Bioactive Amines Screening in Four Genotypes of Thermally Processed Cauliflower
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Biogenic Amine Analysis by HPLC
2.3. Chemical Quality Index—CQI
2.4. Statistical Analysis
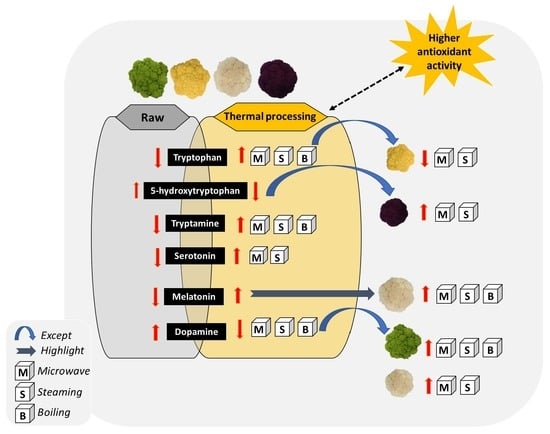
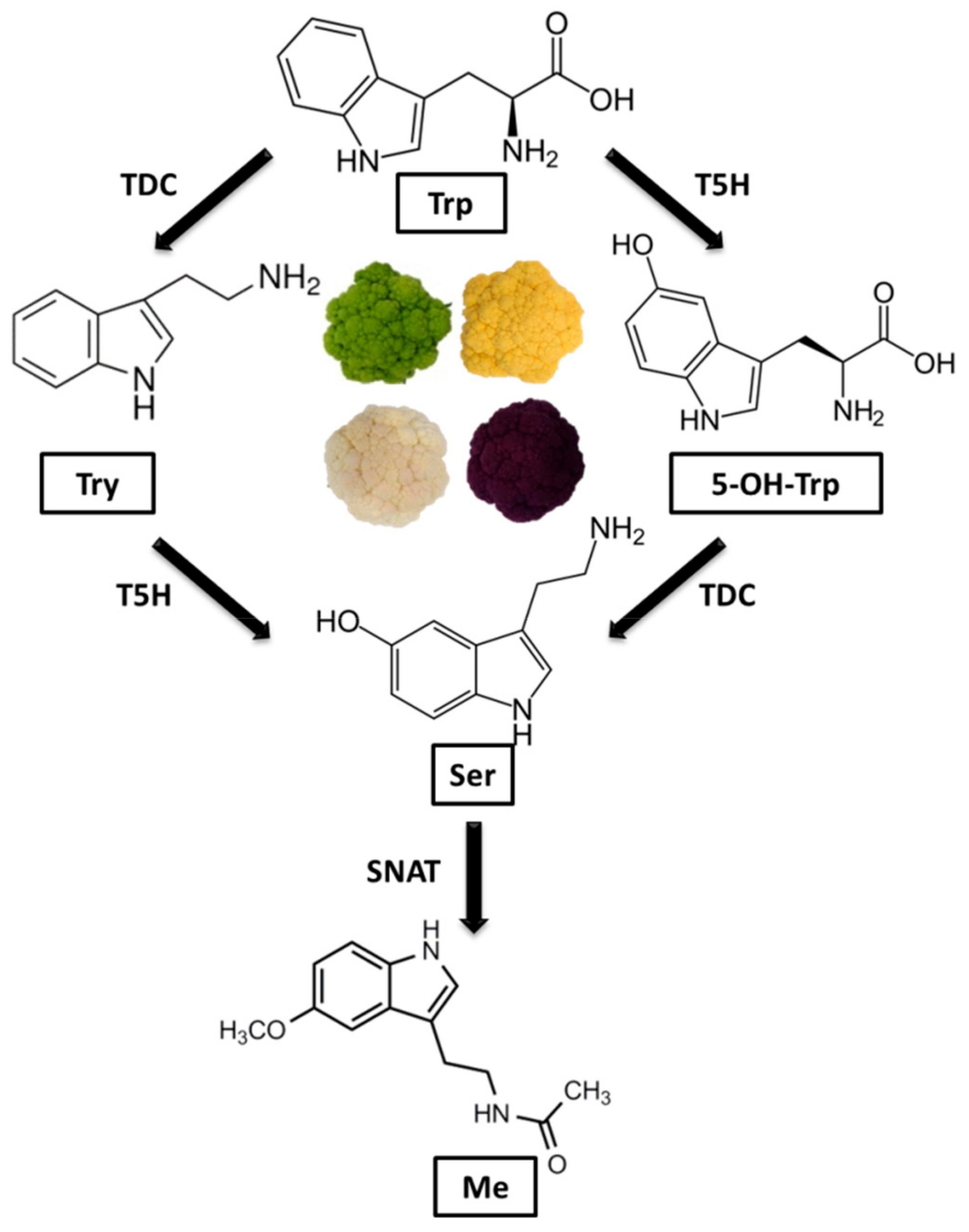
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Strasser, B.; Gostner, J.M.; Fuchs, D. Mood, food, and cognition: Role of tryptophan and serotonin. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food-existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [PubMed]
- Płonka, J.; Michalski, A. The influence of processing technique on the catecholamine and indolamine contents of fruits. J. Food Compos. Anal. 2017, 57, 102–108. [Google Scholar] [CrossRef]
- Borges, C.V.; Belin, M.A.F.; Amorim, E.P.; Minatel, I.O.; Monteiro, G.C.; Gomes, H.A.G.; Monar, G.R.S.; Lima, G.P.P. Bioactive amines changes during the ripening and thermal processes of bananas and plantains. Food Chem. 2019, 298, 125020. [Google Scholar] [CrossRef] [PubMed]
- Pelagio-Flores, R.; Ortíz-Castro, R.; Méndez-Bravo, A.; MacÍas-Rodríguez, L.; López-Bucio, J. Serotonin, a tryptophan-derived signal conserved in plants and animals, regulates root system architecture probably acting as a natural auxin inhibitor in arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 490–508. [Google Scholar] [CrossRef] [PubMed]
- Erland, L.A.E.; Turi, C.E.; Saxena, P.K. Serotonin: An ancient molecule and an important regulator of plant processes. Biotechnol. Adv. 2016, 34, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Corrigendum: Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 7, 1–2. [Google Scholar] [CrossRef]
- Kang, S.; Kang, K.; Lee, K.; Back, K. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 2007, 227, 263–272. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.; Kim, Y.S.; Back, K. Tryptamine 5-hydroxylase-deficient Sekiguchi rice induces synthesis of 5-hydroxytryptophan and N-acetyltryptamine but decreases melatonin biosynthesis during senescence process of detached leaves. J. Pineal Res. 2012, 52, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Normanly, J. Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harb. Perspect. Biol. 2010, 2, a001594. [Google Scholar] [CrossRef] [PubMed]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan biochemistry: Structural, nutritional, metabolic, and medical aspects in humans. J. Amino Acids 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, D.; Troost, F.J.; Masclee, A.A.M. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil. 2009, 21, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, H.A.; Borges, C.V.; Minatel, I.O.; Luvizon, A.C.; Lima, G.P.P. Health benefits of dietary phenolic compounds and biogenic amines. In Bioactive Molecules in Food, 1st ed.; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer Nature: Basel, Switzerland, 2018; pp. 1–25. [Google Scholar]
