Relationships Between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Instrumentation
2.4. Chromatographic and UV Conditions
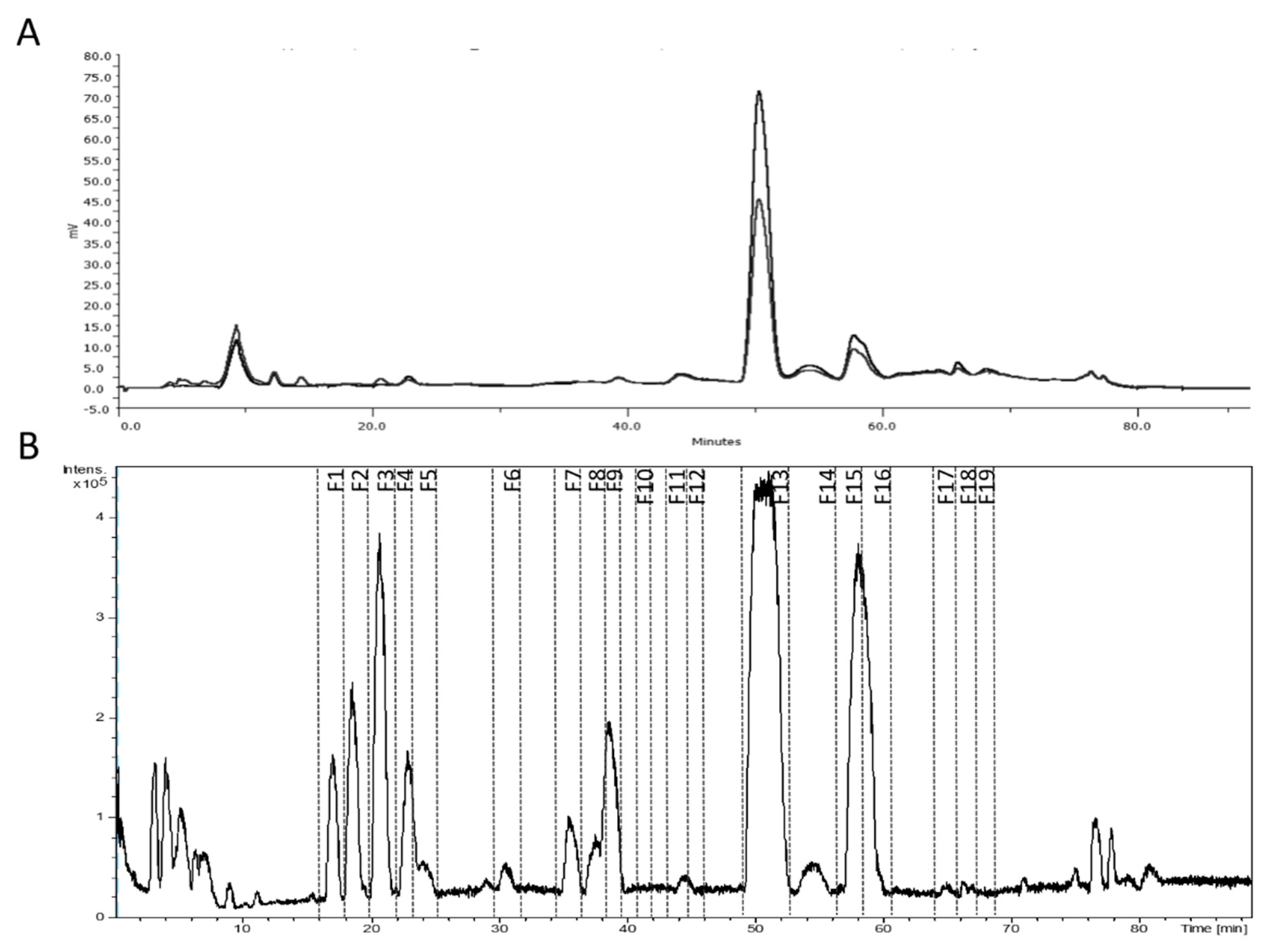
2.5. Fractionation of Phytochemicals
2.6. ESI-TOF-MS Detection
2.7. Antioxidant Activity Assays
2.7.1. Ferric-Reducing Ability Power Assay (FRAP)
2.7.2. Trolox Equivalent Antioxidant Capacity (TEAC)
2.7.3. Oxygen Radical Absorbance Capacity (ORAC)
3. Results and Discussion
3.1. Characterization of A L. citriodora Extract by HPLC-ESI-TOF-MS
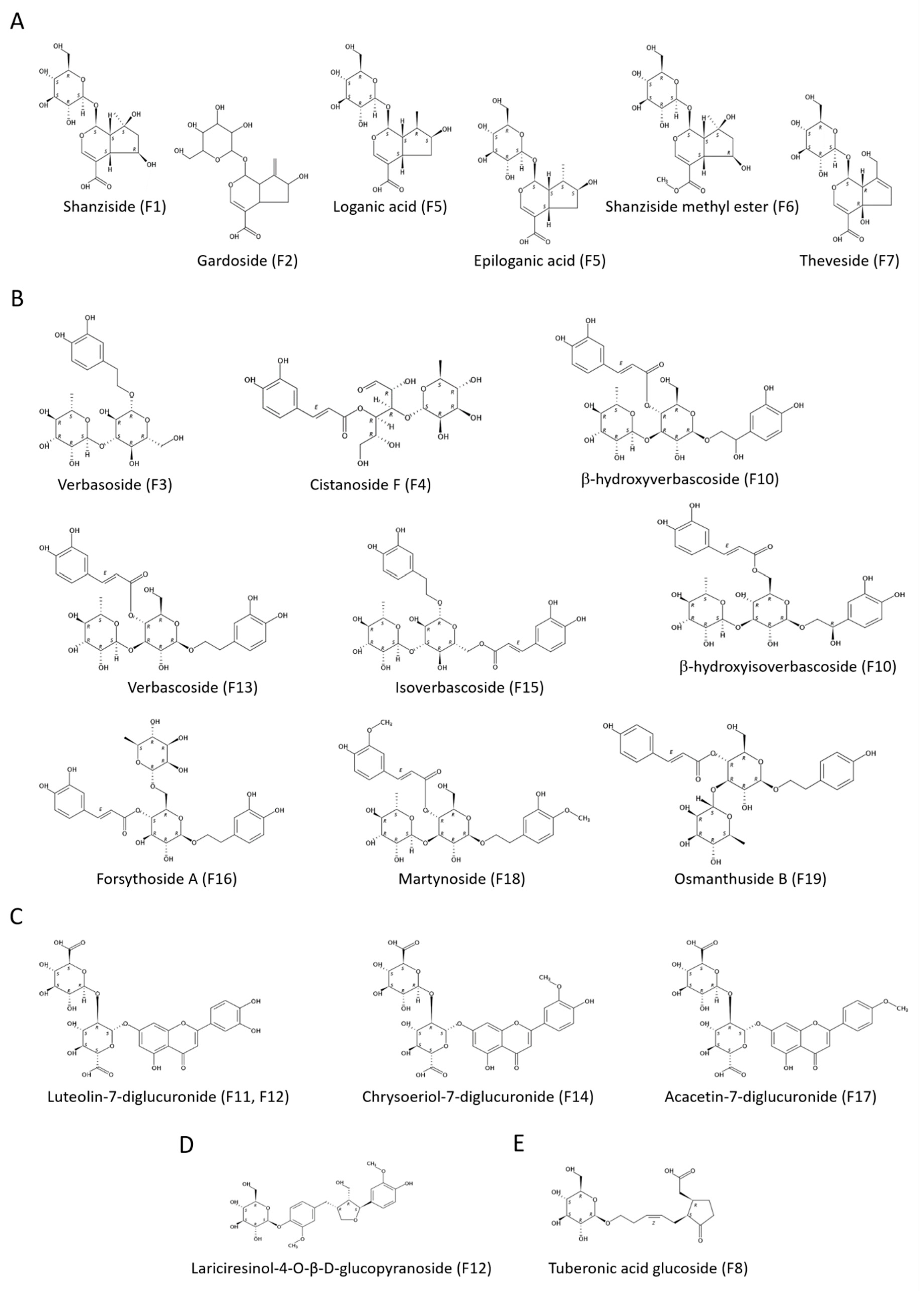
3.1.1. Iridoids
3.1.2. Glycosylated Phenylpropanoids
3.1.3. Flavonoids
3.1.4. Other Compounds
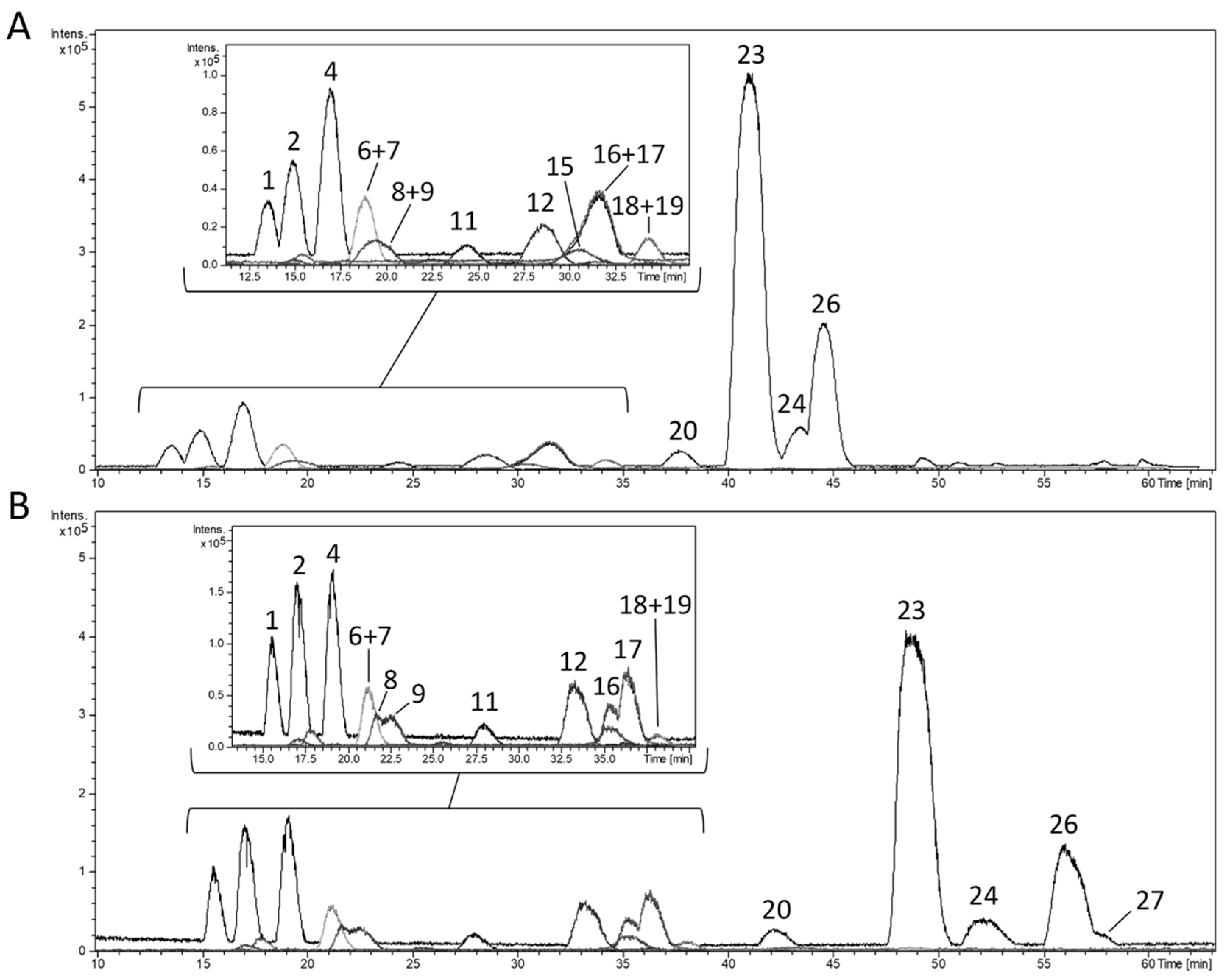
3.2. Fractionation of the Lemon Verbena Extract Using Semi-Preparative Chromatography
3.3. Antioxidant Evaluation and Structure-Activity Relationships (SAR) of the Isolated Compounds
3.3.1. Iridoids
3.3.2. Glycosylated Phenylpropanoids
3.3.3. Flavonoids
3.3.4. Other Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Elechosa, M.A.; Di Leo Lira, P.; Juarez, M.A.; Viturro, C.I.; Heit, C.I.; Molina, A.C.; Martínez, A.J.; Lopez, S.; Molina, A.M.; van Baren, C.M.; et al. Essential oil chemotypes of Aloysia citrodora (Verbenaceae) in Northwestern Argentina. Biochem. Syst. Ecol. 2017, 74, 19–29. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Palau (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, L.; Joubert-Zakeyh, J.; Texier, O.; Lamaison, J.L.; Vasson, M.P.; Felgines, C. Aloysia triphylla infusion protects rats against dextran sulfate sodium-induced colonic damage. J. Sci. Food Agric. 2012, 92, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, A.; Farhang, F.; Vahedi, H.; Dehpour, A.R.; Ejtemai Mehr, S.; Mehrzadi, S.; Rezayat, S.M. Anticonvulsant Effects of Lippia citriodora (Verbenaceae) Leaves Ethanolic Extract in Mice: Role of GABAergic System. Int. J. Prev. Med. 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Alfaro, A.; Ferrer, M.D.; Sureda, A.; Tauler, P.; Martinez, E.; Bibiloni, M.M.; Micol, V.; Tur, J.A.; Pons, A. Phytoestrogens enhance antioxidant enzymes after swimming exercise and modulate sex hormone plasma levels in female swimmers. Eur. J. Appl. Physiol. 2011, 111, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Quintanar, L.; Funes, L.; Vicente-Salar, N.; Blasco-Lafarga, C.; Pons, A.; Micol, V.; Roche, E. Effect of polyphenol supplements on redox status of blood cells: A randomized controlled exercise training trial. Eur. J. Nutr. 2015, 54, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Quintanar, L.; Funes, L.; Viudes, E.; Tur, J.; Micol, V.; Roche, E.; Pons, A. Antioxidant effect of lemon verbena extracts in lymphocytes of university students performing aerobic training program. Scand. J. Med. Sci. Sports 2012, 22, 454–461. [Google Scholar] [CrossRef]
