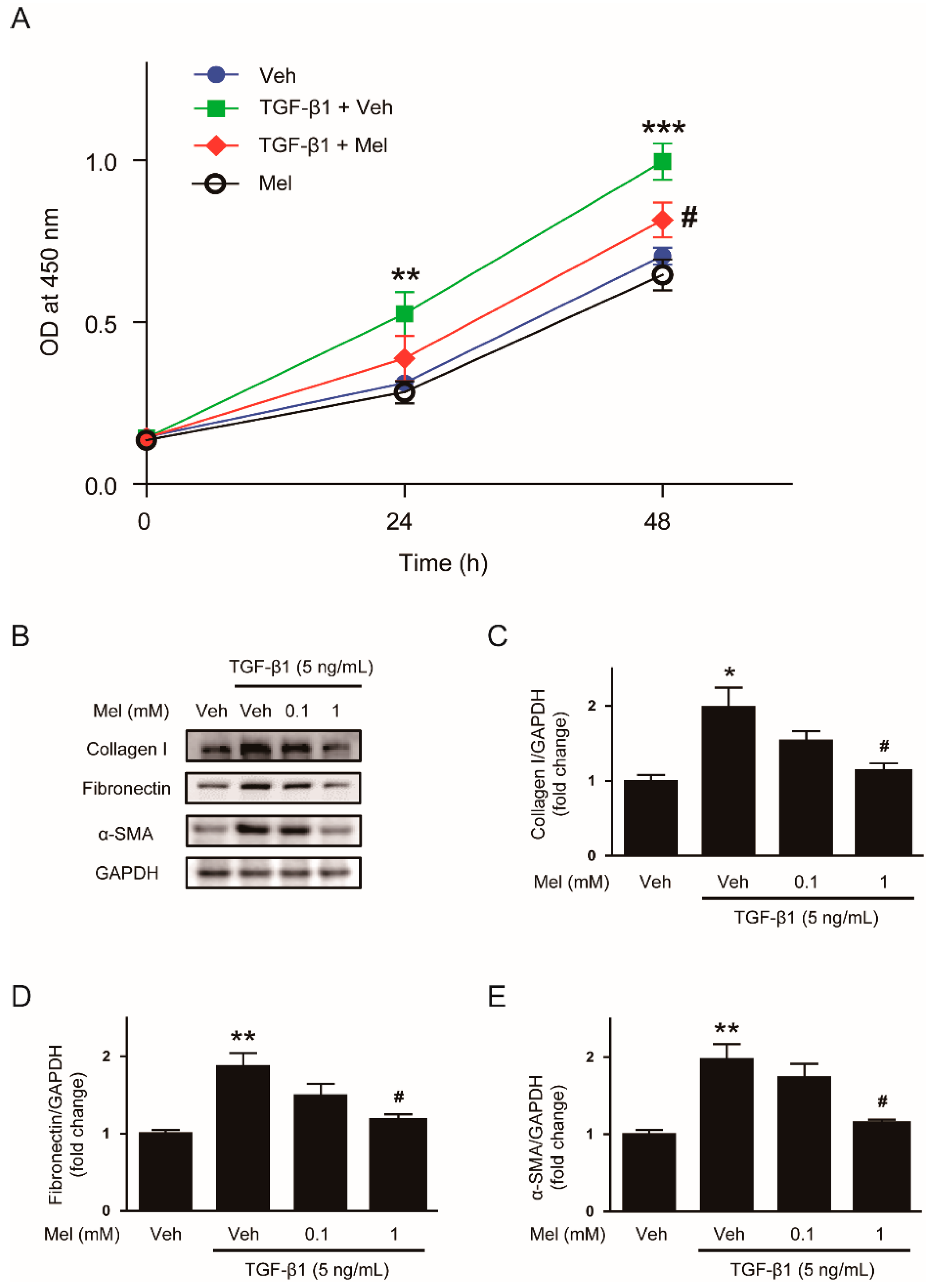
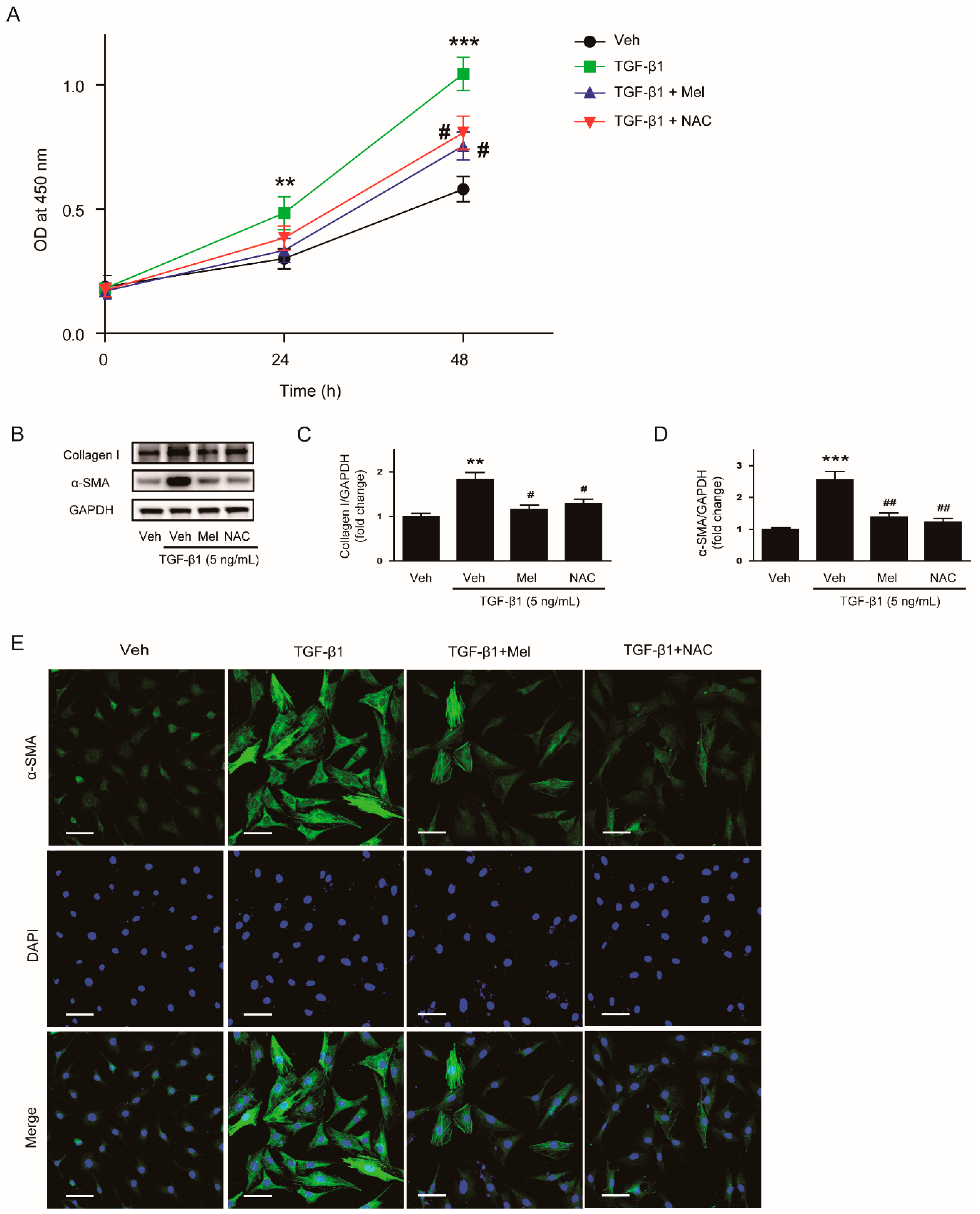
2.2. Cell Viability Assay
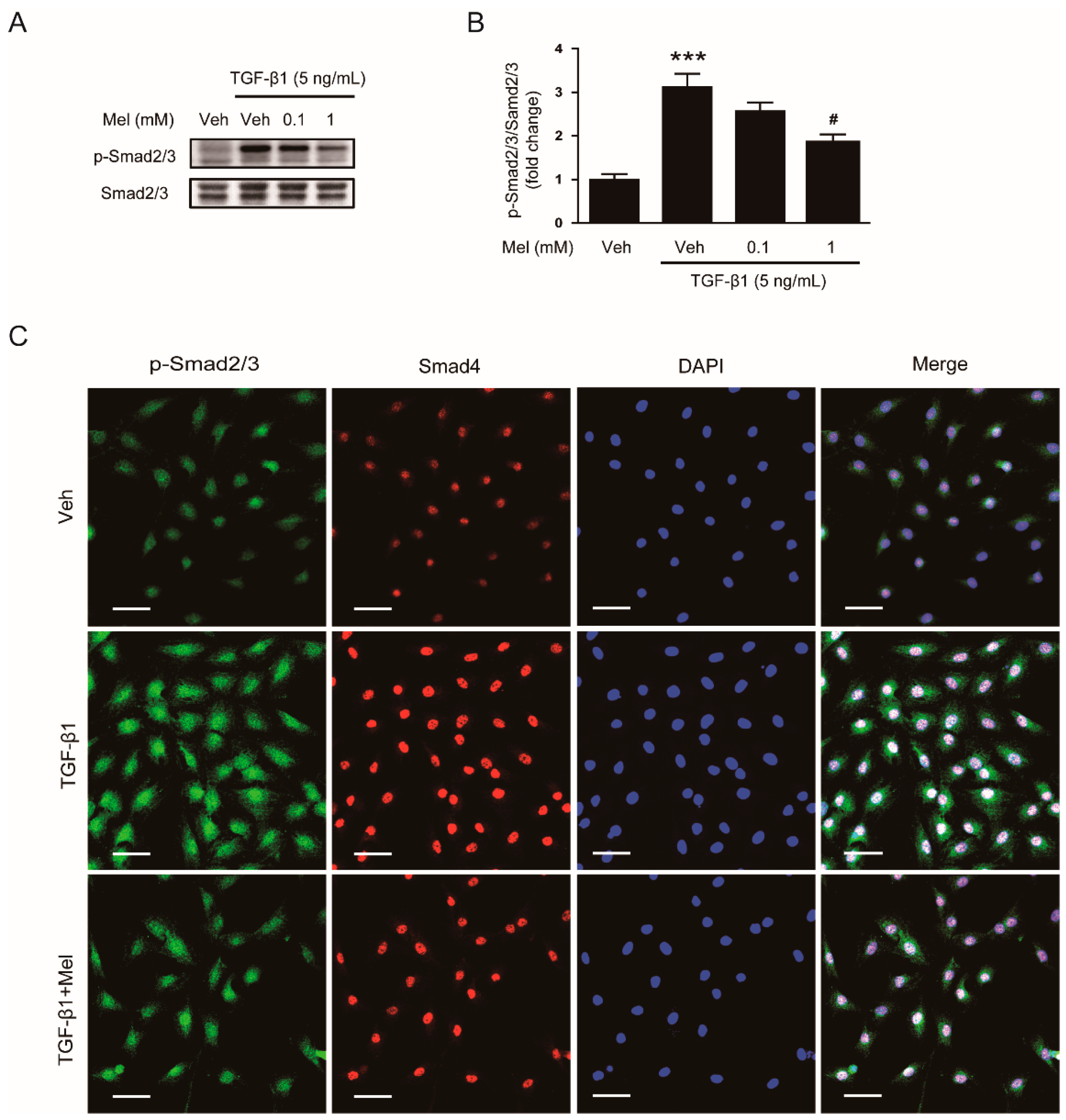
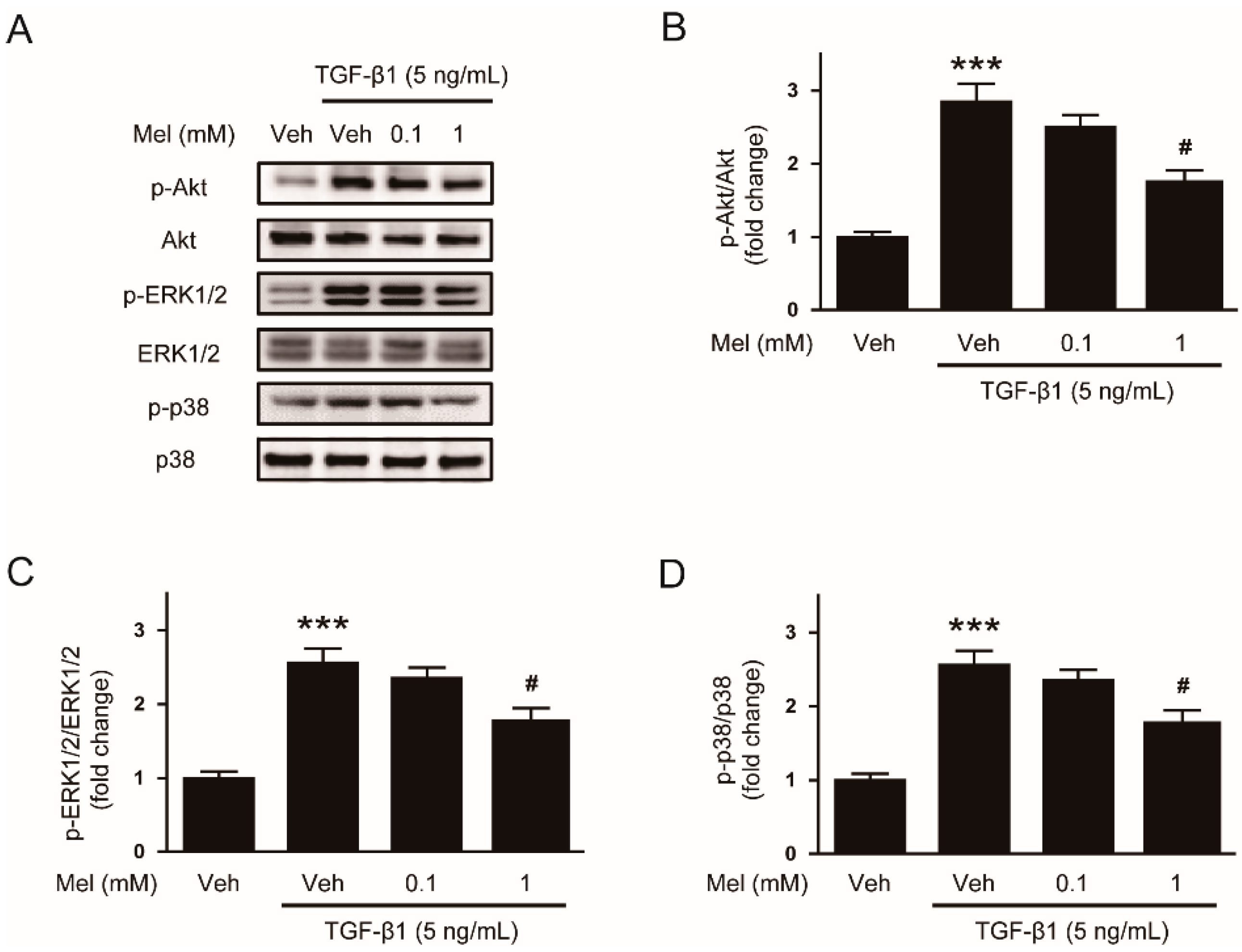
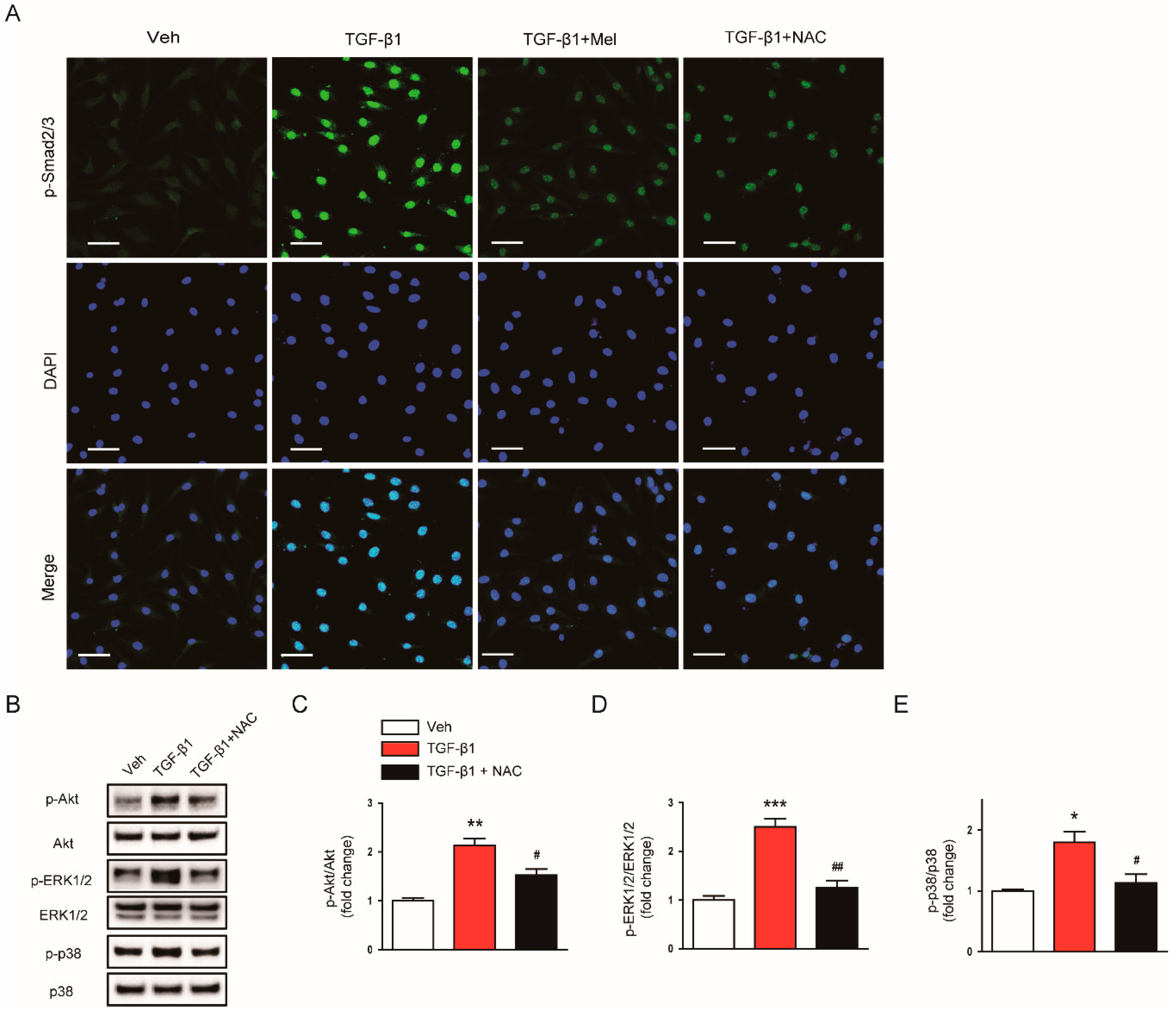
2.3. Western Blot Analysis
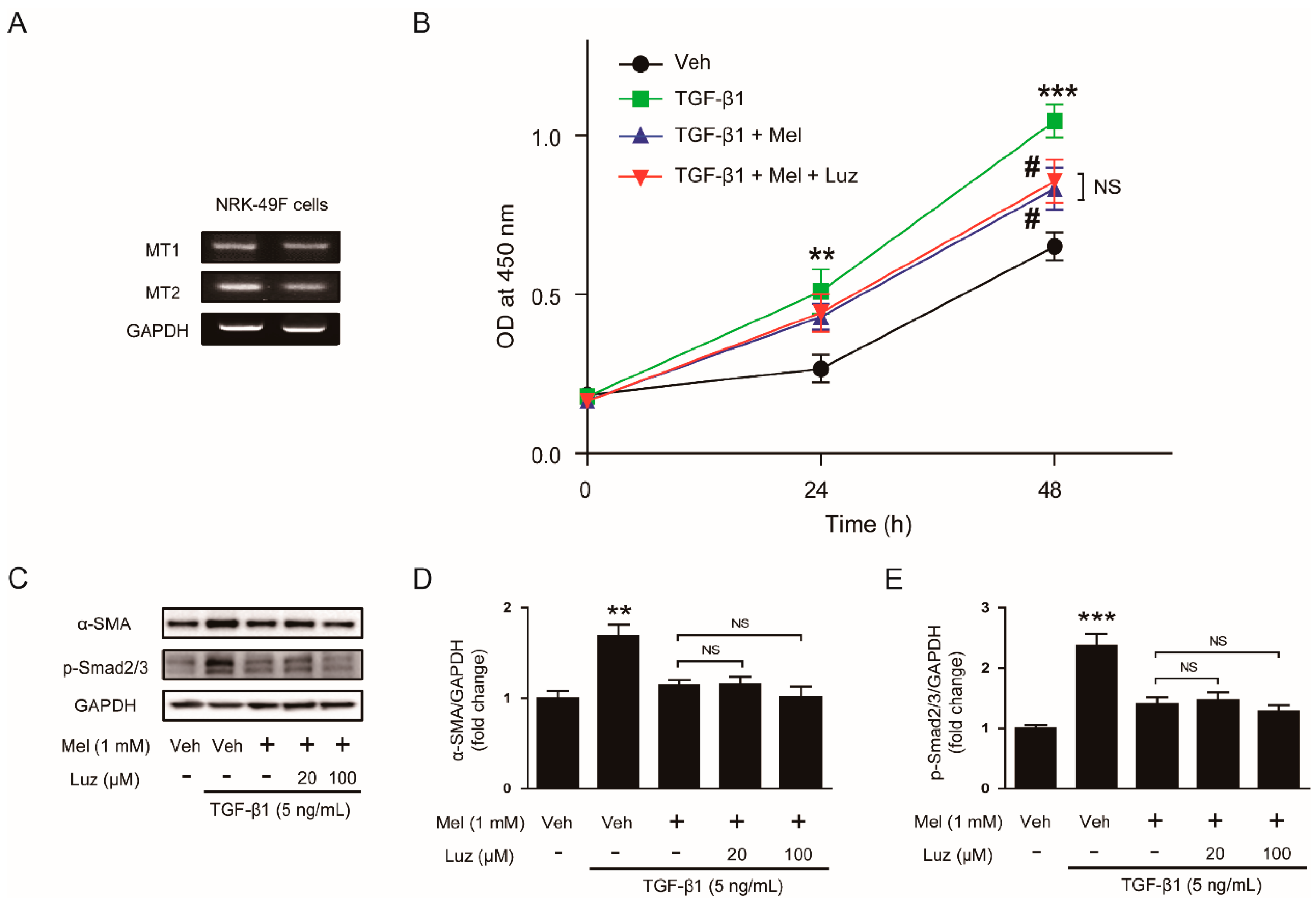
2.4. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
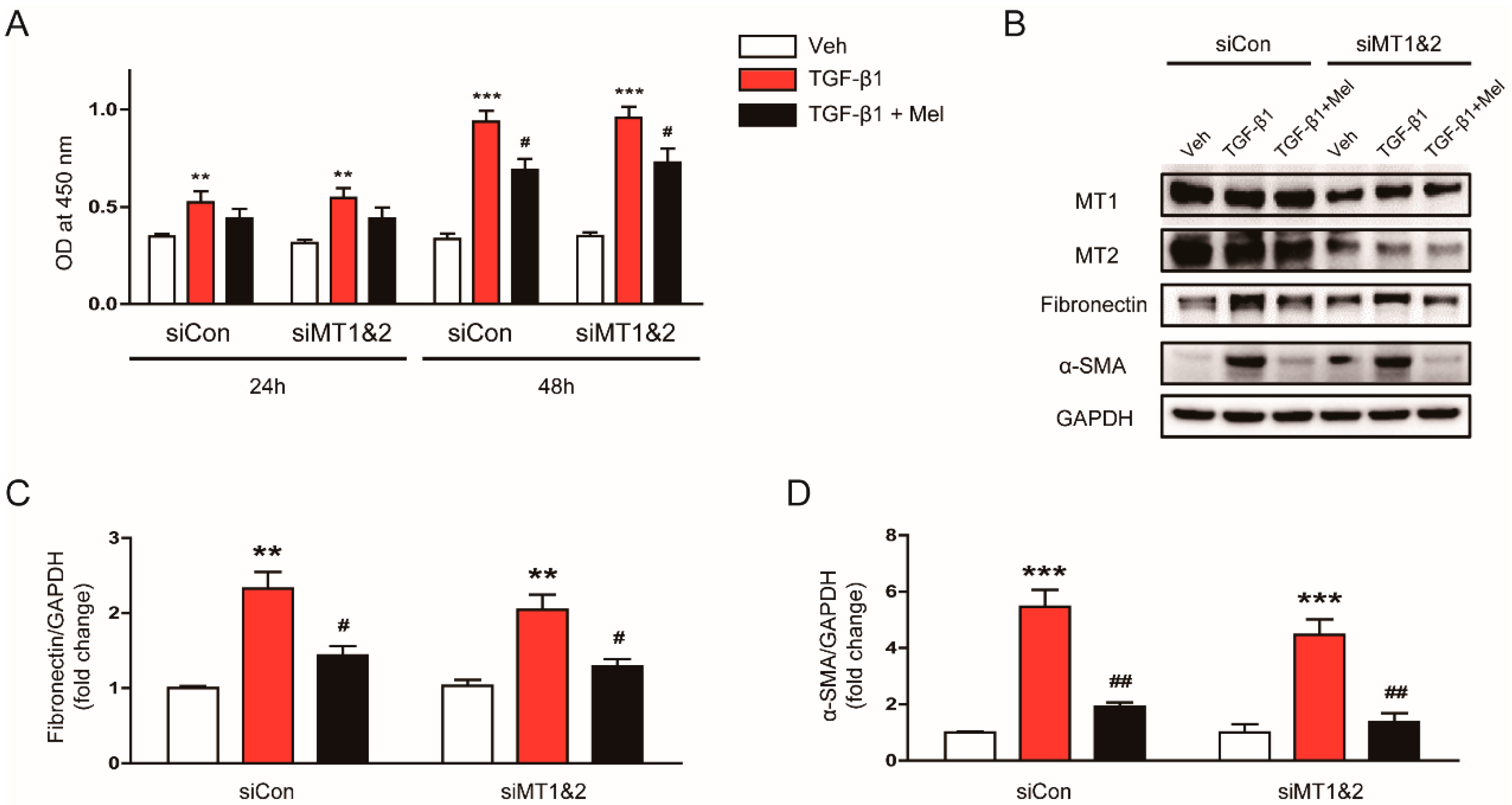
2.5. Knockdown of MT1 and MT2
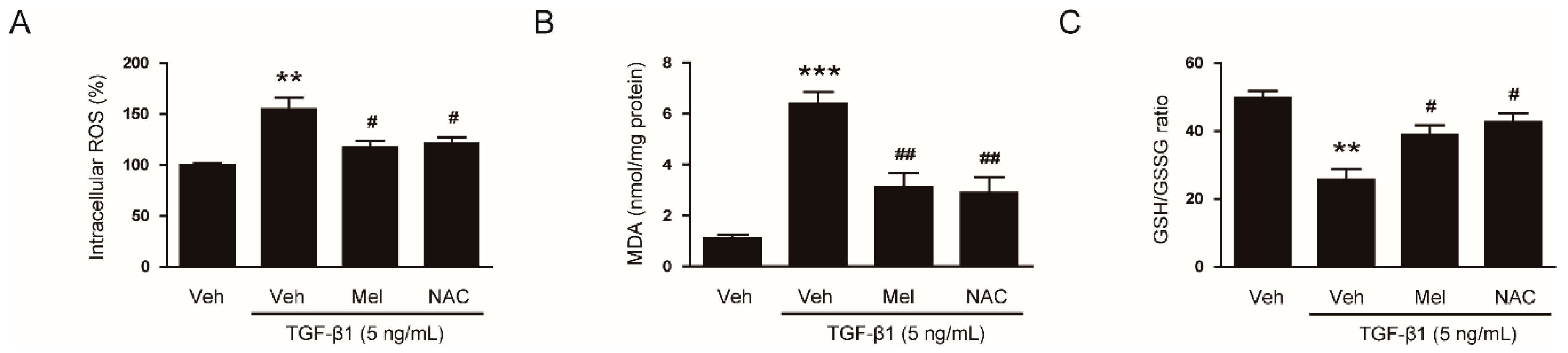
2.6. Evaluation of Iintracellular ROS and Redox Status
2.7. Immunofluorescence Analysis
2.8. Statistical Analysis
3. Results
3.1. Melatonin Inhibits TGF-β1-Induced Proliferation and Activation in NRK-49F Cells
3.2. Melatonin Suppresses TGF-β1-Induced Smad and Non-Smad Signaling Cascades
3.3. Inhibitory Effects of Melatonin on TGF-β1-Induced Proliferation and Activation Is Independent of Its Membrane Receptors
3.4. Inhibitory Effects of Melatonin on TGF-β1-Induced Proliferation and Activation Is attributed to the Inhibition of ROS-Mediated Mechanisms
4. Discussion
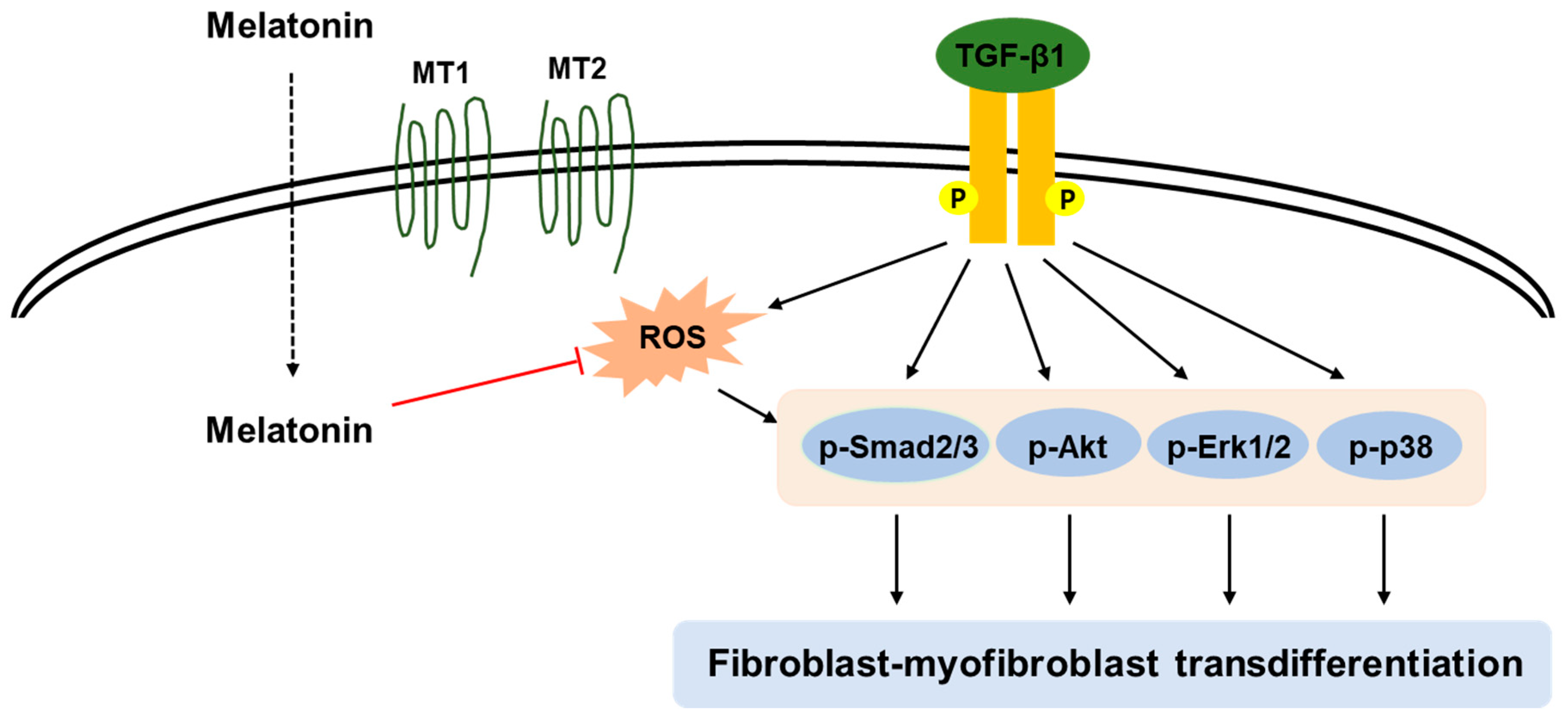
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farris, A.B.; Colvin, R.B. Renal interstitial fibrosis: Mechanisms and evaluation. Curr. Opin. Nephrol. Hypertens. 2012, 21, 289–300. [Google Scholar] [CrossRef]
