The Therapeutic Effect of Curcumin in Quinolinic Acid-Induced Neurotoxicity in Rats is Associated with BDNF, ERK1/2, Nrf2, and Antioxidant Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
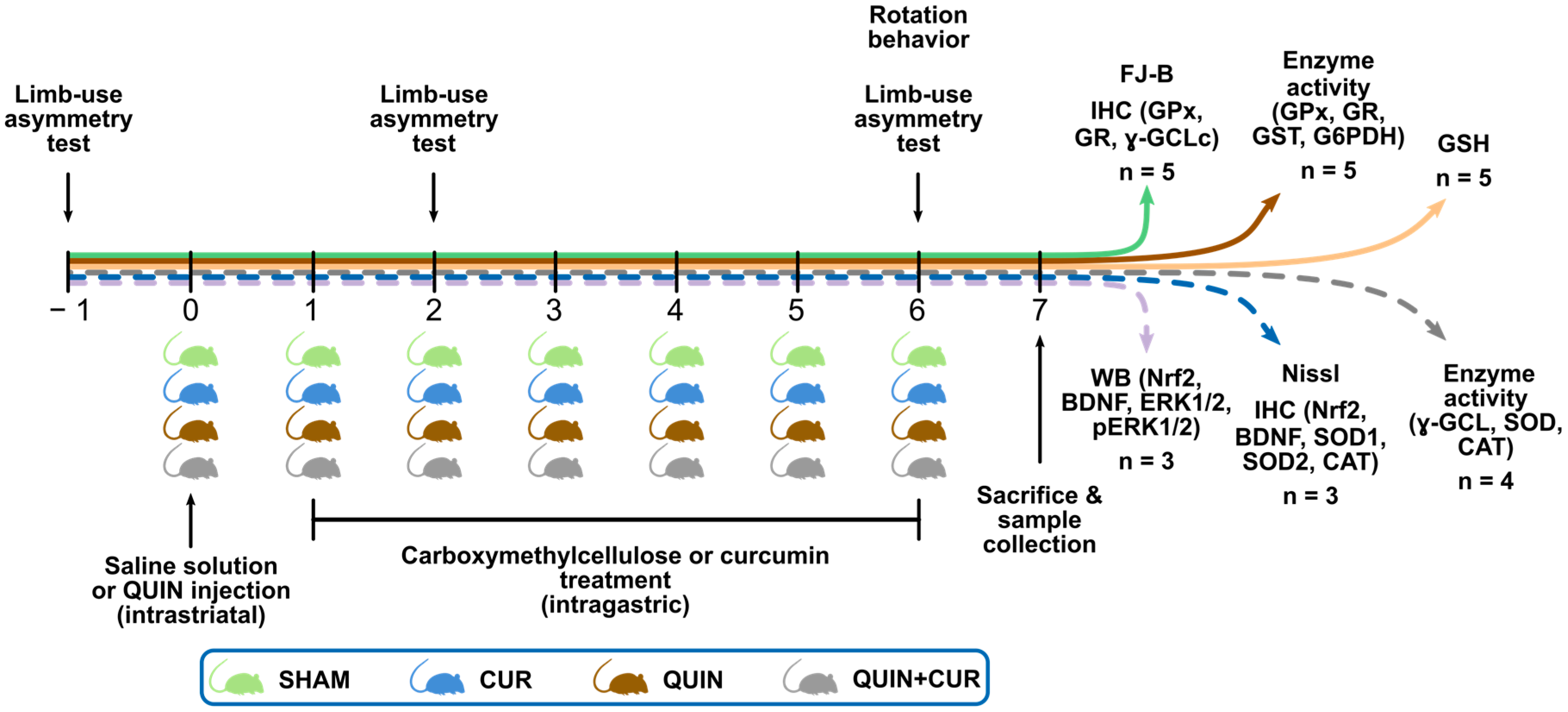
2.3. Experimental Design
2.4. Striatal Lesion
2.5. Motor Assessment
2.5.1. Limb-Use Asymmetry Test
2.5.2. Behavioral Test
2.6. Histological and Immunohistochemical Analyses