- Kulma, A.; Szopa, J. Catecholamines are active compounds in plants. Plant Sci. 2007, 172, 433–440. [Google Scholar] [CrossRef]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 89–95. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Preti, R.; Rapa, M.; Vinci, G. Effect of steaming and boiling on the antioxidant properties and biogenic amines content in green bean (Phaseolus vulgaris) varieties of different colours. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Mietz, J.L.; Karmas, E. Chemical quality index of canned tuna as dtermined by high-pressure liquid chromatography. J. Food Sci. 1977, 42, 155–158. [Google Scholar] [CrossRef]
- Badria, F.A. Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J. Med. Food 2002, 5, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Shirakawa, H.; Nguyen, T.K.; Aso, H.; Komai, M. Simultaneous analysis of serotonin, tryptophan and tryptamine levels in common fresh fruits and vegetables in Japan using fluorescence HPLC. Food Biosci. 2016, 13, 56–59. [Google Scholar] [CrossRef]
- Kalisz, A.; Sękara, A.; Smoleń, S.; Grabowska, A.; Gil, J.; Cebula, S. Mineral composition of cauliflowers with differently coloured curds modified by the chilling of juvenile plants. Sci. Hortic. (Amst.) 2018, 232, 216–225. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Costa, S.M.; de Monaco, K.A.; Uliana, M.R.; Fernandez, R.M.; Correa, C.R.; Vianello, F.; Cisneros-Zevallos, L.; Minatel, I.O. Cooking processes increase bioactive compounds in organic and conventional green beans. Int. J. Food Sci. Nutr. 2017, 7486, 1–12. [Google Scholar]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Borges, C.V.; Minatel, I.O.; Amorim, E.P.; Belin, M.A.F.; Gomez-Gomez, H.A.; Correa, C.R.; Lima, G.P.P. Ripening and cooking processes influence the carotenoid content in bananas and plantains (Musa spp.). Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Lima, G.P.P.; da Rocha, S.A.; Takaki, M.; Ramos, P.R.R.; Ono, E.O. Comparison of polyamine, phenol and flavonoid contents in plants grown under conventional and organic methods. Int. J. Food Sci. Technol. 2008, 43, 1838–1843. [Google Scholar] [CrossRef]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J.; et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 133, 164–176. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Protein and Amino acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Technical Report Series 935; WHO: Geneva, Switzerland, 2002; Available online: https://apps.who.int/iris/handle/10665/43411 (accessed on 29 July 2019).
