3.2. Cell Viability Assessment of the PANC-1 Cells Treated with γ-Conglutin Protein
In this study, we assessed the viability of PANC-1 cells under treatment of the γ-conglutin protein and the potential cytotoxicity of this protein. In order to evaluate whether inflammation inductor LPS and γ-conglutin produce cell cytotoxicity effects, the viability MTT assay was achieved on PANC-1 cells under separate treatments with LPS adding γ-conglutin, at increasing concentrations to complete the conditions of DMEM culture medium + FBS + antibiotic for 24 h. The LPS plus γ-conglutin had no significant (
p > 0.05) effects on cell viability (
Supplementary Table S2), when compared with the control (untreated) group. The cell cultures used as positive control lacked LPS and γ-conglutin protein. In order to complete the usefulness of the γ-conglutin protein study, trypan blue staining was also used for assessing PANC-1-pancreatic cells viability after treatment with LPS (1 μg/μL) and increasing concentrations (from 10 to 50 μg) of γ-conglutin for 24 h, finding significant differences (
p < 0.05) in cell viability after 24 h of incubation only at 50 μg compared to the control (
Supplementary Table S2).
Furthermore, a parallel study was made to assess the cell viability and cytotoxicity of increasing concentrations of insulin in order to know whether an insulin resistance model could be performed in PANC-1 pancreatic cells and to know the actual insulin concentration that should be used to stablish the model. An MTT assay was developed on PANC-1 cell finding that an important change in the percentage of viability was induced for insulin concentrations higher than 10
−7 nmol L
−1 (
Supplementary Table S3). Afterward, IR-C cells were assayed for viability using MTT kit when performed the addition of γ-conglutin protein for 24 h. No significant (
p > 0.05) effect on cell viability (when treated with 25 μg of γ-conglutin protein) (
Supplementary Table S4) was found after comparison with unchallenged IR-C group. When insulin was added alone (in the absence of γ-conglutin), these samples were used as a positive control. We also performed the cell viability assessment using trypan blue exclusion in IR-C pancreatic cells treated with increasing concentrations of this protein for a period of 24 h. No cell viability differences were found after 24 h of incubation in the presence of γ-conglutin.
These results suggest that γ-conglutin do not affect to the PANC-1 pancreatic cell integrity in both, the induced (LPS treatment) inflammation and the IR-C cell models.
3.3. Effect of γ-Conglutin Protein on the Inflammatory Process
Inflammatory-related illnesses as metabolic syndrome, T2DM, obesity and cardiovascular diseases are well known to be developed and chronically associated to a continuously sustained inflammatory state. Among different mechanisms hidden in the inflammatory-based diseases, different molecules namely stressors affect functional pancreatic tissues physiology, particularly β-islets, promoting the course of pathology, which also of course mainly depend of particular genetic backgrounds and environmental factors [
16].
Nowadays, there is an increasing number of diabetes associated to obesity named “Diabesity epidemic”, which is frequently coincidental with a pancreatic islet cells failure unable to generate enough amount of insulin and/or a developed decreasing sensitivity to insulin by tissues able to metabolize glucose. During the establishment of T2DM, sustained high levels of glucose may lead to organ damage, which is mediated by pancreatic β-cells tissue damage, and the enhancement of immune system inflammatory response because the synthesis and release of pro-inflammatory mediators as cytokines and chemokines (cells chemotactic factors). These processes create feed-forward progressive steps that further increases immune system cell content, promoting a chronic inflammatory state [