- Meng, X.M.; Tang, P.M.; Li, J.; Lan, H.Y. TGF-β/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Isaka, Y. Targeting TGF-β signaling in kidney fibrosis. Int. J. Mol. Sci. 2018, 19, 2532. [Google Scholar] [CrossRef]
- Kim, M.K.; Maeng, Y.I.; Sung, W.J.; Oh, H.K.; Park, J.B.; Yoon, G.S.; Cho, C.H.; Park, K.K. The differential expression of TGF-β1, ILK and wnt signaling inducing epithelial to mesenchymal transition in human renal fibrogenesis: An immunohistochemical study. Int. J. Clin. Exp. Pathol. 2013, 6, 1747–1758. [Google Scholar] [PubMed]
- Thomsen, L.H.; Fog-Tonnesen, M.; Nielsen Fink, L.; Norlin, J.; García de Vinuesa, A.; Hansen, T.K.; de Heer, E.; Ten Dijke, P.; Rosendahl, A. Disparate phospho-Smad2 levels in advanced type 2 diabetes patients with diabetic nephropathy and early experimental db/db mouse model. Ren. Fail. 2017, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jang, K.M.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Park, B.; Park, K.K. Pomolic Acid Ameliorates Fibroblast Activation and Renal Interstitial Fibrosis through Inhibition of SMAD-STAT Signaling Pathways. Molecules 2018, 23, 2236. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.J.; Kim, K.H.; Kim, Y.J.; Chang, Y.C.; Lee, I.H.; Park, K.K. Antifibrotic effect of synthetic Smad/Sp1 chimeric decoy oligodeoxynucleotide through the regulation of epithelial mesenchymal transition in unilateral ureteral obstruction model of mice. Exp. Mol. Pathol. 