2.6.1. Obtaining and Treatment of Samples to Histological Analysis
2.6.2. Nissl Staining
2.6.3. Fluoro-Jade B (FJ-B) Staining
2.6.4. Immunohistochemistry
2.7. Preparation of Total Homogenates Used for Enzyme Activity and Western Blot Assays
2.8. Western Blot Assay
2.9. Activity of Antioxidant Enzymes
2.10. GSH Levels
2.11. Statistical Analysis
3. Results
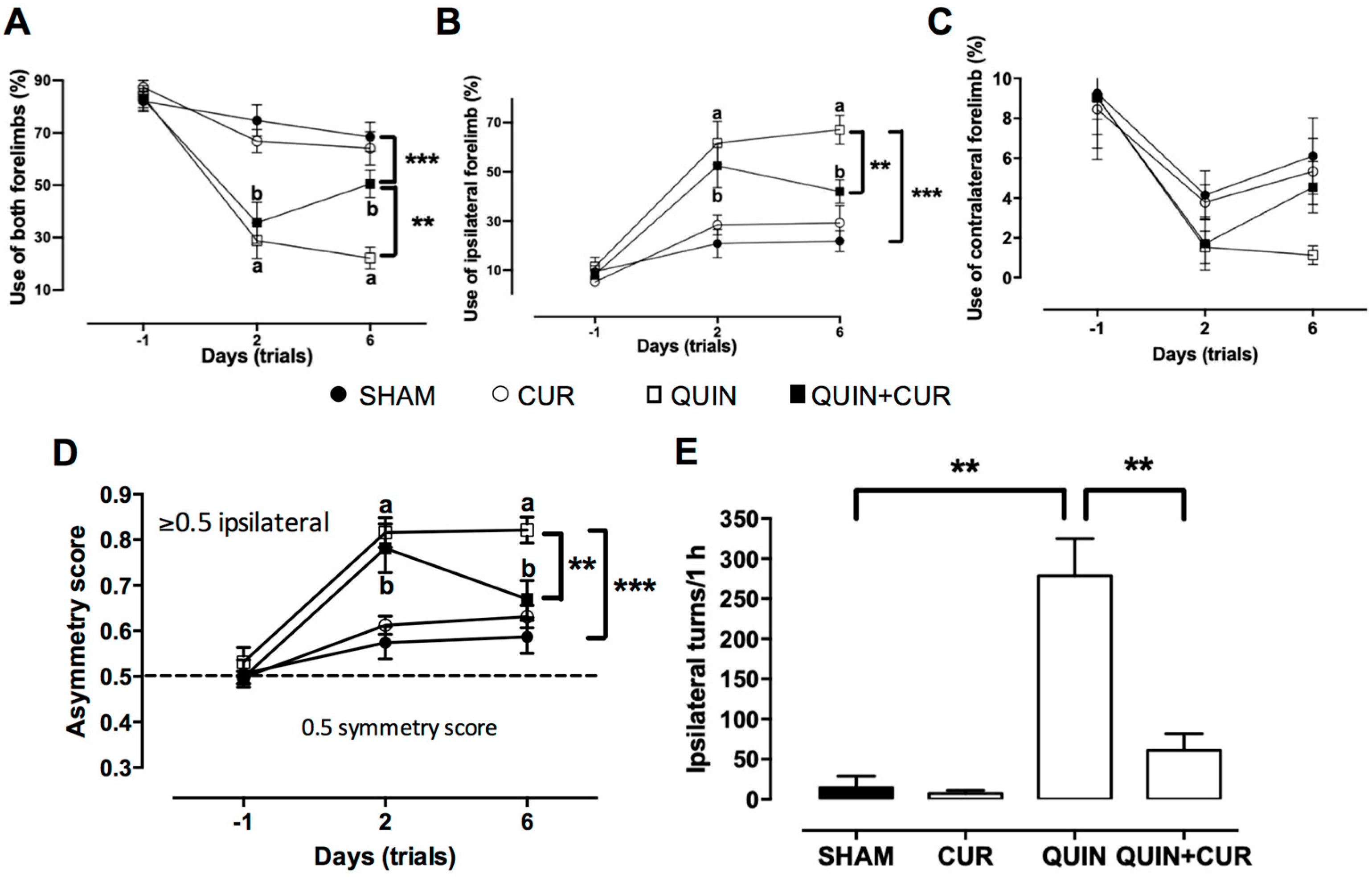