- González-Montelongo, R.; Gloria Lobo, M.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Kanazawa, K.; Sakakibara, H. High content of dopamine, a strong antioxidant, in cavendish banana. J. Agric. Food Chem. 2000, 48, 844–848. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Fish and Fishery Products Hazards and Controls Guidance; U.S. Department of Health and Human Services: Washington, DC, USA. Available online: https://www.fda.gov/media/80637/download (accessed on 29 July 2019).
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. TrAC Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef] [Green Version]
- Karovicová, J.; Kohajdová, Z. Biogenic amines in foods. Chem. Pap. 2005, 59, 70–79. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Saaid, M.; Saad, B.; Rahman, I.A.; Ali, A.S.M.; Saleh, M.I. Extraction of biogenic amines using sorbent materials containing immobilized crown ethers. Talanta 2010, 80, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kalac̆, P.; Krausová, P. A review of dietary polyamines: Formation, implications for growth and health and occurrence in foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine contents in current foods: A basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids 2007, 33, 203–212. [Google Scholar] [CrossRef]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef]
- Magwamba, C.; Matsheka, M.I.; Mpuchane, S.; Gashe, B.A. Detection and quantification of biogenic amines in fermented food products sold in Botswana. J. Food Prot. 2010, 73, 1703–1708. [Google Scholar] [CrossRef]
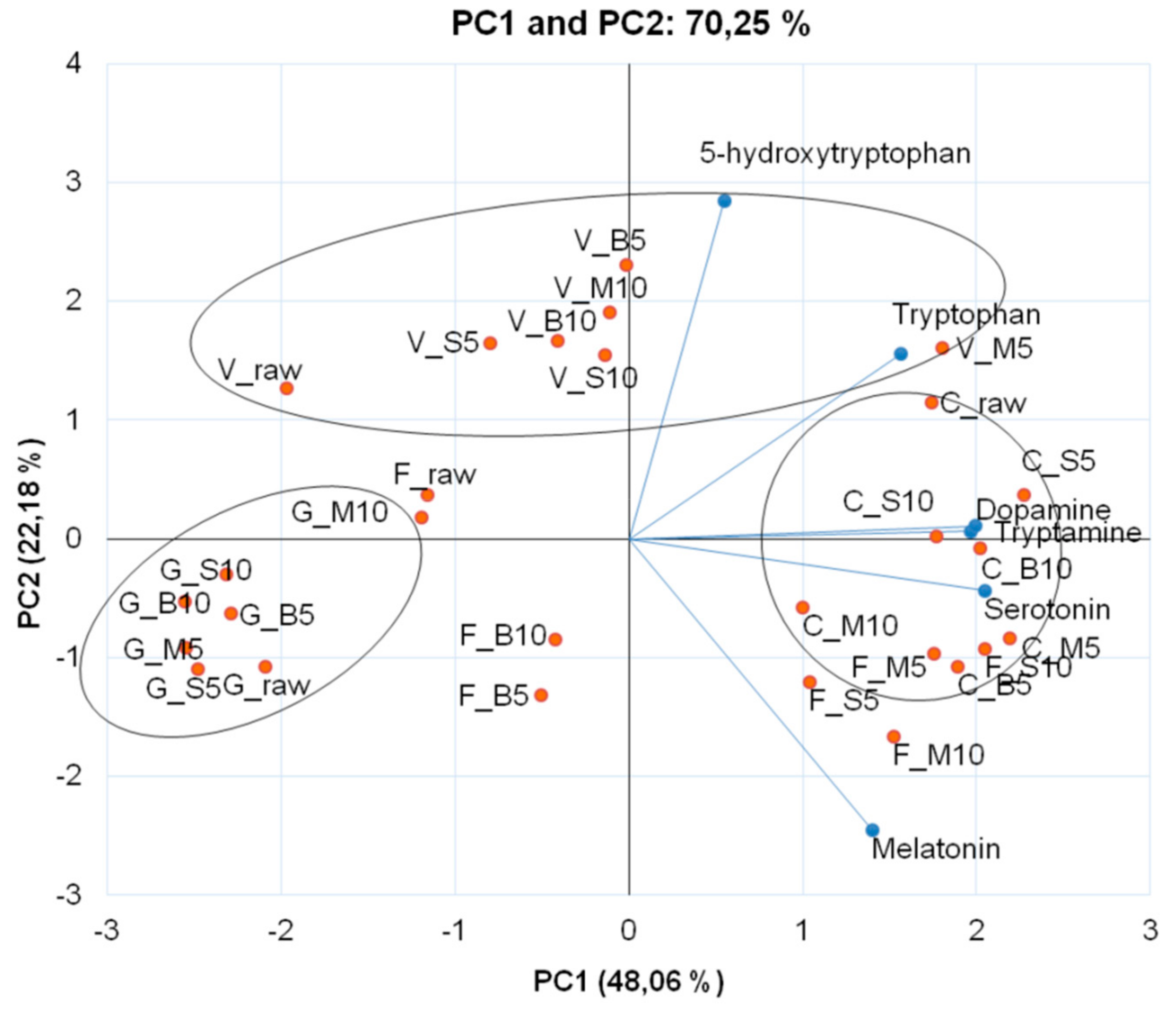