- Funes, L.; Fernández-Arroyo, S.; Laporta, O.; Pons, A.; Roche, E.; Segura-Carretero, A.; Fernández-Gutierrez, A.; Micol, V. Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem. 2009, 117, 589–598. [Google Scholar] [CrossRef]
- Malekirad, A.A.; Hosseini, N.; Bayrami, M.; Hashemi, T.; Rahzani, K.; Abdollahi, M. Benefit of lemon verbena in healthy subjects; targeting diseases associated with oxidative stress. Asian J. Anim. Vet. Adv. 2011, 6, 953–957. [Google Scholar] [CrossRef]
- Mauriz, E.; Vallejo, D.; Tunon, M.J.; Rodriguez-Lopez, J.M.; Rodriguez-Perez, R.; Sanz-Gomez, J.; Garcia-Fernandez Mdel, C. Effects of dietary supplementation with lemon verbena extracts on serum inflammatory markers of multiple sclerosis patients. Nutr. Hosp. 2014, 31, 764–771. [Google Scholar] [CrossRef]
- Campo, G.; Pavasini, R.; Biscaglia, S.; Ferri, A.; Andrenacci, E.; Tebaldi, M.; Ferrari, R. Platelet aggregation values in patients with cardiovascular risk factors are reduced by verbascoside treatment. A randomized study. Pharmacol. Res. 2015, 97, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Caturla, N.; Funes, L.; Perez-Fons, L.; Micol, V. A randomized, double-blinded, placebo-controlled study of the effect of a combination of lemon verbena extract and fish oil omega-3 fatty acid on joint management. J. Altern. Complement. Med. 2011, 17, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Konig, J.; Grune, T. Protein oxidation in aging and the removal of oxidized proteins. J. Proteom. 2013, 92, 132–159. [Google Scholar] [CrossRef]
- Brem, R.; Guven, M.; Karran, P. Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA. Free Radic. Biol. Med. 2017, 107, 101–109. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.O.; Felipe, F.A.; de Melo Costa, A.C.; Teixeira, L.G.; Silva, E.R.; Nunes, P.S.; Shanmugam, S.; de Lucca Junior, W.; Quintans, J.S.; de Souza Araujo, A.A. Inflammatory Mediators and Oxidative Stress in Animals Subjected to Smoke Inhalation: A Systematic Review. Lung 2016, 194, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Lopez, M.; Barrajon-Catalan, E.; Segura-Carretero, A.; Menendez, J.A.; Joven, J.; Micol, V. Lemon verbena (Lippia citriodora) polyphenols alleviate obesity-related disturbances in hypertrophic adipocytes through AMPK-dependent mechanisms. Phytomedicine 2015, 22, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Pine, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernandez-Gutierrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulou, C.; Daferera, D.; Tarantilis, P.A.; Fasseas, C.; Polissiou, M. Chemical composition of the essential oil from leaves of Lippia citriodora H.B.K. (Verbenaceae) at two developmental stages. Biochem. Syst. Ecol. 2007, 35, 831–837. [Google Scholar] [CrossRef]