17]. Thus, increasing levels of multiple factors as IL-1β, TNFα, and iNOS are important contributors for the development of inflammation since IL-1β-mediates β-cell dysfunction during the development of T2DM, while are able to activate the expression of iNOS with the result of an exacerbate synthesis of NO, promoting the up-regulation of pro-inflammatory genes [
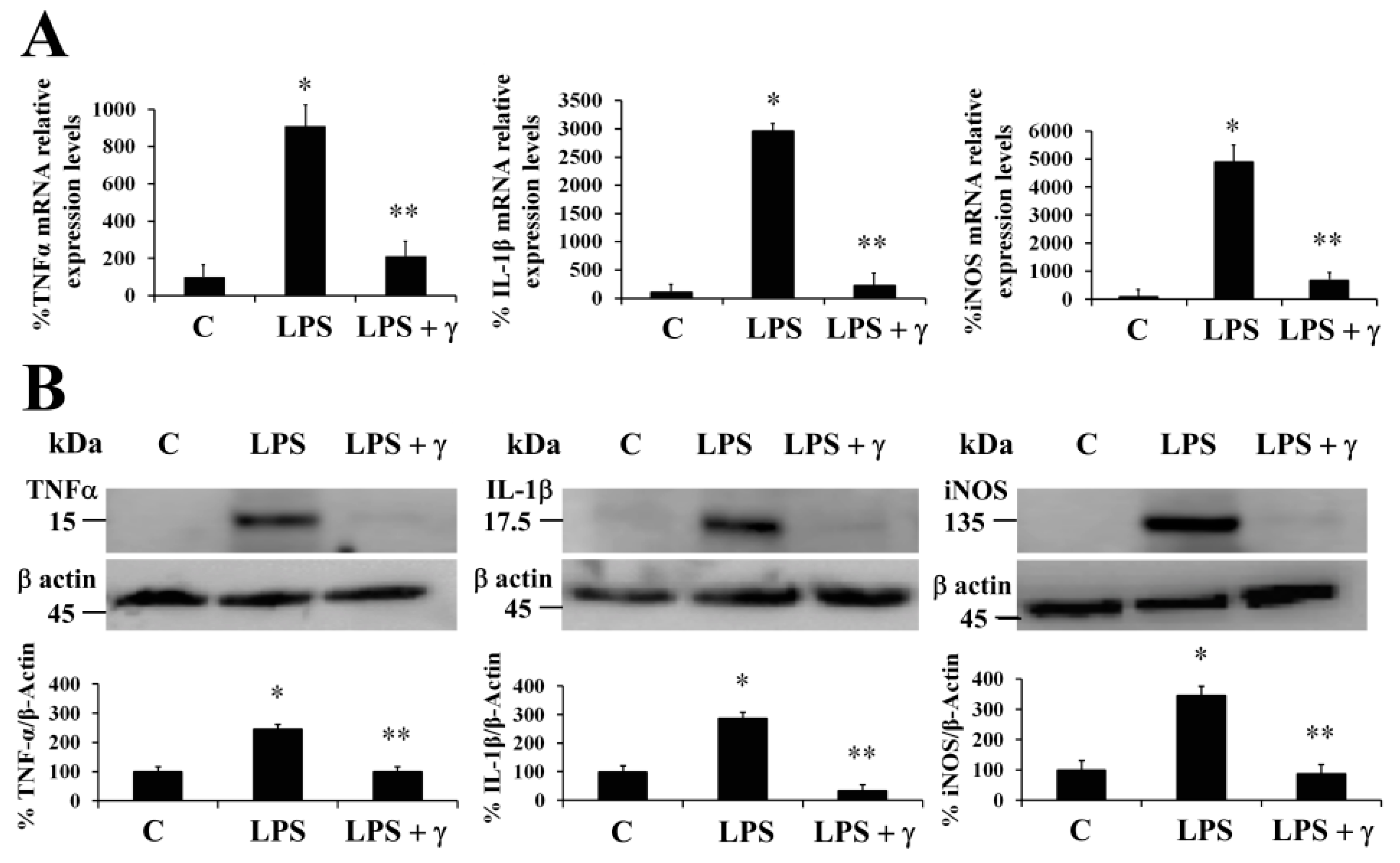
18]. In this regard, we evaluated the ability of γ-conglutin protein to modulate the mRNA levels of genes of pro-inflammatory mediators as potential anti-inflammatory targets (TNFα, IL-1β, and iNOS mRNA) in PANC-1 cells (
Figure 1). Induced inflammatory state by LPS was significantly inhibited (
p < 0.05) by γ-conglutin proteins at mRNA expression level in PANC-1 [−694, −2733, and −4208–fold, respectively,
versus LPS treated culture cells] (
Figure 1A). No statistically significant differences were observed in IL-1β cytokine, TNFα, and iNOS mRNA levels (
p > 0.05) when challenges were performed with γ-conglutin + LPS as compared to the control group (
Figure 1A). These results highlight the potential implications of γ-conglutin to decrease the pro-inflammatory capacity in PANC-1 cells by decreasing cytokines and iNOS genes expression levels, thus supporting the inflammatory process amelioration at molecular level. In this study, this lowering in the cellular pro-inflammatory capacity could be the result of the antioxidant capacity of γ-conglutin since changes in GSH levels, SOD and catalase activities was shown, helping to keep redox homeostasis in T2DM and other inflammatory-dependent diseases also affected by the oxidative stress [
19]. On this line, the above results on PANC-1 pancreatic cells are in agreement with previous studies that shown a similar reduction in the expression levels of iNOS and IL-1β mRNA in T2DM blood culture [
20].
It is well established that systemic production of IL-1β at local tissues plays a fundamental role in the progression of pancreatic dysfunction as β-cell apoptosis in T2DM. The advance of this disease is facilitated by a continued production of inflammatory molecular mediators that would have an initial development stage and further progression promoted by TNFα-/IL-1β-mediated iNOS synthesis and NO production [
21]. We have also demonstrated that NLL γ-conglutin can reverse this state by decreasing the levels of TNFα, IL-1β and iNOS functional protein levels in PANC-1 [−158, −144, and −164–fold, respectively,
versus LPS treated culture cells] (
Figure 1B,
Supplementary Table S5), while no statistically significant differences (
p > 0.05) were observed in TNFα, IL-1β and iNOS protein levels when challenges were accomplished with γ-conglutin (LPS + γ) when compared to the control group (
Figure 1B).
3.4. γ-Conglutin Protein Inhibits the Production of Different Cytokines and Pro-Inflammatory Mediators
Physiological circulating levels of cytokines have important implications in the functional regulation of pancreatic β-cells, although these produce different cytokines itself in response to physio-pathological states, playing also important roles in its own β-cells function [
18]. When insulin resistance is stablished, increasing production of dangerous pro-inflammatory circulating mediators is also stablished. During the T2DM state progression, this non-physiological condition is characterized by an imbalance pro-inflammatory cytokines and mediators profile, led by the β-cell dysfunction and T2DM sustainable situation, which on the other hand, is based on the crosstalk among cytokines in pancreatic β-cells and immune tissues [
22]. Thus, restoring the balance back to the increased levels of protective plasma circulating and β-cells cytokines could prevent and promote the treatment of this β-cell dysfunctional statement, and for extension the T2DM progression.