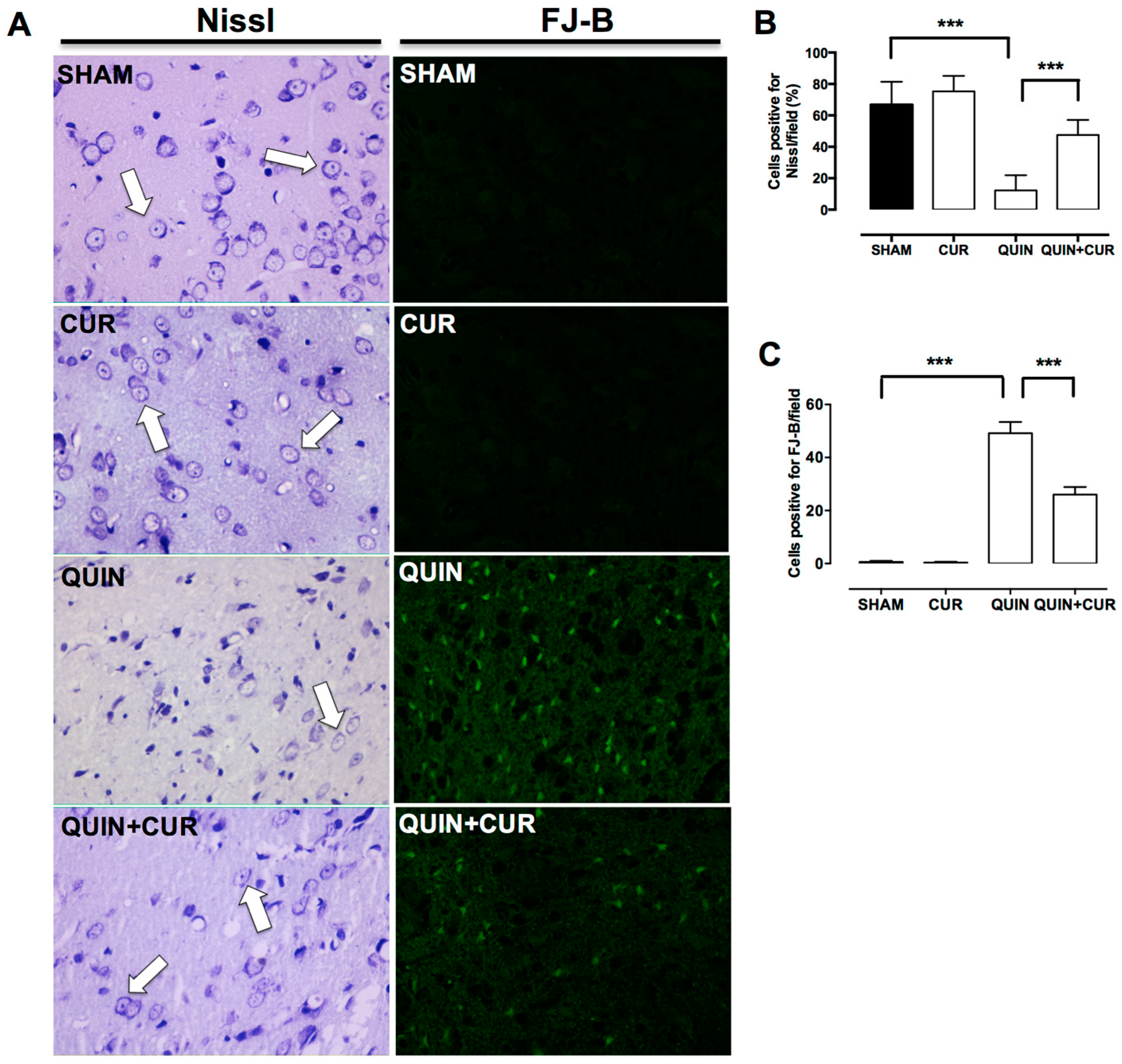
3.1. CUR Improved QUIN-Induced Motor and Histological Impairment
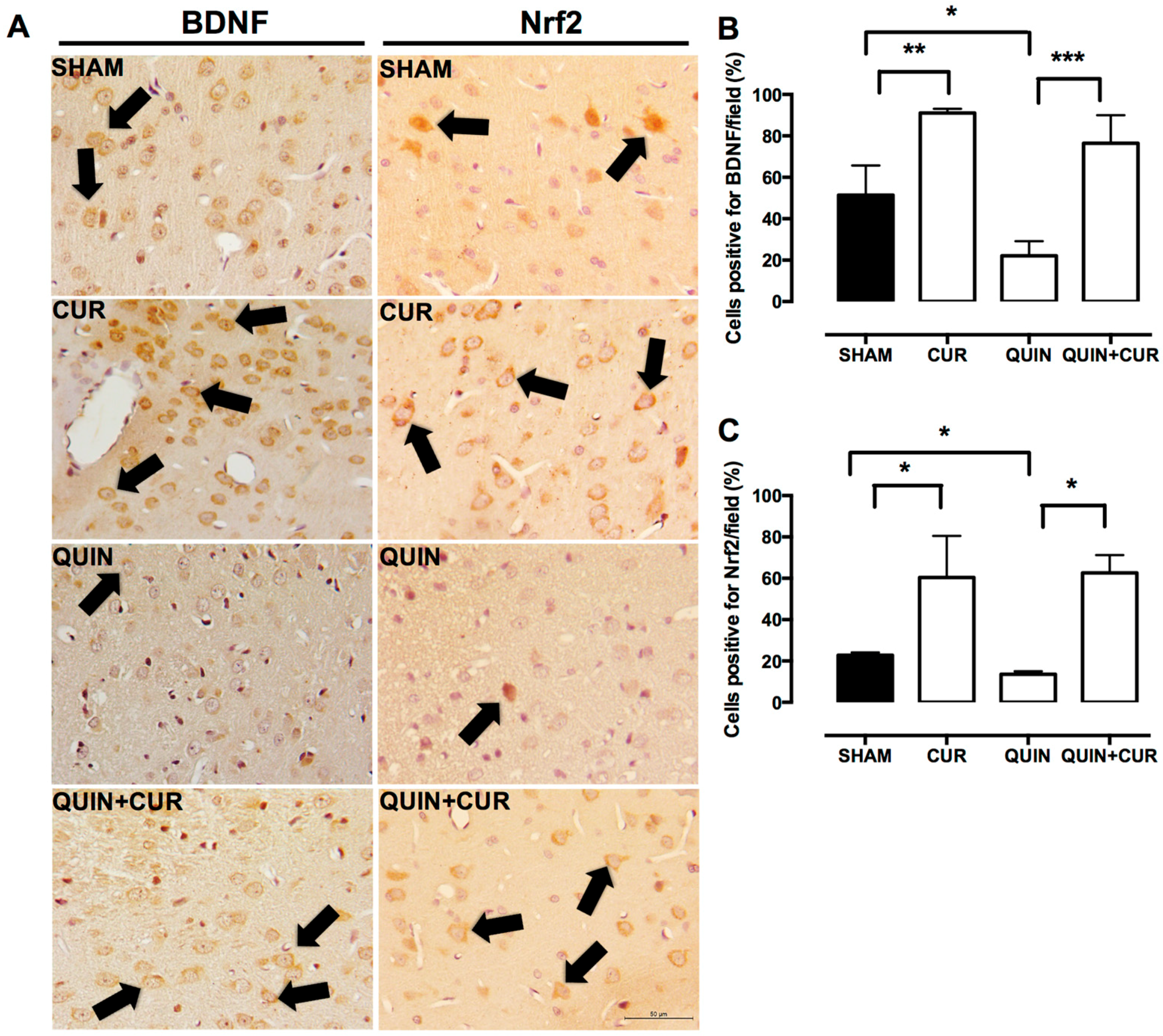
3.2. CUR Increased BDNF, Phospho-ERK1/2, and Nrf2 Levels in the Striatum
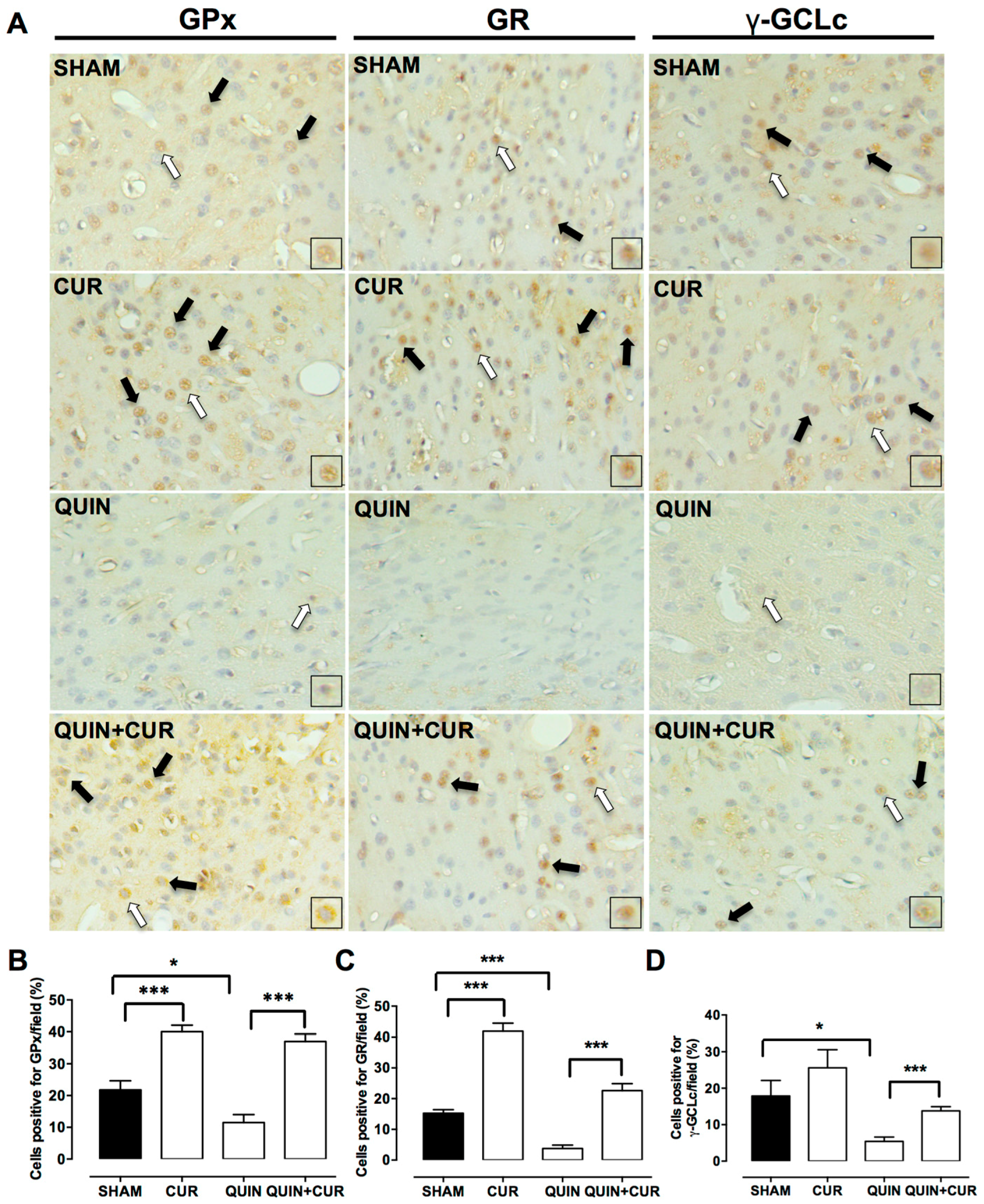
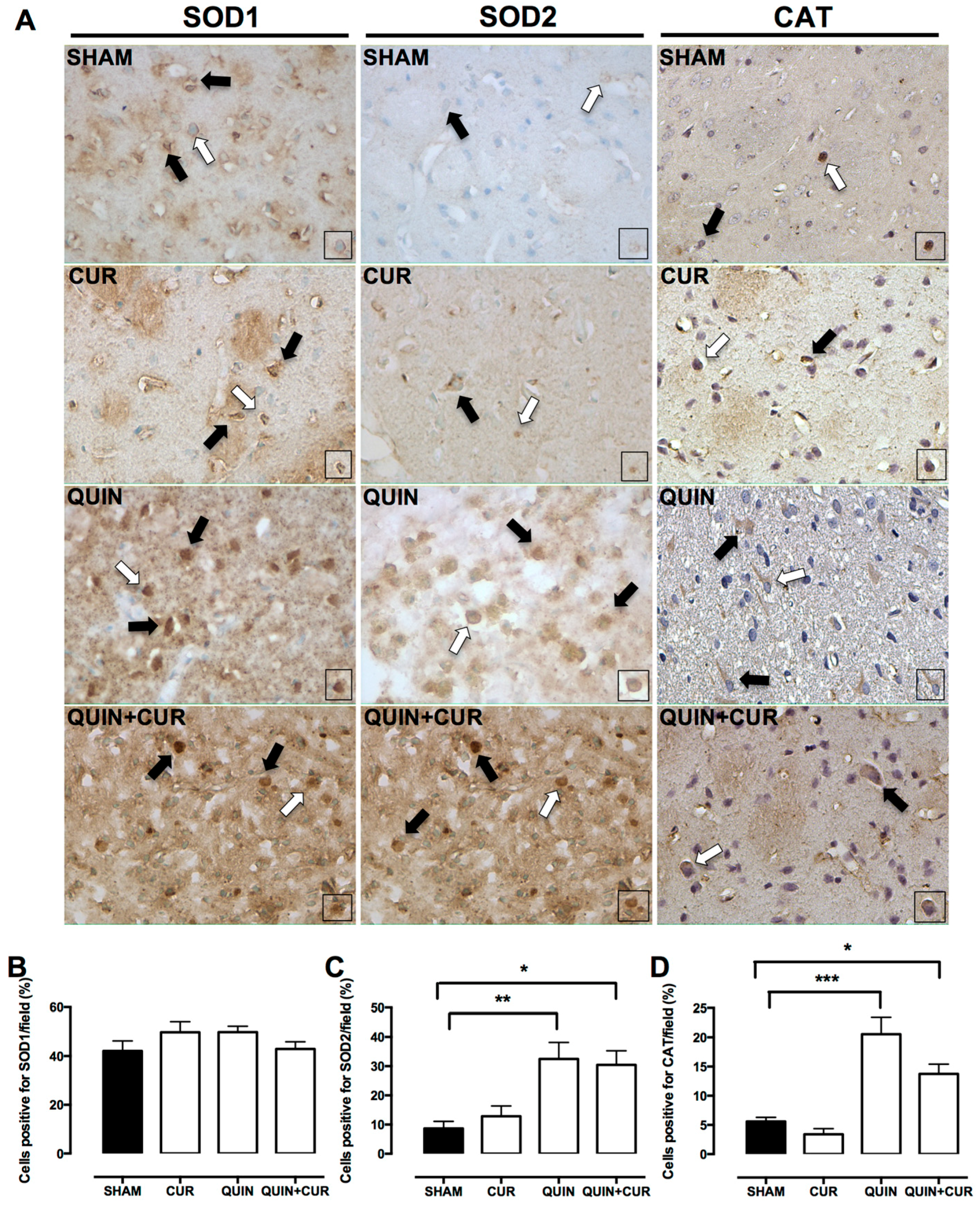
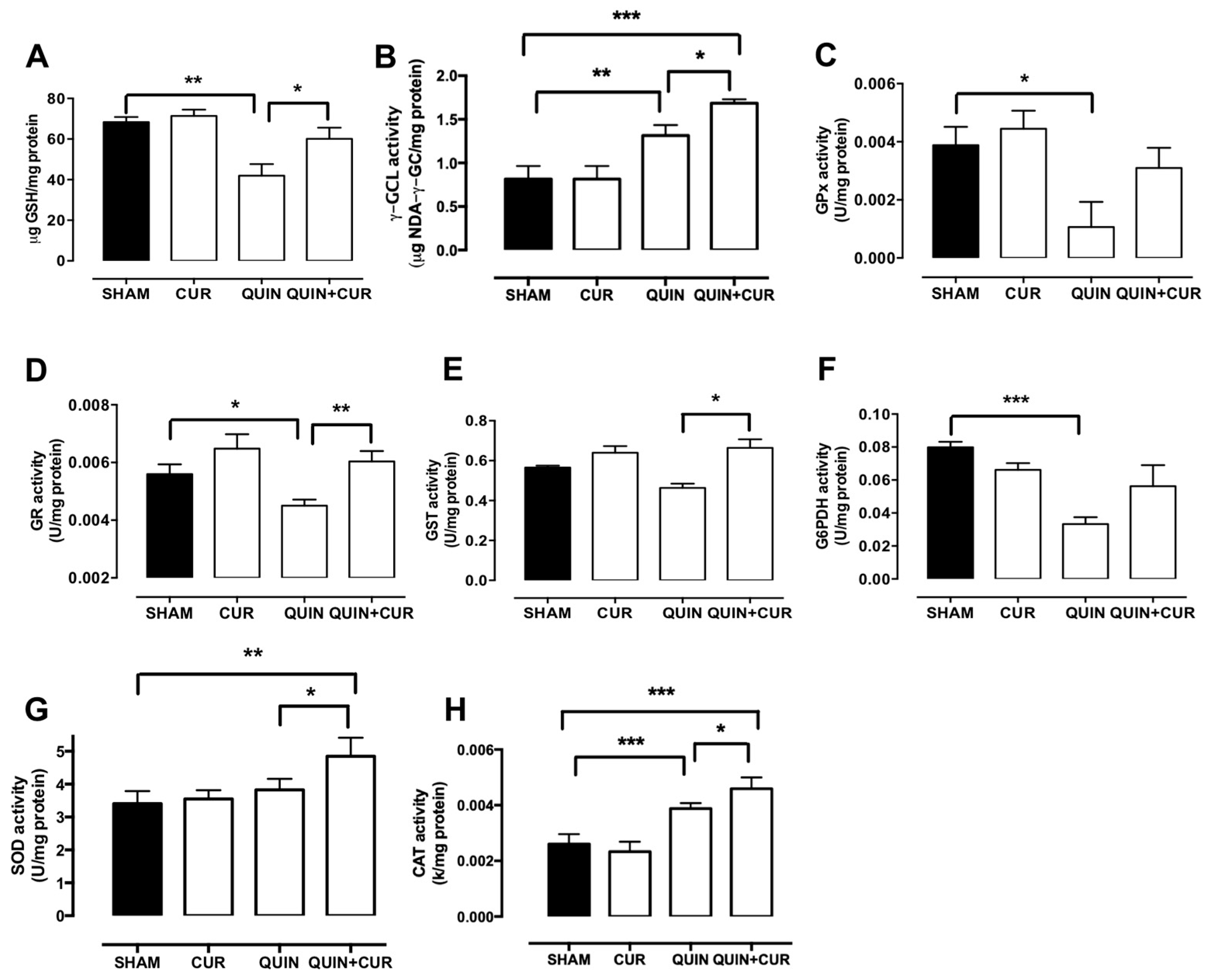