- Parodi, T.V.; Vargas, A.P.D.C.; Krewer, C.; De Moraes Flores, E.M.; Baldisserotto, B.; Heinzmann, B.M.; De Oliveira, J.V.; Popiolski, A.S.; Minozzo, M. Chemical composition and antibacterial activity of Aloysia triphylla (L’Hérit) Britton extracts obtained by pressurized CO2 extraction. Braz. Arch. Biol. Technol. 2013, 56, 283–292. [Google Scholar] [CrossRef]
- Martino, N.A.; Ariu, F.; Bebbere, D.; Uranio, M.F.; Chirico, A.; Marzano, G.; Sardanelli, A.M.; Cardinali, A.; Minervini, F.; Bogliolo, L.; et al. Supplementation with nanomolar concentrations of verbascoside during in vitro maturation improves embryo development by protecting the oocyte against oxidative stress: A large animal model study. Reprod. Toxicol. 2016, 65, 204–211. [Google Scholar] [CrossRef]
- Mosca, M.; Ambrosone, L.; Semeraro, F.; Casamassima, D.; Vizzarri, F.; Costagliola, C. Ocular tissues and fluids oxidative stress in hares fed on verbascoside supplement. Int. J. Food Sci. Nutr. 2014, 65, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.L.; Lozano-Sanchez, J.; Contreras-Gámez, M.; Legeai-Mallet, L.; Fernández-Arroyo, S.; Segura-Carretero, A. Isolation, comprehensive characterization and antioxidant activities of Theobroma cacao extract. J. Funct. Foods 2014, 10, 485–498. [Google Scholar] [CrossRef]
- Quirantes-Pine, R.; Lozano-Sanchez, J.; Herrero, M.; Ibanez, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A. HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Arraez-Roman, D.; Lozano-Sanchez, J.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phytochemical characterisation of green beans (Phaseolus vulgaris L.) by using high-performance liquid chromatography coupled with time-of-flight mass spectrometry. Phytochem. Anal. 2013, 24, 105–116. [Google Scholar] [CrossRef]
- Morales-Soto, A.; García-Salas, P.; Rodríguez-Pérez, C.; Jiménez-Sánchez, C.; Cádiz-Gurrea, M.L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Antioxidant capacity of 44 cultivars of fruits and vegetables grown in Andalusia (Spain). Food Res. Int. 2014, 58, 35–46. [Google Scholar] [CrossRef]
- Bringmann, G.; Kajahn, I.; Neususs, C.; Pelzing, M.; Laug, S.; Unger, M.; Holzgrabe, U. Analysis of the glucosinolate pattern of Arabidopsis thaliana seeds by capillary zone electrophoresis coupled to electrospray ionization-mass spectrometry. Electrophoresis 2005, 26, 1513–1522. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.L.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Pine, R.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Characterization of phenolic and other polar compounds in a lemon verbena extract by capillary electrophoresis-electrospray ionization-mass spectrometry. J. Sep. Sci. 2010, 33, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Cadiz-Gurrea, M.L.; Olivares-Vicente, M.; Herranz-Lopez, M.; Arraez-Roman, D.; Fernandez-Arroyo, S.; Micol, V.; Segura-Carretero, A. Bioassay-guided purification of Lippia citriodora polyphenols with AMPK modulatory activity. J. Funct. Foods 2018, 46, 514–520. [Google Scholar] [CrossRef]