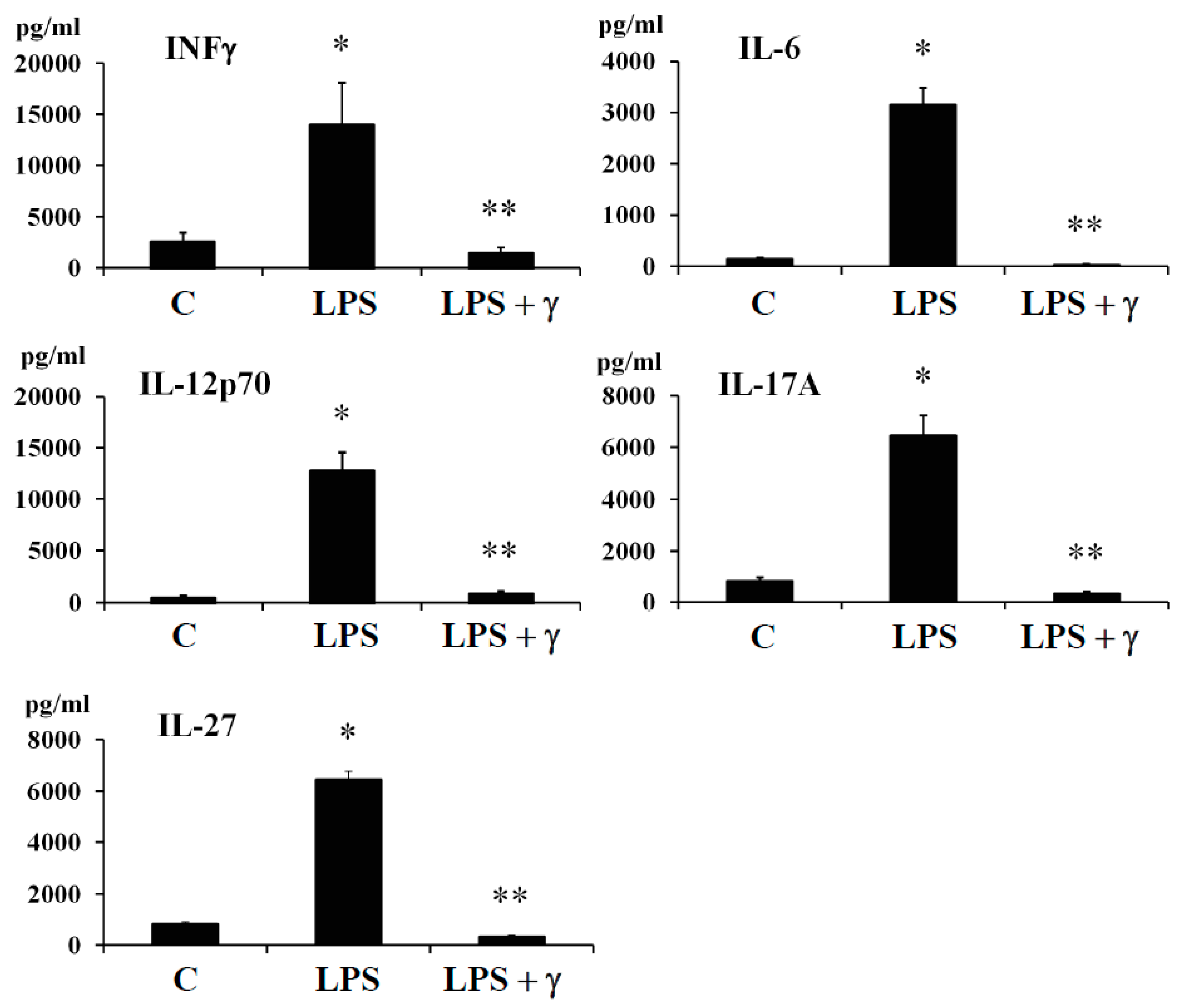
In this regard, we evaluated by ELISA method the potential anti-inflammatory effects of γ-conglutin protein through its capacity to modulate the amount of important pro-inflammatory mediator as INF-γ and cytokines (IL-6, IL-12p70, IL-17A, and IL-27) in both, an induced inflammation model (
Figure 2,
Supplementary Tables S5 and S6), and in an IR-C cell model (
Supplementary Figure S2,
Supplementary Tables S5 and S6) using PANC-1-pancreatic cells. Levels of INFγ and the above cytokines were assessed under basal conditions, after cell treatment with LPS, by challenging the cell culture with γ-conglutin protein after LPS and by adding LPS + γ-conglutin together, or alternatively with γ-conglutin after IR-C model is stablished (as explained in material and methods,
Section 2.6). The protein levels of INF-γ and cytokines (IL-6, IL-12p70, IL-17A, and IL-27) significantly (
p < 0.05) augmented (several-fold) after LPS challenges [LPS: +11335, +2979, +12127, +5632 and +5676-fold
versus C, respectively] (
Figure 2,
Supplementary Table S6); and IR-C model [+8994, +1881, +11592, +5553, +5231-fold
versus C, respectively] (
Supplementary Figure S2,
Supplementary Table S6) whereas the LPS + γ -conglutin protein challenges showed a significant reduction (several-folds) in protein levels [LPS: −256, −1849, −11786, −5339 and −6100-fold
versus LPS treated cells, respectively], and IR-C model [−8644, −1839, −11409, −5659 and −5339-fold
versus IR-C cells, respectively] (
Supplementary Figure S2,
Supplementary Table S5). These results are in agreement with these obtained from RT-qPCR, where reduced mRNA and proteins levels of the pro-inflammatory mediators TNFα, IL-1β, and iNOS were found in PANC-1-cells culture (
Figure 1A,
Figure 1B,
Supplementary Tables S5 and S6). No statistically significant differences (
p > 0.05) were observed in INF-γ and cytokines (IL-6, IL-12p70, IL-17A, and IL-27) protein levels when challenges were accomplished with γ-conglutin (LPS + γ) after comparison to the control group (
Figure 2,
Supplementary Figure S2).
Currently, scarce studies have showed results concerning the anti-inflammatory effects of plant peptides, usually promoted by the modulation of the balance regulation of pro-inflammatory interleukins, INFγ, TNFα and NO. In the case of studied soybean peptides, these inhibited mRNA iNOS expression levels and TNFα and NO production, while also reduced the pro-inflammatory enzymatic activity of COX-2 in LPS-induced macrophages [
8]. Moreover, lunasin was shown to reduce the ROS production in macrophages induced by LPS while inhibiting the release of IL-6 and TNFα [
11,
12]. In this regard, we demonstrated that NLL γ-conglutin protein lowered the pro-inflammatory mediators’ levels assayed. This anti-inflammatory capacity would be capable to manage the diseases developmental states promoting feed-forward process for the establishment of these chronic inflammatory-derived diseases as T2DM. Thus, lupin γ-conglutins may be capable to promote the improvement from the detrimental effects of several inflammatory molecular developments as follows:
(i) Lipotoxicity as a sustained high lipid diet induces the production of IL-1β, IL-6, which β-cells continued exposure induces exacerbate synthesis and release of ROS, while secretion of insulin is also inhibited. This combination promotes the apoptosis of the pancreatic β-cells [
23]. Based in our research, challenging pancreatic β-cells with γ-conglutin decreased the mRNA expression of IL-1β, and protein levels of IL-1β and IL-6 in LPS-induced inflammation [LPS + γ: −2749; −146 and −1100-fold
versus LPS treated cells, respectively] (
Figure 1;
Figure 2,
Supplementary Table S5); and IR-C model [IR-C + γ: −177; −97 and −1849-fold
versus IR-C cells, respectively] (
Figure 3,
Supplementary Figure S2,
Supplementary Table S5).