3.3. CUR Enhanced Cellular Antioxidant Capacity by Increasing Levels and Activity of Antioxidant Enzymes and GSH Levels
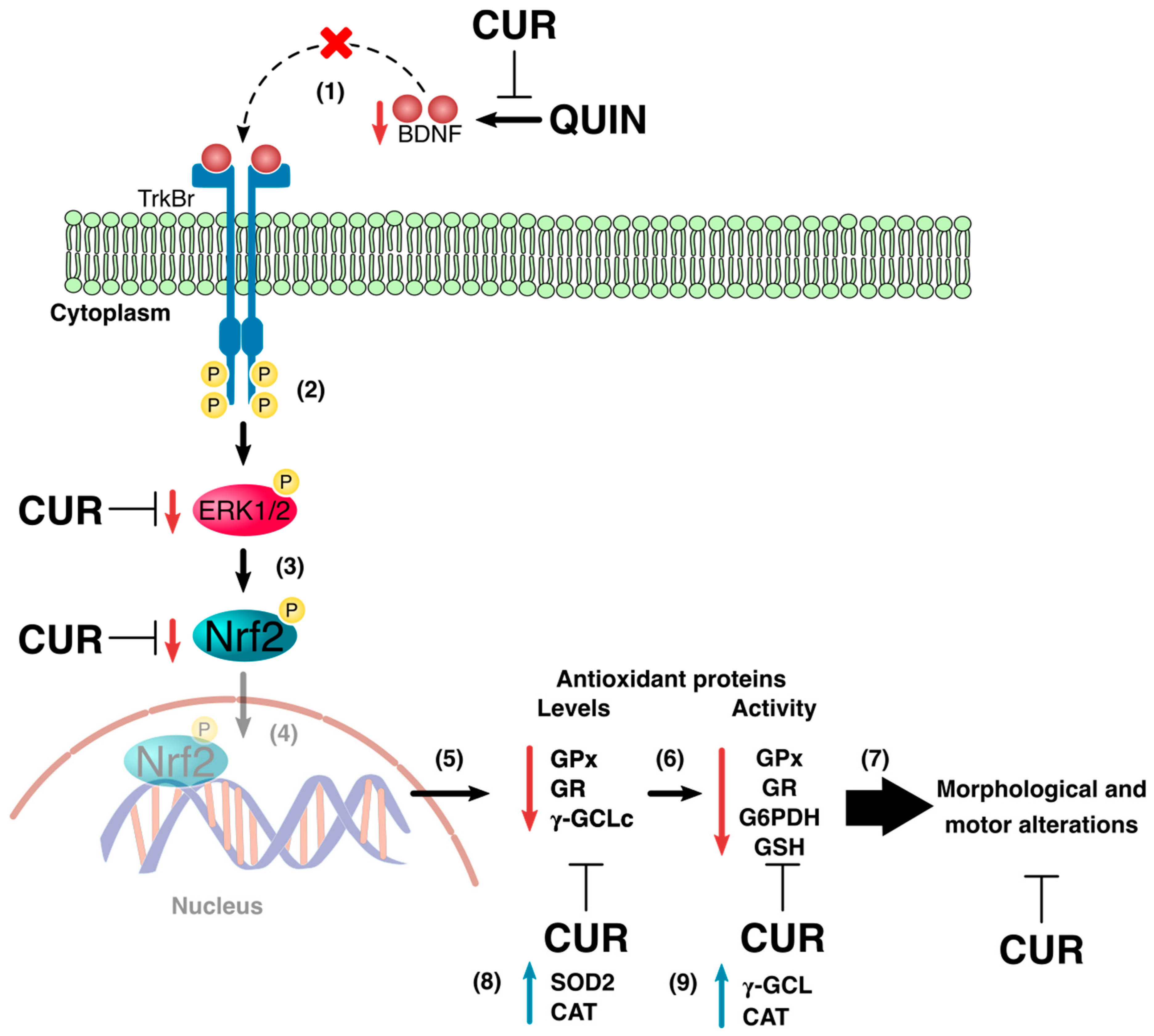
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braidy, N.; Grant, R.; Brew, B.J.; Adams, S.; Jayasena, T.; Guillemin, G.J. Effects of Kynurenine Pathway Metabolites on Intracellular NAD Synthesis and Cell Death in Human Primary Astrocytes and Neurons. Int. J. Tryptophan Res. 2009, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, J.T.; Tan, L. The kynurenine pathway in neurodegenerative diseases: Mechanistic and therapeutic considerations. J. Neurol. Sci. 2012, 323, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Perez-De La Cruz, V.; Santamaria, A. Integrative hypothesis for Huntington’s disease: A brief review of experimental evidence. Physiol. Res. 2007, 56, 513–526. [Google Scholar] [PubMed]
- De Carvalho, L.P.; Bochet, P.; Rossier, J. The endogeneous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem. Int. 1996, 28, 445–452. [Google Scholar] [CrossRef]
- Ghandili, M.; Munakomi, S. Neuroanatomy, Putamen. In StatPearls (Internet); StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542170/ (accessed on 2 September 2019).