- Rastrelli, L.; Caceres, A.; Morales, C.; De Simone, F.; Aquino, R. Iridoids from Lippia graveolens. Phytochemistry 1998, 49, 1829–1832. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokol-Letowska, A.; Oszmianski, J.; Piorecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.G.; Lima, M.A.S.; Braz-Filho, R.; Silveira, E.R. Iridoid and phenylethanoid glycosides from Lippia alba. Biochem. Syst. Ecol. 2006, 34, 819–821. [Google Scholar] [CrossRef]
- Leyva-Jimenez, F.J.; Lozano-Sanchez, J.; Borras-Linares, I.; Arraez-Roman, D.; Segura-Carretero, A. Comparative study of conventional and pressurized liquid extraction for recovering bioactive compounds from Lippia citriodora leaves. Food Res. Int. 2018, 109, 213–222. [Google Scholar] [CrossRef]
- Ivanovic, M.; Alanon, M.E.; Arraez-Roman, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Nakamura, T.; Okuyama, E.; Tsukada, A.; Yamazaki, M.; Satake, M.; Nishibe, S.; Nishimura, H. Acteoside as the analgesic principle of Cedron (Lippia triphylla), a Peruvian medicinal plant. Chem. Pharm. Bull. 1997, 45, 499–504. [Google Scholar] [CrossRef]
- Timoteo, P.; Karioti, A.; Leitao, S.G.; Vincieri, F.F.; Bilia, A.R. A validated HPLC method for the analysis of herbal teas from three chemotypes of Brazilian Lippia alba. Food Chem. 2015, 175, 366–373. [Google Scholar] [CrossRef]
- Koïta, K.; Baissac, Y.; Sanon, E.; Campa, C.; Sankara, P. Phenolics from Lippia multiflora Moldenke as potential bioactive agents against peanut pathogens. Agric. Sci. Res. J. 2017, 7, 267–276. [Google Scholar]
- Saidi, I.; Waffo-Teguo, P.; Ayeb-Zakhama, A.E.L.; Harzallah-Skhiri, F.; Marchal, A.; Ben Jannet, H. Phytochemical study of the trunk bark of Citharexylum spinosum L. growing in Tunisia: Isolation and structure elucidation of iridoid glycosides. Phytochemistry 2018, 146, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Friscic, M.; Bucar, F.; Hazler Pilepic, K. LC-PDA-ESI-MS(n) analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spectrom. 2016, 51, 1211–1236. [Google Scholar] [CrossRef] [PubMed]
- Anhalt, S.; Weissenbock, G. Subcellular localization of luteolin glucuronides and related enzymes in rye mesophyll. Planta 1992, 187, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Funari, C.S.; Eugster, P.J.; Martel, S.; Carrupt, P.A.; Wolfender, J.L.; Silva, D.H. High resolution ultra high pressure liquid chromatography-time-of-flight mass spectrometry dereplication strategy for the metabolite profiling of Brazilian Lippia species. J. Chromatogr. A 2012, 1259, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Rungsimakan, S.; Rowan, M.G. Terpenoids, flavonoids and caffeic acid derivatives from Salvia viridis L. cvar. Blue Jeans. Phytochemistry 2014, 108, 177–188. [Google Scholar] [CrossRef]