2013, 95, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Opie, L.H.; Lecour, S. Melatonin has multiorgan effects. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ma, Z.; Jiang, S.; Fan, C.; Deng, C.; Yan, X.; Di, S.; Lv, J.; Reiter, R.J.; Yang, Y. Melatonin: The dawning of a treatment for fibrosis? J. Pineal Res. 2016, 60, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Eşrefoğlu, M.; Kuruş, M.; Sahna, E. The beneficial effect of melatonin on chronic cyclosporin A nephrotoxicity in rats. J. Int. Med. Res. 2003, 31, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Ogeturk, M.; Kus, I.; Kavakli, A.; Oner, J.; Kukner, A.; Sarsilmaz, M. Reduction of carbon tetrachloride-induced nephropathy by melatonin administration. Cell Biochem. Funct. 2005, 23, 85–92. [Google Scholar] [CrossRef]
- Ozbek, E.; Ilbey, Y.O.; Ozbek, M.; Simsek, A.; Cekmen, M.; Somay, A. Melatonin attenuates unilateral ureteral obstruction-induced renal injury by reducing oxidative stress, iNOS, MAPK, and NF-kB expression. J. Endourol. 2009, 23, 1165–1173. [Google Scholar] [CrossRef]
- Li, J.; Li, N.; Yan, S.; Lu, Y.; Miao, X.; Gu, Z.; Shao, Y. Melatonin attenuates renal fibrosis in diabetic mice by activating the AMPK/PGC1α signaling pathway and rescuing mitochondrial function. Mol. Med. Rep. 2019, 19, 1318–1330. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, J.; Kim, K.; An, H.J.; Gwon, M.G.; Gu, H.; Kim, H.J.; Yang, A.Y.; Kim, S.W.; Jeon, E.J.; et al. Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice. Antioxidants 2019, 8, 322. [Google Scholar] [CrossRef]
- Lin, J.J.; Lin, Y.; Zhao, T.Z.; Zhang, C.K.; Zhang, T.; Chen, X.L.; Ding, J.Q.; Chang, T.; Zhang, Z.; Sun, C.; et al. Melatonin suppresses neuropathic pain via MT2-dependent and -independent pathways in dorsal root ganglia neurons of mice. Theranostics 2017, 7, 2015–2032. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Yu, N.; Sun, Y.T.; Su, X.M.; He, M.; Dai, B.; Kang, J. Melatonin attenuates TGFβ1-induced epithelial-mesenchymal transition in lung alveolar epithelial cells. Mol. Med. Rep. 2016, 14, 5567–5572. [Google Scholar] [CrossRef]
- Chen, D.Q.; Cao, G.; Zhao, H.; Chen, L.; Yang, T.; Wang, M.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Combined melatonin and poricoic acid A inhibits renal fibrosis through modulating the interaction of Smad3 and β-catenin pathway in AKI-to-CKD continuum. Ther. Adv. Chronic Dis. 2019, 10, 2040622319869116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Hong, R.T.; Xie, Y.Y.; Xu, J.M. Melatonin