| Cooking Methods | Time (min) | AA | Monoamines | ||||
|---|---|---|---|---|---|---|---|
| Tryptophan | 5-Hydroxytryptophan | Tryptamine | Serotonin | Melatonin | Dopamine | ||
| ‘Verde di Macerata’ | |||||||
| Raw | 79.30 ± 4.44 oF 1 | 254.39 ± 7.17 bA | 5.18 ± 0.04 oE | 0.87 ± 0.01 mD | 0.40 ± 0.11 kB | 0.08 ± 0.00 mD | |
| Boiling | 5 | 277.09 ± 13.33 fD | 208.74 ± 13.42 cB | 11.88 ± 0.17 bA | 0.85 ± 0.03 mD | 0.31 ± 0.04 kC | 0.06 ± 0.01 nE |
| 10 | 354.91 ± 10.30 cB | 70.05 ± 5.85 hE | 8.18 ± 0.17 jC | 0.81 ± 0.00 nD | 0.31 ± 0.00 kC | 0.22 ± 0.01 hB | |
| Steaming | 5 | 250.65 ± 7.18 gE | 124.95 ± 1.35 eC | 7.66 ± 0.10 kD | 1.25 ± 0.04 kC | 0.40 ± 0.02 kB | 0.11 ± 0.00 lC |
| 10 | 302.67 ± 0.88 eC | 128.81 ± 0.41 eC | 8.53 ± 0.07 iB | 1.29 ± 0.00 kC | 0.59 ± 0.17 jA | 0.08 ± 0.01 mD | |
| Microwave | 5 | 238.71 ± 0.64 hE | 90.93 ± 1.26 fD | 7.43 ± 0.01 lD | 3.45 ± 0.14 cA | 0.17 ± 0.03 kC | 0.35 ± 0.00 cA |
| 10 | 432.37 ± 13.12 aA | 136.40 ± 4.85 dC | 8.69 ± 0.20 hB | 1.41 ± 0.04 jB | 0.50 ± 0.03 jA | 0.03 ± 0.00 qF | |
| ‘Cheddar’ | |||||||
| Raw | 337.35 ± 9.88 dC | 384.97 ± 5.84 aA | 9.00 ± 0.22 gD | 1.00 ± 0.02 lF | 5.33 ± 0.01 eC | 0.42 ± 0.00 aA | |
| Boiling | 5 | 362.25 ± 2.60 cB | 36.76 ± 1.23 lE | 9.79 ± 0.05 fC | 2.40 ± 0.01 hE | 6.78 ± 0.08 dB | 0.35 ± 0.01 bB |
| 10 | 399.40 ± 14.07 bA | 45.93 ± 3.02 kD | 11.53 ± 0.49 cB | 2.71 ± 0.10 gD | 4.29 ± 0.43 gD | 0.24 ± 0.01 fD | |
| Steaming | 5 | 244.80 ± 5.28 hE | 71.42 ± 2.54 hB | 13.90 ± 0.34 aA | 3.34 ± 0.09 dC | 4.20 ± 0.13 gD | 0.34 ± 0.01 dC |
| 10 | 301.35 ± 4.48 eD | 70.39 ± 0.09 hB | 8.74 ± 0.09 hD | 3.70 ± 0.02 bB | 5.21 ± 0.01 eC | 0.22 ± 0.00 hE | |