- Mari, A.; Ciocarlan, A.; Aiello, N.; Scartezzini, F.; Pizza, C.; D’Ambrosio, M. Research survey on iridoid and phenylethanoid glycosides among seven populations of Euphrasia rostkoviana Hayne from the Alps. Phytochemistry 2017, 137, 72–80. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, Y.; Li, M.; Xu, T.; Zhang, L.; Lu, B.; Wu, X.; Ge, Z. Degradation of phenylethanoid glycosides in Osmanthus fragrans Lour. flowers and its effect on anti-hypoxia activity. Sci. Rep. 2017, 7, 10068. [Google Scholar] [CrossRef]
- Marston, A. Role of advances in chromatographic techniques in phytochemistry. Phytochemistry 2007, 68, 2786–2798. [Google Scholar] [CrossRef]
- Ono, M.; Oda, E.; Tanaka, T.; Iida, Y.; Yamasaki, T.; Masuoka, C.; Ikeda, T.; Nohara, T. DPPH radical-scavenging effect on some constituents from the aerial parts of Lippia triphylla. J. Nat. Med. 2008, 62, 101–106. [Google Scholar] [CrossRef]
- Vo, T.N.; Nguyen, P.L.; Tuong, L.T.; Vo, P.N.; Nguyen, K.P.P.; Nguyen, N.S. Constituents of the leaves of Pseuderanthemum carruthersii (Seem.) Guill. var. atropurpureum (Bull.) Fosb. Phytochem. Lett. 2012, 5, 673–676. [Google Scholar] [CrossRef]
- Dou, H.; Liao, X.; Peng, S.; Pan, Y.; Ding, L. Chemical Constituents from the Roots of Schnabelia tetradonta. Helv. Chim. Acta. 2033, 86, 2711–2936. [Google Scholar] [CrossRef]
- Arthur, H.; Joubert, E.; De Beer, D.; Malherbe, C.J.; Witthuhn, R.C. Phenylethanoid glycosides as major antioxidants in Lippia multiflora herbal infusion and their stability during steam pasteurisation of plant material. Food Chem. 2011, 127, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Buchwald-Werner, S.; Naka, I.; Wilhelm, M.; Schutz, E.; Schoen, C.; Reule, C. Effects of lemon verbena extract (Recoverben(R)) supplementation on muscle strength and recovery after exhaustive exercise: A randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Kirmizibekmez, H.; Ariburnu, E.; Masullo, M.; Festa, M.; Capasso, A.; Yesilada, E.; Piacente, S. Iridoid, phenylethanoid and flavonoid glycosides from Sideritis trojana. Fitoterapia 2012, 83, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Sun, Y.; Shu, Y.; Tan, S.; Yin, L.; Guo, Y.; Tang, L. HSCCC Separation of the Two Iridoid Glycosides and Three Phenolic Compounds from Veronica ciliata and Their in Vitro Antioxidant and Anti-Hepatocarcinoma Activities. Molecules 2016, 21, 1234. [Google Scholar] [CrossRef] [PubMed]