(ii) Apoptosis of islets β-cells prompted by IL-1β and INFγ is stimulated by endoplasmic reticulum stress [
24]. In this regard, β-cell apoptosis is also activated by the join action of INFγ and TNFα, together with the activation of Ca
2+ channels. This situation induces the NO synthesis and consequently the endoplasmic reticulum stress pathway activation [
25], leading to caspases activation and mitochondrial dysfunction [
26]. In this concern, γ -conglutin may be able to prevent these mechanisms by suppressing the TNFα, IL-1β and INFγ mRNA and protein levels (
Figure 1,
Figure 2 and
Figure 3,
Supplementary Figure S2,
Supplementary Tables S5 and S6).
(iii) The synergistic action of IL-1β + INFγ, or even IL-1β + INFγ + TNFα cytokines in pancreatic tissues increases NO production as consequence of direct increasing of iNOS, resulting in islet β-cell destruction [
27]. We have shown that mRNA expression levels of TNFα and IFNγ (apoptosis mediated molecules) were lowered after treatment with γ-conglutin (
Figure 1,
Figure 2 and
Figure 3,
Supplementary Figure S2,
Supplementary Tables S5 and S6), which may have a positive effect on the survival of islet β-cells [
28].
(iv) IL-12 mRNA expression levels are increased by the effect of INFγ, while IL-12 promote signaling positive feed-back effect for raising levels of INFγ [
29]. The γ-conglutin protein reduction effect of IL-12 mRNA levels (
Figure 2,
Supplementary Figure S2,
Supplementary Tables S5 and S6) may decrease INFγ levels and its negative inflammatory effects.
(v) Important inflammatory cytokine, IL-17A, involved in the T2DM progressing, is able to induce ROS production, which also greatly affects to insulin resistance. A join action from IL-17 and INFγ acts as diabetes chronic state development [
30]. Overall, IL-17A has pleiotropic functional effects comprising synthesis of IL-6 and TNFα, and chemokines (chemotaxis effect) on a diversity of cells [
31]. Thus, the lowering of the IL-17 protein level (
Figure 2,
Supplementary Figure S2,
Supplementary Tables S5 and S6) might reduce pro-inflammatory effects of IL-6 and TNFα (
Figure 2,
Supplementary Figure S2,
Supplementary Tables S5 and S6), avoiding islet β-cell apoptosis and the recruitment of immune cells to local tissues, enhancing feed-forward mechanism of inflammation progression in islets [
32] as preventive action for inflammation based T2DM progression.
3.5. γ-Conglutin Reverses the Insulin Resistance through Inflammation Amelioration while Improving Insulin Signalling Pathway in Pancreatic IR-C Cells
Insulin resistance is another consequence of a sustained inflammation, which has been observed in several pathophysiological processes, including metabolic disorders as hyperinsulinemia, hyperglycemia, and hypertriglyceridemia, being IR also an important cause of pre-diabetes establishment and T2DM development and obesity [