Ameliorates Liver Fibrosis Induced by Carbon Tetrachloride in Rats via Inhibiting TGF-β1/Smad Signaling Pathway. Curr. Med. Sci. 2018, 38, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Peña, A.B.; Grande, M.T.; Eleno, N.; Arévalo, M.; Guerrero, C.; Santos, E.; López-Novoa, J.M. Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int. 2008, 74, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Stambe, C.; Atkins, R.C.; Tesch, G.H.; Masaki, T.; Schreiner, G.F.; Nikolic-Paterson, D.J. The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. J. Am. Soc. Nephrol. 2004, 15, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Okumura, Y.; Sato, H.; Hamaoka, K. Delayed inhibition of p38 mitogen-activated protein kinase ameliorates renal fibrosis in obstructive nephropathy. Nephrol. Dial. Transplant. 2008, 23, 2520–2524. [Google Scholar] [CrossRef]
- Sugiyama, N.; Kohno, M.; Yokoyama, T. Inhibition of the p38 MAPK pathway ameliorates renal fibrosis in an NPHP2 mouse model. Nephrol. Dial. Transplant. 2012, 27, 1351–1358. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G.; Ai, L.; Shi, J.; Zhang, J.; Chen, Y.X. Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci. Rep. 2018, 8, 15579. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.J.; Bae, M.K.; Kim, Y.D. Suppression of osteoclastogenesis by melatonin: A melatonin receptor-independent action. Int. J. Mol. Sci. 2017, 18, 1142. [Google Scholar] [CrossRef]