| Microwave | 5 | 207.22 ± 2.26 iF | 57.03 ± 3.55 iC | 11.40 ± 0.16 cB | 3.68 ± 0.14 bB | 8.25 ± 0.16 cA | 0.34 ± 0.01 dC |
| 10 | 211.49 ± 10.28 iF | 55.90 ± 0.06 iC | 8.63 ± 0.34 hD | 4.26 ± 0.14 aA | 4.48 ± 0.26 gD | 0.13 ± 0.01 kF | |
| ‘Forata’ | |||||||
| Raw | 36.53 ± 0.56 pF | 203.25 ± 0.72 cA | 4.69 ± 0.03 pE | 2.03 ± 0.02 hC | 0.97 ± 0.05 iE | 0.12 ± 0.01 kD | |
| Boiling | 5 | 167.97 ± 3.42 lE | 45.50 ± 0.56 kE | 8.60 ± 0.15 hD | 1.86 ± 0.05 iD | 4.79 ± 0.32 fC | 0.07 ± 0.00 mE |
| 10 | 184.81 ± 2.83 kD | 45.81 ± 1.30 kE | 8.84 ± 0.11 gC | 1.81 ± 0.10 iD | 4.28 ± 0.02 gD | 0.05 ± 0.00 oF | |
| Steaming | 5 | 169.47 ± 1.58 lE | 66.83 ± 0.81 hB | 10.26 ± 0.10 eB | 2.83 ± 0.02 gB | 9.34 ± 0.26 aA | 0.13 ± 0.00 kD |
| 10 | 256.65 ± 2.21 gA | 49.66 ± 1.71 jD | 10.89 ± 0.16 dA | 2.79 ± 0.02 gB | 9.02 ± 0.04 bA | 0.28 ± 0.01 eA | |
| Microwave | 5 | 216.63 ± 2.37 iC | 52.68 ± 1.37 jC | 10.40 ± 0.02 eB | 3.04 ± 0.06 eA | 8.39 ± 0.00 cB | 0.23 ± 0.00 gB |
| 10 | 235.09 ± 1.22 hB | 42.50 ± 0.63 kF | 10.48 ± 0.07 eE | 2.91 ± 0.04 fB | 9.25 ± 0.11 aA | 0.15 ± 0.00 jC | |
| ‘Graffiti’ | |||||||
| Raw | 21.29 ± 0.06 qG | 42.90 ± 0.42 kC | 5.46 ± 0.01 nG | 0.45 ± 0.00 oB | 1.06 ± 0.12 iB | 0.18 ± 0.00 iA | |
| Boiling | 5 | 175.98 ± 0.29 kB | 27.78 ± 2.10 mE | 7.72 ± 0.16 kC | 0.75 ± 0.06 nA | 0.49 ± 0.04 jD | 0.04 ± 0.00 pC |
| 10 | 193.56 ± 2.86 jA | 27.57 ± 0.17 mE | 6.28 ± 0.04 mF | 0.70 ± 0.02 nA | 0.46 ± 0.09 jD | 0.05 ± 0.00 oB | |
| Steaming | 5 | 87.45 ± 0.33 oF | 42.33 ± 0.13 kC | 7.55 ± 0.03 kD | 0.81 ± 0.09 nA | 0.94 ± 0.04 iB | 0.02 ± 0.00 rD |
| 10 | 159.22 ± 2.02 mD | 50.49 ± 1.97 jB | 7.24 ± 0.06 lE | 0.80 ± 0.01 nA | 0.90 ± 0.02 iB | 0.02 ± 0.00 rC | |