- Gousiadou, C.; Gotfredsen, C.H.; Matsa, M.; Hadjipavlou-Litina, D.; Skaltsa, H. Minor iridoids from Scutellaria albida ssp. albida. Inhibitory potencies on lipoxygenase, linoleic acid lipid peroxidation and antioxidant activity of iridoids from Scutellaria sp. J. Enzym. Inhib. Med. Chem. 2013, 28, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; D’Abrosca, B.; Pascarella, M.T.; Letizia, M.; Uzzo, P.; Piscopo, V.; Fiorentino, A. Antioxidant efficacy of iridoid and phenylethanoid glycosides from the medicinal plant Teucrium chamaedris in cell-free systems. Bioorg. Med. Chem. 2009, 17, 6173–6179. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.F.; Landbo, A.K.; Christensen, L.P.; Hansen, A.; Meyer, A.S. Antioxidant effects of phenolic rye (Secale cereale L.) extracts, monomeric hydroxycinnamates, and ferulic acid dehydrodimers on human low-density lipoproteins. J. Agric. Food Chem. 2001, 49, 4090–4096. [Google Scholar] [CrossRef]
- Plaza, A.; Montoro, P.; Benavides, A.; Pizza, C.; Piacente, S. Phenylpropanoid glycosides from Tynanthus panurensis: Characterization and LC-MS quantitative analysis. J. Agric. Food Chem. 2005, 53, 2853–2858. [Google Scholar] [CrossRef]
- Fuji, Y.; Uchida, A.; Fukahori, K.; Chino, M.; Ohtsuki, T.; Matsufuji, H. Chemical characterization and biological activity in young sesame leaves (Sesamum indicum L.) and changes in iridoid and polyphenol content at different growth stages. PLoS ONE 2018, 13, e0194449. [Google Scholar] [CrossRef] [PubMed]
- Allouche, N.; Feki, M.; Damak, M.; Sayadi, S. Isolation of hydroxytyrosol 4-b-D-glucoside and 3,4-dihydroxyphenylglycol with antioxidant activity from olive mill wastewaters. J. Tunis. Chem. Soc. 2005, 7, 2031–2238. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant. Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Bouaziz, M.; Lassoued, S.; Engasser, J.M.; Ghoul, M.; Sayadi, S. Hydroxytyrosol acyl esters: Biosynthesis and activities. Appl. Biochem. Biotechnol. 2011, 163, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Tejero, I.; Gonzalez-Garcia, N.; Gonzalez-Lafont, A.; Lluch, J.M. Tunneling in green tea: Understanding the antioxidant activity of catechol-containing compounds. A variational transition-state theory study. J. Am. Chem. Soc. 2007, 129, 5846–5854. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
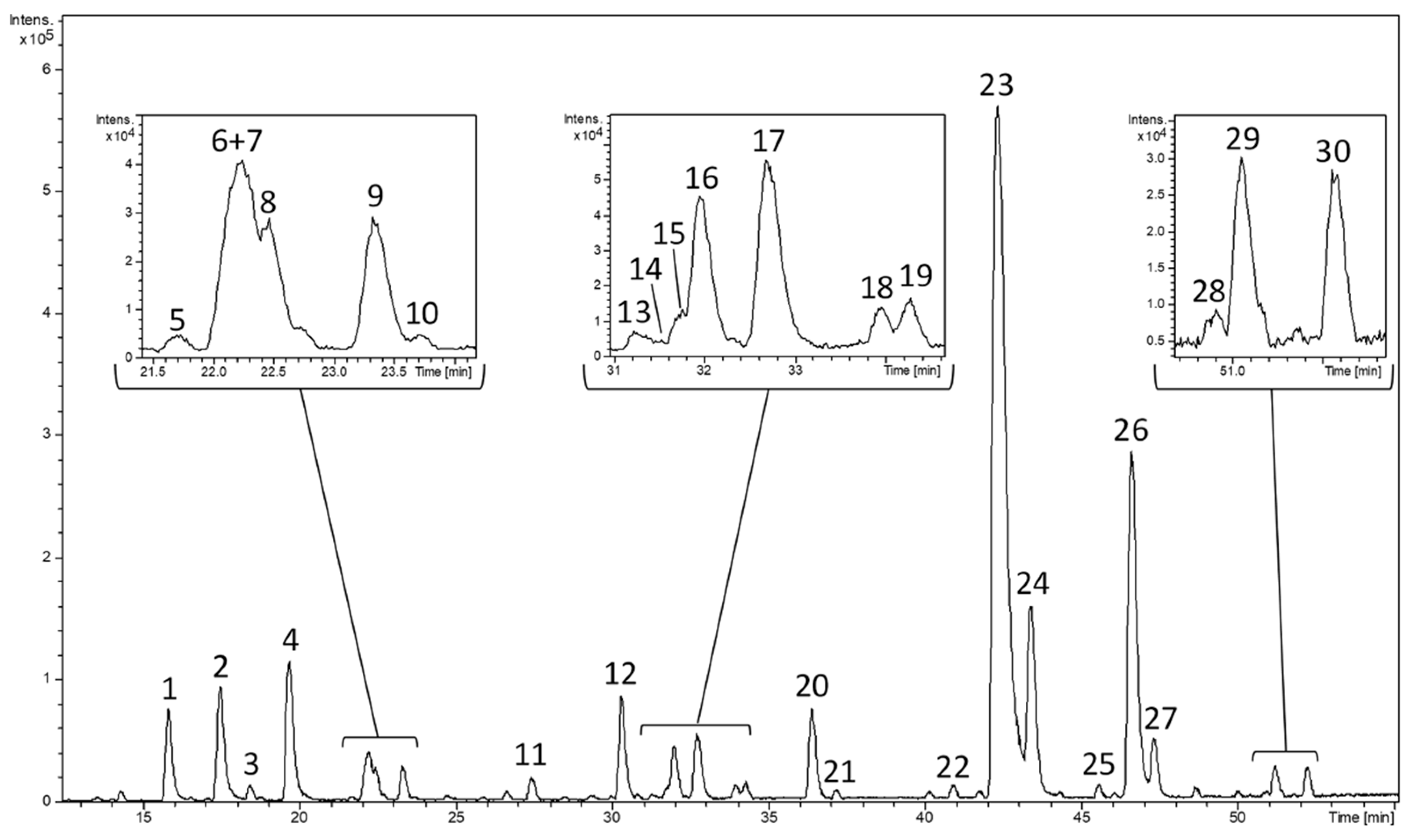

| Peak | RT (min) | [M-H]- Measured | [M-H]- Calculated | Error (ppm) | mSigma | Molecular Formula | Proposed Compound | Matrix |
|---|---|---|---|---|---|---|---|---|
| 1 | 15.85 | 391.1236 | 391.1246 | 2.5 | 1.9 | C 16 H 24 O11 | Shanziside | L. citriodora |
| 2 | 17.51 | 373.1122 | 373.114 | 4.9 | 3.1 | C 16 H 22 O 10 | Gardoside | L. citriodora |
| 3 | 18.46 | 387.0934 | 387.0933 | –0.3 | 5.7 | C 16 H 20 O 11 | Ixoside | L. citriodora |
| 4 | 19.62 | 461.1654 | 461.1664 | 2.3 | 3.2 | C 20 H 30 O 12 | Verbasoside | L. citriodora |