33], affecting to different insulin target organs. Thus, amelioration of IR by NLL γ-conglutin may constitute a major approach to prevent and treat these metabolic disorders.
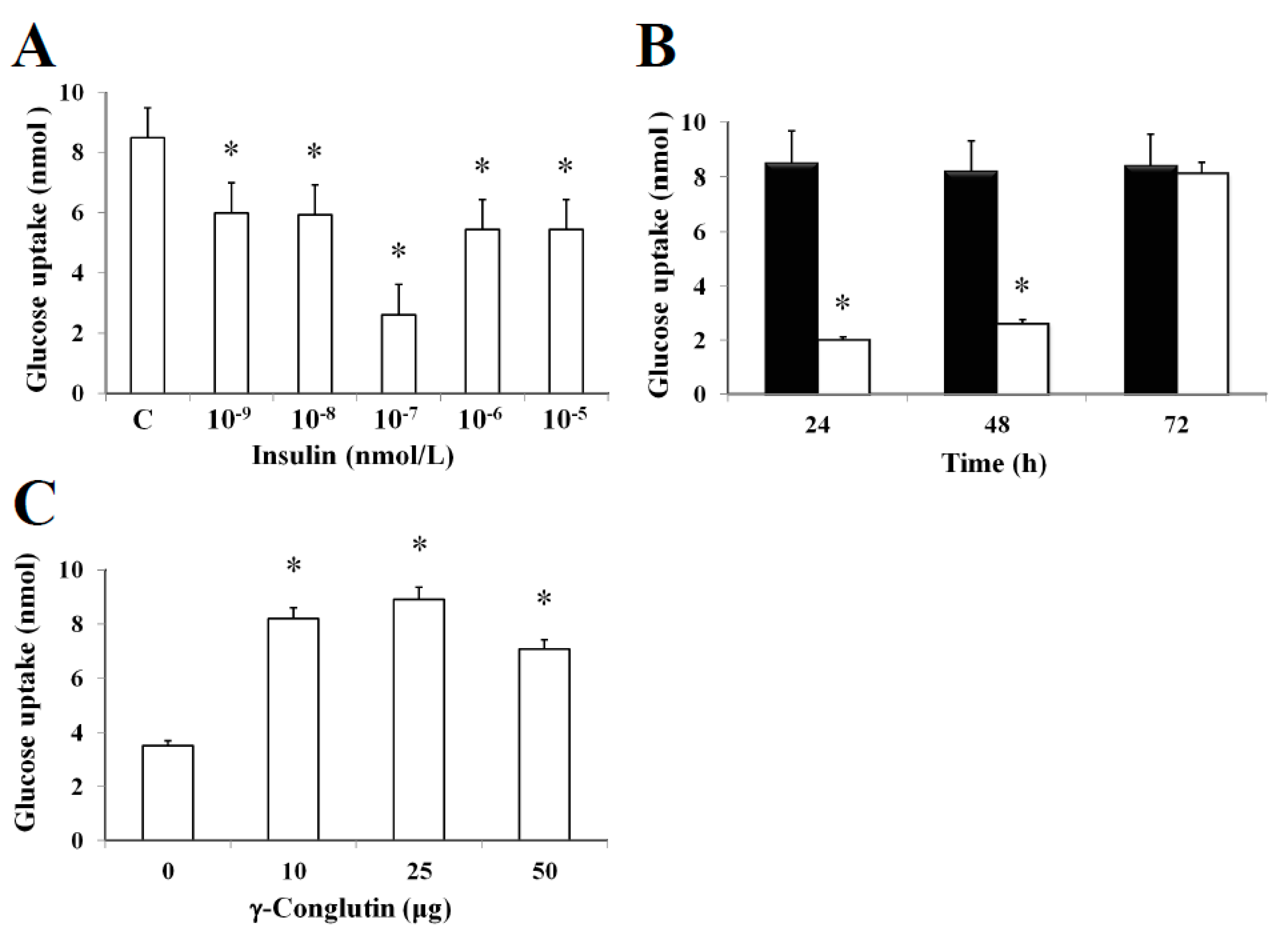
In this study, it was established an in vitro insulin-resistant (IR-C) cell model using PANC-1 cells to evaluate the insulin effects on glucose uptake and metabolism in IR-C cell. To evaluate glucose uptake, control cells were incubated with a range of insulin concentrations (between 10
−5 to 10
−9 nmol L
−1) for 24 h (
Figure 4). Following an insulin concentration of 10
−7 nmol L
−1, we found the most statistically significant reduction in the extracellular glucose depletion (
p < 0.05) in comparison to control cells (without insulin treatment) (
Figure 4A). The addition of 10
−7 nmol L
−1 of insulin promoted a time-dependent lowering (
p < 0.05) of glucose consumption between 24–48 h when compared to control cells (
Figure 4B). These results clearly showed the maintenance of the insulin resistance by IR-C cells for a period of 48 h after insulin treatment. Following 48 h, cells acquired a normal condition as control cells (C). These results are consistent with the increasing glucose uptake shown in
Figure 3B after 72 h, while no statistically significant differences (
p > 0.05) in glucose consumption was observed when compared to control cells without insulin treatment. Furthermore, the molecular mechanisms leading to glucose homeostasis and/or IR are still uncertain. However, NLL γ-conglutin might be able to contribute in this process of glucose homeostasis, as we have demonstrated in the current study that glucose uptake by IR-C cells is clearly induced by treatment with γ-conglutin protein, reaching higher glucose uptake levels after IR-C cells challenged with 25 μg of γ-conglutin protein, which glucose uptake increased more than 60% in comparison to IR-C cells (
p < 0.05), which were assayed without γ-conglutin protein challenge (
Figure 4C).
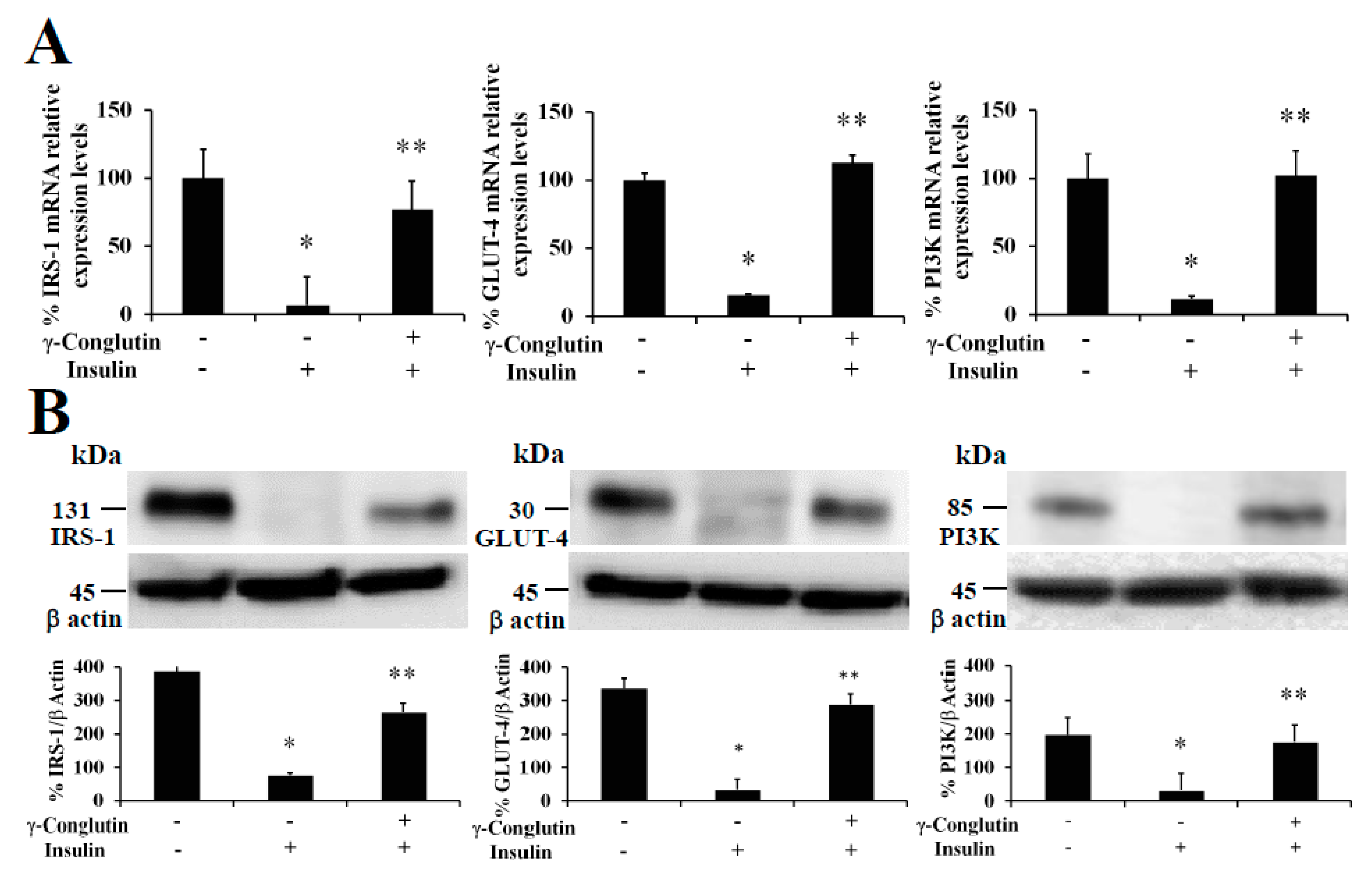
The treatment of pancreatic IR-C cells with γ-conglutin was also accomplished to determine whether this protein had effects on insulin resistance improvement throughout recovering the control-like associated mRNA expression levels of IRS-1, GLUT-4, and PI3K, key upstream and glucose transport mediators in the insulin signaling pathway [
20], which would also be the reflect of a potential improvement in the glucose uptake and the inflammatory state on IR-C cells. The analysis of IRS-1, GLUT-4 and PI3K showed their up-regulation in their mRNA expression after γ-conglutin treatment in IR-C cells (
Figure 5) [IRS-1: +70; GLUT-4: +97%; and PI3K: +90-fold, respectively], which differences were statistically significant compared to IR-C untreated cells (
p < 0.05) (
Figure 5A), as well as the mRNA expression level reduction of IRS-1, GLUT-4 and PI3K in IR-C cells [IRS-1: −93; GLUT-4: −84%; and PI3K: −89-fold, respectively] compared to control cells PANC-1 (