| Microwave | 5 | 147.81 ± 0.59 nE | 37.11 ± 1.65 lD | 8.48 ± 0.08 iB | 0.73 ± 0.00 nA | 0.68 ± 0.03 jC | 0.02 ± 0.00 rD |
| 10 | 165.08 ± 0.42 lC | 77.13 ± 0.11 gA | 10.37 ± 0.02 eA | 0.77 ± 0.01 nA | 1.63 ± 0.26 hA | 0.02 ± 0.00 rD | |
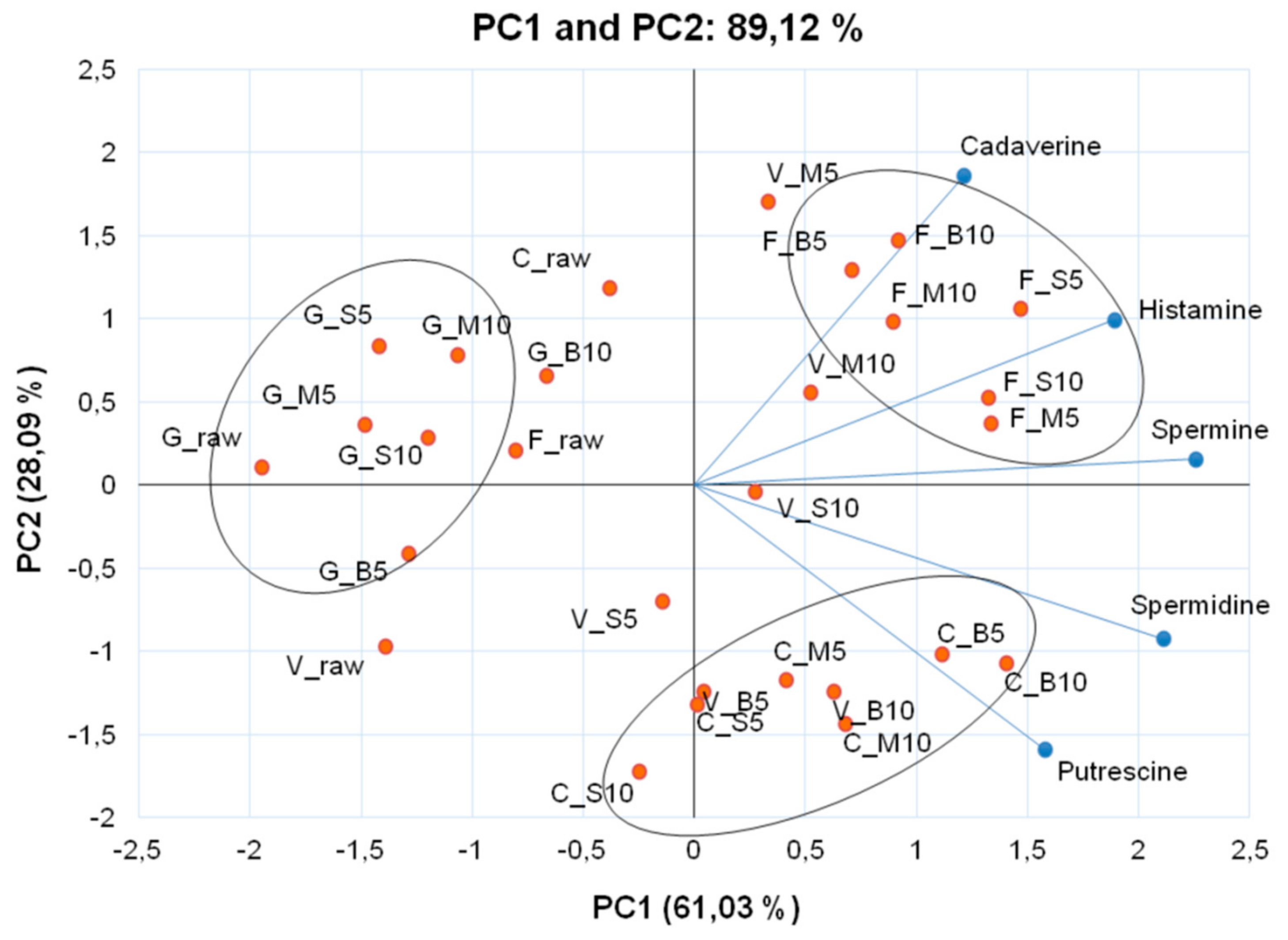
| Cooking Methods | Time (min) | Monoamine | Diamines | Polyamines | CQI | ||
|---|---|---|---|---|---|---|---|
| Histamine | Cadaverine | Putrescine | Spermidine | Spermine | |||
| ‘Verde di Macerata’ | |||||||
| Raw | 0.06 ± 0.00 kE 1 | 0.03 ± 0.00 nF | 3.58 ± 0.05 iE | 1.84 ± 0.04 nG | 0.29 ± 0.02 pE | 1.17 ± 0.03 | |
| Boiling | 5 | 0.10 ± 0.00 jD | 0.08 ± 0.03 kE | 5.87 ± 0.10 fC | 14.86 ± 0.31 gB | 1.83 ± 0.02 jB | 0.34 ± 0.00 |
| 10 | 0.12 ± 0.00 jD | 0.10 ± 0.01 jE | 7.68 ± 0.06 dA | 21.38 ± 0.22 cA | 3.08 ± 0.03 iA | 0.31 ± 0.00 | |
| Steaming | 5 | 0.11 ± 0.01 jD | 0.12 ± 0.01 iD | 6.24 ± 0.07 eB | 13.29 ± 0.17 iD | 1.37 ± 0.02 lD | 0.41 ± 0.00 |
| 10 | 0.21 ± 0.02 iC | 0.18 ± 0.01 gC | 4.49 ± 0.03 gD | 13.66 ± 0.09 iC | 1.68 ± 0.01 kC | 0.30 ± 0.00 | |
| Microwave | 5 | 0.56 ± 0.01 cA | 0.39 ± 0.02 bA | 1.45 ± 0.03 lF | 9.74 ± 0.01 kF | 1.81 ± 0.01 jB | 0.19 ± 0.00 |
| 10 | 0.37 ± 0.02 gB | 0.31 ± 0.01 cB | 5.95 ± 0.13 fC | 11.54 ± 0.26 jE | 1.69 ± 0.04 kC | 0.47 ± 0.00 | |
| ‘Cheddar’ | |||||||
| Raw | 0.46 ± 0.02 eA | 0.27 ± 0.02 dA | 2.04 ± 0.03 kD | 3.50 ± 0.02 mF | 0.35 ± 0.02 pF | 0.57 ± 0.01 | |
| Boiling | 5 | 0.41 ± 0.01 fB | 0.09 ± 0.00 kC | 7.71 ± 0.06 dC | 27.70 ± 0.13 bB | 4.15 ± 0.00 fB | 0.25 ± 0.00 |
| 10 | 0.48 ± 0.05 eA | 0.10 ± 0.01 nB | 12.04 ± 0.48 aA | 37.44 ± 1.45 aA | 5.29 ± 0.17 eA | 0.29 ± 0.00 | |