| 5 | 21.72 | 299.1116 | 299.1136 | 6.7 | 3.9 | C 14 H 20 O 7 | Salidroside | S. viridis, E. rostkoviana, O. fragans |
| 6 | 22.14 | 487.1435 | 487.1457 | 4.6 | 1.6 | C 21 H 28 O 13 | Cistanoside F (isomer) | L. citriodora |
| 7 | 22.22 | 487.1433 | 487.1457 | 5 | 4.1 | C 21 H 28 O 13 | Cistanoside F (isomer) | L. citriodora |
| 8 | 22.46 | 375.1285 | 375.1297 | 3 | 4.3 | C 16 H 24 O 10 | Epiloganic acid | L. graveolens |
| 9 | 23.33 | 375.1279 | 375.1297 | 4.8 | 3.9 | C 16 H 24 O 10 | Loganic acid | L. citriodora |
| 10 | 23.71 | 373.1124 | 373.114 | 8.8 | 160.1 | C 16 H 22 O 10 | Secologanic acid | L. graveolens |
| 11 | 27.74 | 405.139 | 405.1402 | 3 | 2.9 | C 17 H 26 O 11 | Shanziside methyl ester | L. citriodora, L. alba |
| 12 | 30.28 | 389.1088 | 389.1089 | 0.3 | 3.1 | C 16 H 22 O 11 | Theveside | L. citriodora |
| 13 | 31.29 | 489.1608 | 489.1614 | 1.1 | 3.3 | C 21 H 30 O 13 | Teucardoside | L. citriodora, T. polium |
| 14 | 31.51 | 387.1654 | 387.1661 | 1.7 | 18.9 | C 18 H 28 O 9 | Tuberonic acid glucoside (isomer) | L. citriodora |
| 15 | 31.76 | 445.2068 | 445.2079 | 2.5 | 24.6 | C 21 H 34 O 10 | Sacranoside A | L. citriodora |
| 16 | 31.96 | 387.1654 | 387.1661 | 1.8 | 3.5 | C 18 H 28 O 9 | Tuberonic acid glucoside (isomer) | L. citriodora |
| 17 | 32.69 | 387.2007 | 387.2024 | 4.6 | 1.4 | C 19 H 32 O 8 | UK | |
| 18 | 33.93 | 639.1928 | 639.1931 | 0.5 | 30.6 | C 29 H 36 O 16 | b-hydroxy-(iso)-verbascoside | L. citriodora |
| 19 | 34.25 | 639.1931 | 639.1931 | 0 | 41.7 | C 29 H 36 O 16 | b-hydroxy-(iso)-verbascoside | L. citriodora |
| 20 | 36.36 | 637.1049 | 637.1046 | –0.5 | 2.1 | C 27 H 26 O 18 | Luteolin-7-diglucuronide | L. citriodora |
| 21 | 37.12 | 521.2016 | 521.2028 | 2.5 | 3.4 | C 26 H 34 O 11 | Lariciresinol-4-O-β-D-glucopyranoside | L. graveolens |
| 22 | 40.85 | 621.1102 | 621.1097 | –0.8 | 7 | C 27 H 26 O 17 | Apigenin-7-diglucuronide | L. citriodora |
| 23 | 42.24 | 623.1999 | 623.1981 | –2.9 | 3 | C 29 H 36 O 15 | Verbascoside | L. citriodora |
| 24 | 43.33 | 651.1228 | 651.1203 | –3.9 | 4.2 | C 28 H 28 O 18 | Chrysoeriol-7-diglucuronide | L. citriodora |
| 25 | 45.29 | 621.1827 | 621.1766 | –9.9 | 83.4 | C 36 H 30 O 10 | Diooflavone | L. citriodora |
| 26 | 46.54 | 623.1988 | 623.1981 | –1 | 3.3 | C 29 H 36 O 15 | Isoverbascoside | L. citriodora |
| 27 | 47.24 | 637.215 | 637.2138 | –2 | 5.2 | C 30 H 38 O 15 | Leucosceptoside A | L. citriodora, L. alba, L. multiflora |
| 28 | 50.8 | 637.2142 | 637.2138 | –0.6 | 4.8 | C 30 H 38 O 15 | Isoleucosceptoside A | C. spinosum, Globularia spp. |
| 29 | 51.1 | 635.1271 | 635.1254 | –2.7 | 6.1 | C 28 H 28 O 17 | Acacetin-7-diglucuronide | L. citriodora |
| 30 | 54.63 | 651.2299 | 651.2294 | –0.7 | 3.8 | C 31 H 40 O 15 | Martynoside | L. citriodora |