- Zielińska, M.; Jarmuż, A.; Sałaga, M.; Kordek, R.; Laudon, M.; Storr, M.; Fichna, J. Melatonin, but not melatonin receptor agonists Neu-P11 and Neu-P67, attenuates TNBS-induced colitis in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 511–519. [Google Scholar] [CrossRef]
- Cheng, X.P.; Sun, H.; Ye, Z.Y.; Zhou, J.N. Melatonin modulates the GABAergic response in cultured rat hippocampal neurons. J. Pharmacol. Sci. 2012, 119, 177–185. [Google Scholar] [CrossRef]
- Bondi, C.D.; Manickam, N.; Lee, D.Y.; Block, K.; Gorin, Y.; Abboud, H.E.; Barnes, J.L. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J. Am. Soc. Nephrol. 2010, 21, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Manickam, N.; Patel, M.; Griendling, K.K.; Gorin, Y.; Barnes, J.L. RhoA/Rho kinase mediates TGF-β1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am. J. Physiol.-Ren. Physiol. 2014, 307, F159–F171. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Ma, S.K.; Bae, E.H.; Lee, J.; Kim, S.W. Peroxiredoxin 5 Protects TGF-β Induced Fibrosis by Inhibiting Stat3 Activation in Rat Kidney Interstitial Fibroblast Cells. PLoS ONE 2016, 11, e0149266. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 2004, 101, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.L.; Gorin, Y. Myofibroblast differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney Int. 2011, 79, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Cucoranu, I.; Clempus, R.; Dikalova, A.; Phelan, P.J.; Ariyan, S.; Dikalov, S.; Sorescu, D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005, 97, 900–907. [Google Scholar] [CrossRef]
- Hecker, L.; Vittal, R.; Jones, T.; Jagirdar, R.; Luckhardt, T.R.; Horowitz, J.C.; Pennathur, S.; Martinez, F.J.; Thannickal, V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009, 15, 1077–1081. [Google Scholar] [CrossRef]
- Hotta, Y.; Uchiyama, K.; Takagi, T.; Kashiwagi, S.; Nakano, T.; Mukai, R.; Toyokawa, Y.; Yasuda, T.; Ueda, T.; Suyama, Y.; et al. Transforming growth factor β1-induced collagen production in myofibroblasts is mediated by reactive oxygen species derived from NADPH oxidase 4. Biochem. Biophys. Res. Commun. 2018, 506, 557–562. [Google Scholar] [CrossRef]
- Dosoki, H.; Stegemann, A.; Taha, M.; Schnittler, H.; Luger, T.A.; Schröder, K.; Distler, J.H.; Kerkhoff, C.; Böhm, M. Targeting of NADPH oxidase in vitro and in vivo suppresses fibroblast activation and experimental skin fibrosis. Exp. Dermatol. 2017, 26, 73–81. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Park, J.