| Steaming | 5 | 0.22 ± 0.02 iD | 0.03 ± 0.00 nE | 7.56 ± 0.20 dC | 13.54 ± 0.33 iD | 1.37 ± 0.03 lD | 0.49 ± 0.00 |
| 10 | 0.20 ± 0.01 iD | 0.02 ± 0.00 nE | 8.90 ± 0.10 bB | 11.74 ± 0.13 jE | 0.77 ± 0.01 nE | 0.68 ± 0.00 | |
| Microwave | 5 | 0.27 ± 0.01 hC | 0.07 ± 0.00 lD | 8.08 ± 0.13 cC | 14.08 ± 0.28 hD | 1.42 ± 0.02 lD | 0.51 ± 0.00 |
| 10 | 0.27 ± 0.05 hC | 0.07 ± 0.00 lD | 8.96 ± 0.42 bB | 16.87 ± 0.55 fC | 1.89 ± 0.05 jC | 0.47 ± 0.00 | |
| ‘Forata’ | |||||||
| Raw | 0.04 ± 0.00 kD | 0.20 ± 0.00 fE | 3.22 ± 0.02 jF | 4.60 ± 0.05 lG | 0.85 ± 0.01 nG | 0.54 ± 0.00 | |
| Boiling | 5 | 0.60 ± 0.06 bB | 0.28 ± 0.02 dC | 3.37 ± 0.07 jD | 10.29 ± 0.17 kE | 4.04 ± 0.06 gE | 0.28 ± 0.00 |
| 10 | 0.71 ± 0.01 aA | 0.45 ± 0.02 aA | 4.24 ± 0.02 hB | 10.02 ± 0.09 kF | 3.87 ± 0.03 hF | 0.36 ± 0.00 | |
| Steaming | 5 | 0.65 ± 0.04 bA | 0.32 ± 0.00 cB | 3.48 ± 0.05 iC | 20.58 ± 0.20 dA | 7.60 ± 0.08 bB | 0.15 ± 0.00 |
| 10 | 0.60 ± 0.06 bB | 0.24 ± 0.00 eD | 4.16 ± 0.04 hB | 19.11 ± 0.24 eB | 8.17 ± 0.11 aA | 0.18 ± 0.00 | |
| Microwave | 5 | 0.62 ± 0.03 bB | 0.21 ± 0.00 fE | 4.64 ± 0.06 gA | 18.73 ± 0.04 eC | 6.98 ± 0.01 cC | 0.20 ± 0.00 |
| 10 | 0.52 ± 0.03 dC | 0.27 ± 0.01 dC | 3.32 ± 0.01 jE | 14.02 ± 0.08 hD | 5.80 ± 0.03 dD | 0.20 ± 0.00 | |
| ‘Graffiti’ | |||||||
| Raw | 0.09 ± 0.00 kC | 0.05 ± 0.00 mD | 0.34 ± 0.01 nF | 0.52 ± 0.00 pD | 0.13 ± 0.01 qE | 0.29 ± 0.00 | |
| Boiling | 5 | 0.11 ± 0.01 jB | 0.03 ± 0.01 nE | 0.96 ± 0.01 mB | 1.46 ± 0.01 oB | 0.82 ± 0.02 nB | 0.34 ± 0.01 |
| 10 | 0.13 ± 0.01 jA | 0.16 ± 0.02 hA | 1.38 ± 0.02 lA | 1.91 ± 0.25 nA | 1.10 ± 0.03 mA | 0.42 ± 0.02 | |