| Fraction | Peak(s) | Major Compound(s) | Residue Weight (mg) | Relative Amount (%) |
|---|---|---|---|---|
| F1 | 1 | Shanziside | 0.7 | 2.8 |
| F2 | 2, 3 | Gardoside | 1.0 | 4.0 |
| F3 | 4 | Verbasoside | 0.9 | 3.6 |
| F4 | 5, 6, 7, 8 | Cistanoside F isomers | 0.5 | 2.0 |
| F5 | 8, 9, 10 | Loganic and epiloganic acids | 0.9 | 3.6 |
| F6 | 11 | Shanziside methyl ester | 0.6 | 2.4 |
| F7 | 12 | Theveside | 0.6 | 2.4 |
| F8 | 13, 14, 15, 16, 17 | Tuberonic acid glucoside + UK | 0.6 | 2.4 |
| F9 | 17, 18, 19 | UK | 0.4 | 1.6 |
| F10 | 17, 18, 19 | β-hydroxy(iso)verbascoside | 0.4 | 1.6 |
| F11 | 20 | Luteolin-7-diglucuronide | 0.5 | 2.0 |
| F12 | 20, 21 | Luteolin-7-diglucuronide | 0.2 | 0.8 |
| F13 | 22, 23, 26, A | Verbascoside | 2.3 | 9.2 |
| F14 | 24, 25 | Chrysoeriol-7-diglucuronide | 0.9 | 3.6 |
| F15 | 26, A | Isoverbascoside | 1.1 | 4.4 |
| F16 | A, 27, 28 | Forsythoside A | 0.7 | 2.8 |
| F17 | 29 | Acacetin-7-diglucuronide | 0.4 | 1.6 |
| F18 | 30, B | Martynoside | 0.7 | 2.8 |
| F19 | B, C | Osmanthuside B | 0.3 | 1.2 |
| Peak | RT (min) | [M-H]- Measured | [M-H]- Calculated | Error (ppm) | mSigma | Molecular Formula | Proposed Compound | Reference | Matrix |
|---|---|---|---|---|---|---|---|---|---|
| A | 49.84 | 623.1986 | 623.1981 | –0.7 | 4.3 | C 29 H 35 O 15 | Forsythoside A | [17,38] | L. citriodora |
| B | 56.59 | 651.2292 | 651.2294 | 0.3 | 5.1 | C 31 H 40 O 15 | Isomartynoside | [50,51,52] | L. citriodora, P. carruthersii, S. tetradonta |
| C | 58.03 | 591.1972 | 591.2083 | 18.9 | 5.6 | C 29 H 36 O 13 | Osmanthuside B | [37] | L. citriodora, C. tubulosa |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Marzo, N.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.d.l.L.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships Between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena. Antioxidants 2019, 8, 324. https://doi.org/10.3390/antiox8080324
Sánchez-Marzo N, Lozano-Sánchez J, Cádiz-Gurrea MdlL, Herranz-López M, Micol V, Segura-Carretero A. Relationships Between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena. Antioxidants. 2019; 8(8):324. https://doi.org/10.3390/antiox8080324
Chicago/Turabian StyleSánchez-Marzo, Noelia, Jesús Lozano-Sánchez, María de la Luz Cádiz-Gurrea, María Herranz-López, Vicente Micol, and Antonio Segura-Carretero. 2019. "Relationships Between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena" Antioxidants 8, no. 8: 324. https://doi.org/10.3390/antiox8080324
APA StyleSánchez-Marzo, N., Lozano-Sánchez, J., Cádiz-Gurrea, M. d. l. L., Herranz-López, M., Micol, V., & Segura-Carretero, A. (2019). Relationships Between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena. Antioxidants, 8(8), 324. https://doi.org/10.3390/antiox8080324