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef]
- Maldonado, P.D.; Molina-Jijón, E.; Villeda-Hernández, J.; Galván-Arzate, S.; Santamaría, A.; Pedraza-Chaverrí, J. NAD(P)H oxidase contributes to neurotoxicity in an excitotoxic/prooxidant model of Huntington’s disease in rats: Protective role of apocynin. J. Neurosci. Res. 2010, 88, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, A.; Jiménez-Capdeville, M.E.; Camacho, A.; Rodríguez-Martínez, E.; Flores, A.; Galvan-Arzate, S. In vivo hydroxyl radical formation after quinolinic acid infusion into rat corpus striatum. NeuroReport 2001, 12, 2693–2696. [Google Scholar]
- Gandhi, S.; Abramov, A.Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related Factor-2-dependent Genes Conferring Protection against Oxid. Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [Green Version]
- Holland, R.; Fishbein, J.C. Chemistry of the Cysteine Sensors in Kelch-Like ECH-Associated Protein 1. Antioxid. Redox Signal. 2010, 13, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yuan, X.; Pan, Z.; Shen, G.; Kim, J.H.; Yu, S.; Khor, T.O.; Li, W.; Ma, J.; Kong, A.N. Mechanism of action of isothiocyanates: The induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther. 2006, 5, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Zamanian-Daryoush, M.; Xu, Z.; Kadereit, S.; Williams, B.R. Protein kinase PKR is required for platelet-derived growth factor signaling of c-fos gene expression via Erks and Stat3. EMBO J. 2001, 20, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Peus, D.; Vasa, R.A.; Beyerle, A.; Meves, A.; Krautmacher, C.; Pittelkow, M.R. UVB Activates ERK1/2 and p38 Signaling Pathways via Reactive Oxygen Species in Cultured Keratinocytes. J. Investig. Dermatol. 1999, 112, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eigner, D.; Scholz, D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J. Ethnopharmacol. 1999, 67, 1–6. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, P. Neuroprotective Activity of Curcumin in Combination with Piperine against Quinolinic Acid Induced Neurodegeneration in Rats. Pharmacology 2016, 97, 151–160. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A Potential Role of the Curry Spice Curcumin in Alzheimer’s Disease. Curr. Alzheimer Res. 2005, 2, 131–136. [Google Scholar] [CrossRef]
- Ji, H.F.; Shen, L. The multiple pharmaceutical potential of curcumin in Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2014, 13, 369–373. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer Is A Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- González-Reyes, S.; Guzman-Beltran, S.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Curcumin Pretreatment Induces Nrf2 and an Antioxidant Response and Prevents Hemin-Induced Toxicity in Primary Cultures of Cerebellar Granule Neurons of Rats. Oxid. Med. Cell. Longev. 2013, 2013, 801418. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Drago, F.; Maisto, R.; Romano, G.L.; D’Agata, V.; Maugeri, G.; Giunta, S. Curcumin prevents high glucose damage in retinal pigment epithelial cells through ERK1/2-mediated activation of the Nrf2/HO-1 pathway. J. Cell Physiol. 2019, 234, 17295–17304. [Google Scholar] [CrossRef] [PubMed]
- Na Kim, A.; Jeon, W.-K.; Lee, J.J.; Kim, B.C. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic. Biol. Med. 2010, 49, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Motaghinejad, M.; Motevalian, M.; Fatima, S.; Hashemi, H.; Gholami, M. Curcumin confers neuroprotection against alcohol-induced hippocampal neurodegeneration via CREB-BDNF pathway in rats. Biomed. Pharmacother. 2017, 87, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Dhuriya, Y.K.; Gupta, R.; Shukla, R.K.; Yadav, R.S.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. Protective effect of curcumin by modulating BDNF/DARPP32/CREB in arsenic-induced alterations in dopaminergic signaling in rat corpus striatum. Mol. Neurobiol. 2018, 55, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xu, B. BDNF signaling and survival of striatal neurons. Front. Cell. Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- Soulé, J.; Messaoudi, E.; Bramham, C.; Bramham, C. Brain-derived neurotrophic factor and control of synaptic consolidation in the adult brain. Biochem. Soc. Trans. 2006, 34, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [Green Version]
- Stroppolo, A.; Guinea, B.; Tian, C.; Sommer, J.; Ehrlich, M.E. Role of phosphatidylinositide 3-kinase in brain-derived neurotrophic factor-induced DARPP-32 expression in medium size spiny neurons in vitro. J. Neurochem. 2001, 79, 1027–1032. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press Inc.: San Diego, CA, USA, 1998. [Google Scholar]