-H.; Jeon, E.J.; Leem, J.; Park, K.-K. Melatonin Prevents Transforming Growth Factor-β1-Stimulated Transdifferentiation of Renal Interstitial Fibroblasts to Myofibroblasts by Suppressing Reactive Oxygen Species-Dependent Mechanisms. Antioxidants 2020, 9, 39. https://doi.org/10.3390/antiox9010039
Kim J-Y, Park J-H, Jeon EJ, Leem J, Park K-K. Melatonin Prevents Transforming Growth Factor-β1-Stimulated Transdifferentiation of Renal Interstitial Fibroblasts to Myofibroblasts by Suppressing Reactive Oxygen Species-Dependent Mechanisms. Antioxidants. 2020; 9(1):39. https://doi.org/10.3390/antiox9010039
Chicago/Turabian StyleKim, Jung-Yeon, Jae-Hyung Park, Eon Ju Jeon, Jaechan Leem, and Kwan-Kyu Park. 2020. "Melatonin Prevents Transforming Growth Factor-β1-Stimulated Transdifferentiation of Renal Interstitial Fibroblasts to Myofibroblasts by Suppressing Reactive Oxygen Species-Dependent Mechanisms" Antioxidants 9, no. 1: 39. https://doi.org/10.3390/antiox9010039
APA StyleKim, J.-Y., Park, J.-H., Jeon, E. J., Leem, J., & Park, K.-K. (2020). Melatonin Prevents Transforming Growth Factor-β1-Stimulated Transdifferentiation of Renal Interstitial Fibroblasts to Myofibroblasts by Suppressing Reactive Oxygen Species-Dependent Mechanisms. Antioxidants, 9(1), 39. https://doi.org/10.3390/antiox9010039