| Steaming | 5 | 0.12 ± 0.00 jA | 0.10 ± 0.00 jB | 0.71 ± 0.01 mE | 0.63 ± 0.05 pD | 0.33 ± 0.02 pD | 0.48 ± 0.01 |
| 10 | 0.13 ± 0.00 jA | 0.05 ± 0.00 mD | 0.78 ± 0.01 mD | 1.36 ± 0.02 oB | 0.56 ± 0.00 oC | 0.33 ± 0.00 | |
| Microwave | 5 | 0.11 ± 0.00 jB | 0.09 ± 0.00 kC | 0.81 ± 0.01 mC | 0.68 ± 0.02 pD | 0.32 ± 0.02 pD | 0.51 ± 0.00 |
| 10 | 0.13 ± 0.01 jA | 0.11 ± 0.01 jB | 0.83 ± 0.00 mC | 1.20 ± 0.00 oC | 0.55 ± 0.00 oC | 0.39 ± 0.01 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamante, M.S.; Borges, C.V.; da Silva, M.B.; Minatel, I.O.; Corrêa, C.R.; Gomez Gomez, H.A.; Lima, G.P.P. Bioactive Amines Screening in Four Genotypes of Thermally Processed Cauliflower. Antioxidants 2019, 8, 311. https://doi.org/10.3390/antiox8080311
Diamante MS, Borges CV, da Silva MB, Minatel IO, Corrêa CR, Gomez Gomez HA, Lima GPP. Bioactive Amines Screening in Four Genotypes of Thermally Processed Cauliflower. Antioxidants. 2019; 8(8):311. https://doi.org/10.3390/antiox8080311
Chicago/Turabian StyleDiamante, Marla Silvia, Cristine Vanz Borges, Mônica Bartira da Silva, Igor Otavio Minatel, Camila Renata Corrêa, Hector Alonzo Gomez Gomez, and Giuseppina Pace Pereira Lima. 2019. "Bioactive Amines Screening in Four Genotypes of Thermally Processed Cauliflower" Antioxidants 8, no. 8: 311. https://doi.org/10.3390/antiox8080311
APA StyleDiamante, M. S., Borges, C. V., da Silva, M. B., Minatel, I. O., Corrêa, C. R., Gomez Gomez, H. A., & Lima, G. P. P. (2019). Bioactive Amines Screening in Four Genotypes of Thermally Processed Cauliflower. Antioxidants, 8(8), 311. https://doi.org/10.3390/antiox8080311