- Woodlee, M.T.; Asseo-García, A.M.; Zhao, X.; Liu, S.J.; Jones, T.A.; Schallert, T. Testing forelimb placing? Across the midline? Reveals distinct, lesion-dependent patterns of recovery in rats. Exp. Neurol. 2005, 191, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Santana-Martínez, R.; Galván-Arzate, S.; Hernandez-Pando, R.; Chánez-Cárdenas, M.; Avila-Chavez, E.; López-Acosta, G.; Pedraza-Chaverri, J.; Santamaría, A.; Maldonado, P. Sulforaphane reduces the alterations induced by quinolinic acid: Modulation of glutathione levels. Neuroscience 2014, 272, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Cruz-Álvarez, S.; Santana-Martínez, R.; Avila-Chávez, E.; Barrera-Oviedo, D.; Hernández-Pando, R.; Pedraza-Chaverri, J.; Maldonado, P.D. Apocynin protects against neurological damage induced by quinolinic acid by an increase in glutathione synthesis and Nrf2 levels. Neuroscience 2017, 350, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Glock, G.E.; McLean, P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem. J. 1953, 55, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Zhang, L.Y.; Chen, Y.L.; Yu, S.S.; Zhao, Y.; Zhao, J. Curcumin pretreatment and post-treatment both improve the antiOxid. ability of neurons with oxygen-glucose deprivation. Neural Regen. Res. 2015, 10, 481–489. [Google Scholar]
- Ishii, T.; Warabi, E.; Mann, G.E. Circadian control of p75 neurotrophin receptor leads to alternate activation of Nrf2 and c-Rel to reset energy metabolism in astrocytes via brain-derived neurotrophic factor. Free Radic. Biol. Med. 2018, 119, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wu, Y.; Wang, Y.; Zhu, J.; Chu, H.; Kong, L.; Yin, L.; Ma, H. Brain-Derived Neurotrophic Factor Increases Synaptic Protein Levels via the MAPK/Erk Signaling Pathway and Nrf2/Trx Axis Following the Transplantation of Neural Stem Cells in a Rat Model of Traumatic Brain Injury. Neurochem. Res. 2017, 42, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Massiah, M.A.; Bozak, R.E.; Hicks, R.J.; Talalay, P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA 2001, 98, 3404–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by Curcumin in Ischemic Brain Injury Involves the Akt/Nrf2 Pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Watabe, M.; Nakaki, T. Regulation of neuronal glutathione synthesis. J. Pharmacol. Sci. 2008, 108, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Stridh, M.H.; Correa, F.; Nodin, C.; Weber, S.G.; Blomstrand, F.; Nilsson, M.; Sandberg, M. Enhanced glutathione efflux from astrocytes in culture by low extracellular Ca2+ and curcumin. Neurochem. Res. 2010, 35, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Khanna, V.K.; Khan, M.Y.; Srimal, R.C. Protective effect of curcumin against lead neurotoxicity in rat. Hum. Exp. Toxicol. 2003, 22, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Chen, Y.; Dalton, T.P.; Gysin, R.; Cuénod, M.; Steullet, P.; Do, K.Q. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: Importance of the glutamate cysteine ligase modifier subunit. J. Neurochem. 2009, 108, 1410–1422. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, X.J.; Wang, Z.Q.; Xu, F.S.; Zhu, Y.T. A study on neuroprotective effects of curcumin on the diabetic rat brain. J. Nutr. Health Aging 2016, 20, 835–840. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar]
- Vandresen-Filho, S.; Herculano, B.D.A.; Franco, J.L.; Boeck, C.R.; Dafre, A.L.; Tasca, C.I. Evaluation of glutathione metabolism in NMDA preconditioning against quinolinic acid-induced seizures in mice cerebral cortex and hippocampus. Brain Res. 2007, 1184, 38–45. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Martínez, R.A.; Silva-Islas, C.A.; Fernández-Orihuela, Y.Y.; Barrera-Oviedo, D.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Maldonado, P.D. The Therapeutic Effect of Curcumin in Quinolinic Acid-Induced Neurotoxicity in Rats is Associated with BDNF, ERK1/2, Nrf2, and Antioxidant Enzymes. Antioxidants 2019, 8, 388. https://doi.org/10.3390/antiox8090388
Santana-Martínez RA, Silva-Islas CA, Fernández-Orihuela YY, Barrera-Oviedo D, Pedraza-Chaverri J, Hernández-Pando R, Maldonado PD. The Therapeutic Effect of Curcumin in Quinolinic Acid-Induced Neurotoxicity in Rats is Associated with BDNF, ERK1/2, Nrf2, and Antioxidant Enzymes. Antioxidants. 2019; 8(9):388. https://doi.org/10.3390/antiox8090388
Chicago/Turabian StyleSantana-Martínez, Ricardo A., Carlos A. Silva-Islas, Yessica Y. Fernández-Orihuela, Diana Barrera-Oviedo, José Pedraza-Chaverri, Rogelio Hernández-Pando, and Perla D. Maldonado. 2019. "The Therapeutic Effect of Curcumin in Quinolinic Acid-Induced Neurotoxicity in Rats is Associated with BDNF, ERK1/2, Nrf2, and Antioxidant Enzymes" Antioxidants 8, no. 9: 388. https://doi.org/10.3390/antiox8090388
APA StyleSantana-Martínez, R. A., Silva-Islas, C. A., Fernández-Orihuela, Y. Y., Barrera-Oviedo, D., Pedraza-Chaverri, J., Hernández-Pando, R., & Maldonado, P. D. (2019). The Therapeutic Effect of Curcumin in Quinolinic Acid-Induced Neurotoxicity in Rats is Associated with BDNF, ERK1/2, Nrf2, and Antioxidant Enzymes. Antioxidants, 8(9), 388. https://doi.org/10.3390/antiox8090388