Figure 5A).
We have also demonstrated that NLL γ-conglutin can reverse this state by up-regulating the IRS-1, GLUT-4 and PI3K functional protein levels in IR-C cells [IRS-1: +266; GLUT-4: +185; and PI3K: +144-fold, respectively] (
Figure 5B), after decreased proteins levels showed when PANC-1 control cells acquired the IR-C statement compared to the control group [IRS-1: −302; GLUT-4: −310; and PI3K: −166-fold, respectively] (
Figure 5B). These results confirm that γ-conglutin protein would be capable to reduce significantly the blood glucose level by promoting glucose uptake by insulin sensitive tissues while ameliorating hyperglycemia via increasing GLUT-4 glucose transporter protein level and plasma membrane recruitment [
34], and insulin signaling pathway upstream mediators IRS-1 and PI3K [
20].
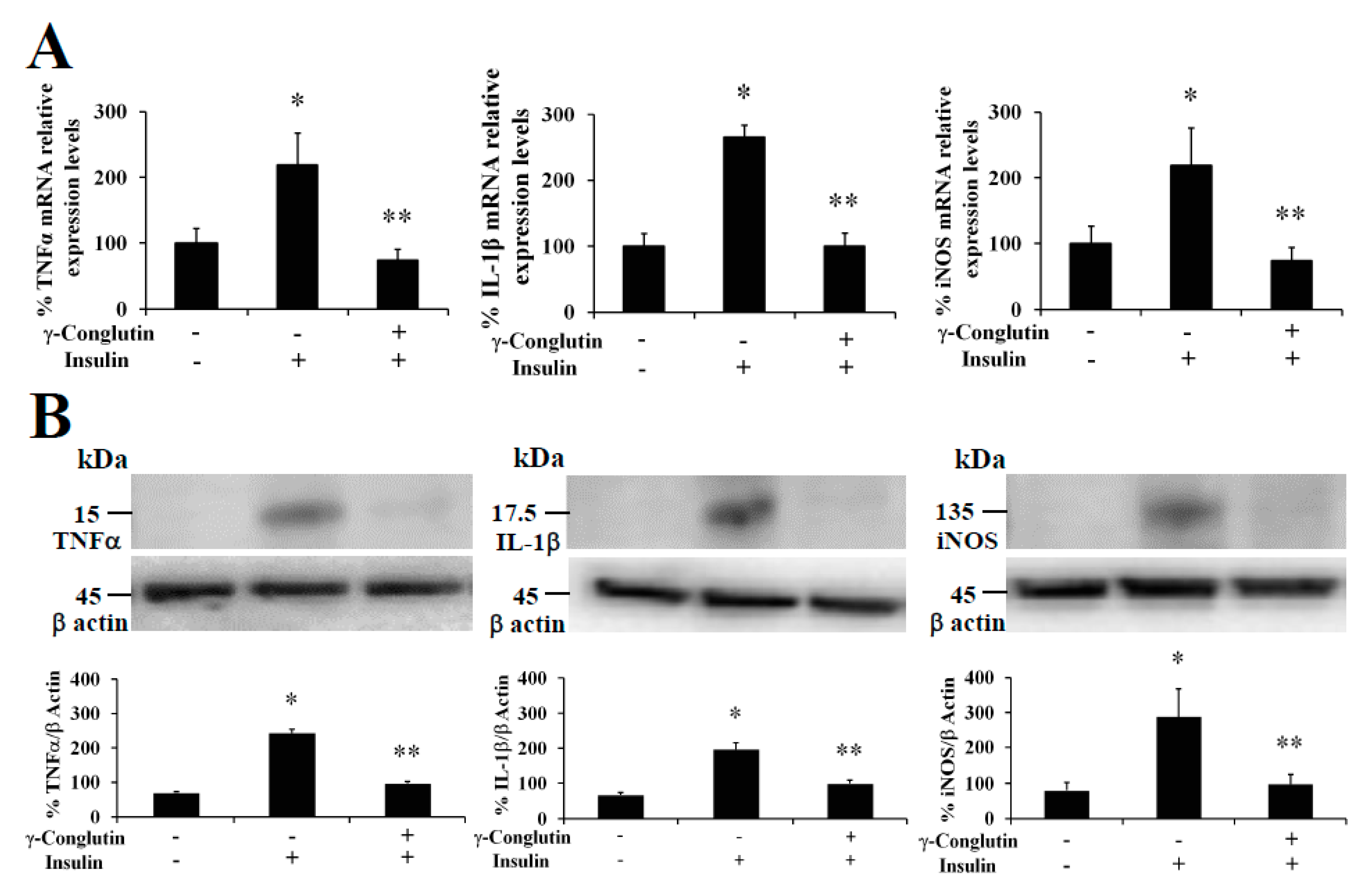
Furthermore, at the same time we also evaluated the capability of γ-conglutin protein to regulate the mRNA and protein levels of pro-inflammatory molecules as potential mechanism helping to reverse the IR-C cell statement. TNFα, IL-1β and iNOS were analyzed in IR-C culture (
Figure 3). These pro-inflammatory mediators were significantly lowered in γ-conglutin protein treated IR-C cells, at the mRNA expression levels [TNFα: −158; IL-1β: −144; and iNOS: −164-fold, respectively,
versus IR-C untreated cells] (
Figure 3A), and at the protein levels [TNFα: −189; IL-1β: −146; and iNOS: −97-fold, respectively,
versus IR-C untreated cells] (
Figure 3B,
Supplementary Table S6). No statistically significant differences (
p > 0.05) were found for TNFα, IL-1β and iNOS levels in IR-C cells treated with γ-conglutin in comparison to the PANC-1 control group (
Figure 3). These results highlight the potential implications of γ-conglutin to improve insulin resistance through inflammation amelioration at molecular level in PANC-1 pancreatic cells by decreasing cytokines and iNOS levels [
20].
In this study, we have demonstrated for the first time that NLL γ-conglutin protein is able to help improving the insulin resistance state in PANC-1 cell line targeting two major molecular signaling cross-roads, restoring functional levels of insulin activation pathway mediators while decreasing several pro-inflammatory mediators’ levels that worthwhile reinforces the first effect on PANC-1 cells. These outcomes are vital knowledge to be considered for successful anti-inflammatory insulin sensitizing new alternative therapies from natural plant sources.
3.6. Oxidative Stress Modulation by γ-Conglutin Protein as Anti-Inflammatory and Insulin Resistance Improvement Mechanism
Oxidative stress, understood as the cellular statement of excess reactive oxygen species (ROS) production, is a main factor in the T2DM development [
35], through promoting IR development. Afterward, high amounts of blood glucose sustained long time causes damage on the enzymes superoxide dismutase (Cu/Zn-SOD), catalase (CAT), and glutathione molecule as the most important elements of the cell antioxidant defense system [
36]. Thus, an excessive ROS production contributes to oxidative stress, a pro-inflammatory state, and mitochondrial dysfunction that in turn exacerbates IR [
37]. It would be necessary a comprehensive knowledge about the relationship between oxidative stress and T2DM risk factors (inflammation and IR) in order to improve diabetes prevention and its associated complications. In this regard, signaling molecules as nitric oxide (NO) play a critical role of the inflammation pathogenesis acting as a pro-inflammatory molecule, together with cytokines and chemokines (e.g., TNFα, IL-6, IL-12), under oxidative stress situations because of the excessive NO and ROS production, i.e., IR [
38], promoting islet β-cell apoptosis [
39] and the progression of diseases concomitant with inflammation [
40].
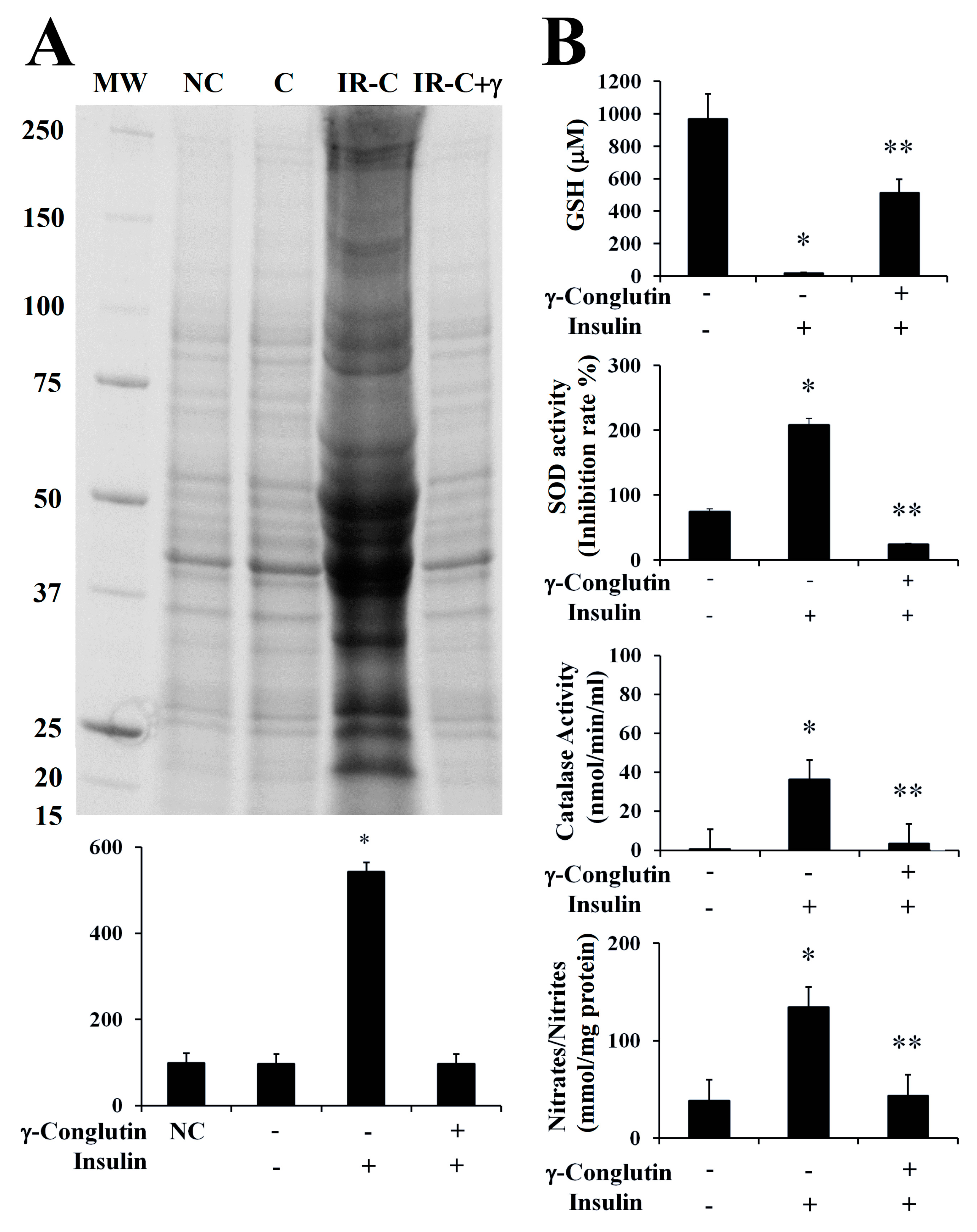
In the present study, we evaluated the oxidative homeostasis in inflammatory LPS-induced PANC-1 cells, as well as in IR-C cell model, after treatment with γ-conglutin protein. In both cases, we assessed the ROS production by measuring the levels of protein carbonylation, the covalent modifications of proteins induced by ROS, i.e., H
2O
2 or other derived molecules from the oxidative stress process by using an OxyBlot protein oxidation detection and immunoassay [
41], and comparing them with control cells, LPS treated cells and IR-C cells, respectively, without any challenge with γ-conglutin. Very low levels of protein oxidation, generated through normal metabolic activity, were observed in untreated (control) cells with LPS (
Supplementary Figure S3A), as well as in control PANC-1 cells before IR-C statement induction (
Figure 6A). However, ROS production was significantly increased (
p < 0.05) after LPS cells treatment (+677-fold,
Supplementary Figure S3A), and in IR-C cells (+445-fold,
Figure 6A), as significant (
p < 0.05) increased levels of proteins carbonylation was detected. Treatments of these type of cells with γ-conglutin protein restored oxidative balance in both situations (LPS-induced cells: −423-fold, and IR-C cells: −445-fold, respectively;
Supplementary Figure S3A,
Figure 6A), in comparison to their respective inflammatory induced stages. These results suggest that γ-conglutin protein efficiently avoid at certain levels the ROS production (oxidative stress) in PANC-1 cells after inflammatory statement incensement, and that γ-conglutin exhibited strong anti-oxidant effect since this protein ameliorated the oxidative stress induced by LPS and in IR-C cell model. Interestingly, the present and future related studies would benefit from the comparative further analyses using other types of cell cultures, like primary islets and/or pancreatic β-cells and/or adipocyte cells to determine actions related to insulin secretion and islet inflammation.
Therefore, removal of free radicals is strongly dependent of enzymatic activities as superoxide dismutase (Cu/Zn-SOD), catalase (CAT) and glutathione (GSH) levels, representing crucial indicators of the cellular anti-oxidant capacity, and the oxidative stress cell state [
35]. In the current study, we assessed the modulation of these antioxidant factors by γ-conglutin in the inflammatory LPS-induced PANC-1 cells, as well as in IR-C cell model, by measuring SOD and catalase activities, GSH levels and NO production, before and after the treatment with γ-conglutin (
Supplementary Figure S3B,
Figure 6B). We found a statistically significant (
p < 0.05) decreased levels of GSH (LPS-induced inflammation cells: −660-fold; IR-C cells: −949-fold, respectively) (
Supplementary Figure S3B,
Figure 6B). Furthermore, the levels of SOD and catalase activity were strongly reduced after the same treatments with γ-conglutin protein in LPS-induced inflammatory statement (SOD: −677-fold; catalase: −142-fold, respectively) (
Supplementary Figure S3B) and IR-C cells (SOD: −183-fold; catalase: −33-fold, respectively) (
Figure 6B). These data showed that high GSH and low SOD levels and catalase activities might be regulated by γ-conglutin protein through direct or indirect marked effects in avoiding lipids and protein oxidative modifications, which is also supported by the concomitant large reduction of oxidative carbonylation (
Supplementary Figure S3B,
Figure 6B), and an overall oxidative stress balance improvement, translated also to an inflammation molecular cellular statement amelioration by γ-conglutin protein as an anti-oxidant protein.
Furthermore, we analyzed the NO production again in both induced inflammation cell models treated with γ-conglutin protein for 24 h. Statistically significant decreased levels of NO were found (
p < 0.05) in the LPS-induced cells (−351-fold,
Supplementary Figure S3B) and IR-C cells (−91-fold,
Figure 6B), in comparison to inflammation induced cells without γ-conglutin protein treatment, showing again how γ-conglutin is able to ameliorate the inflammatory state of cells promoting lowering NO [
42] and iNOS expression levels, showing potential uses in the improvement of T2DM and other inflammatory-based diseases.
These novel results clearly indicated that oxidative stress is a major point targeted by NLL γ-conglutin protein effects causing an improved stress balancing through reduced ROS-related pro-inflammatory mediators and increased anti-oxidative molecules. Indeed, such data can be helpful for the development of future antioxidant and new anti-inflammatory therapeutics avoiding the oxidative stress activation of inflammatory mediators involved in several chronic diseases, with the advantage of being a natural product from lupin seeds that can be implemented as a functional food.