Characterization and Antioxidant Activities of Yellow Strain Flammulina velutipes (Jinhua Mushroom) Polysaccharides and Their Effects on ROS Content in L929 Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
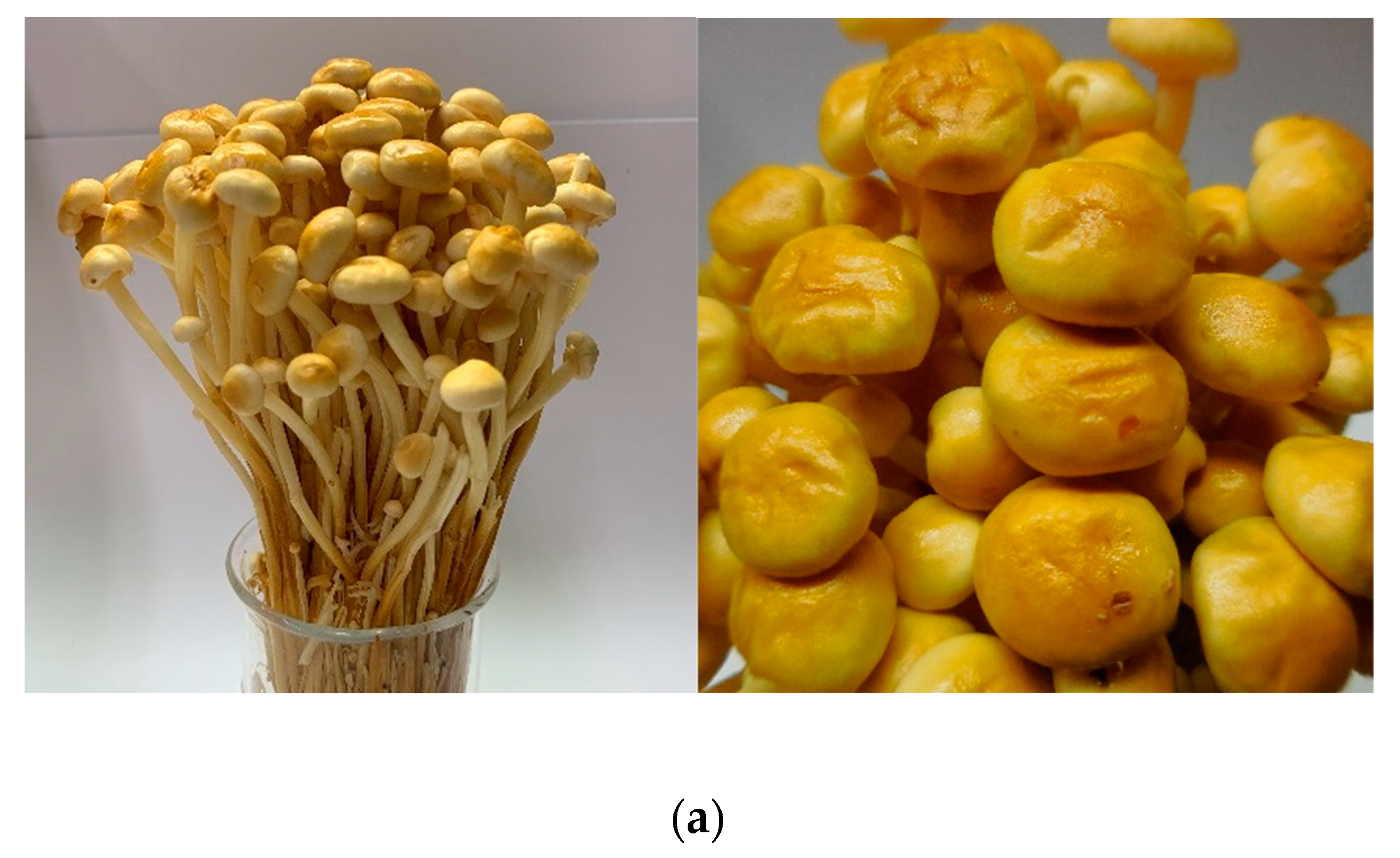
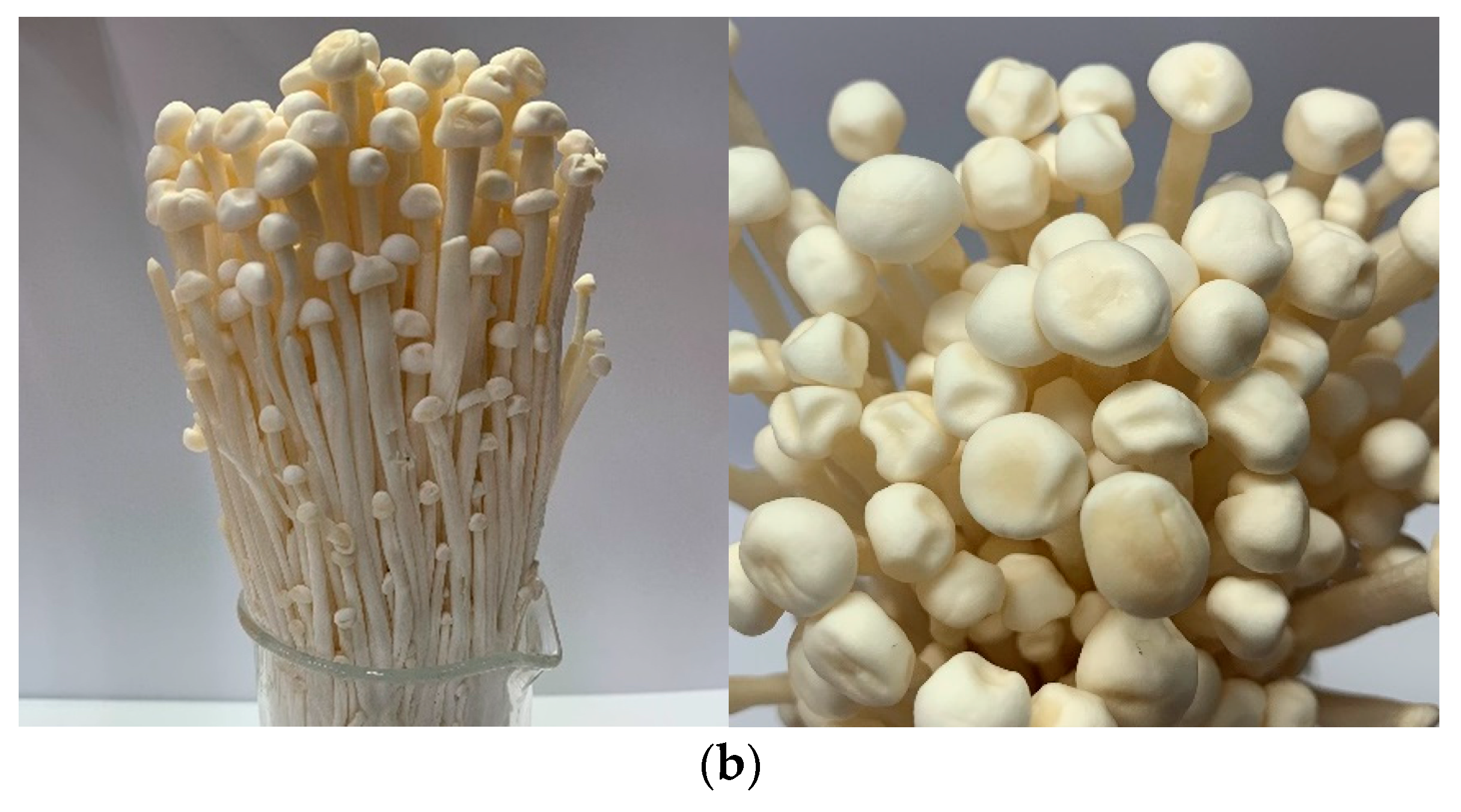
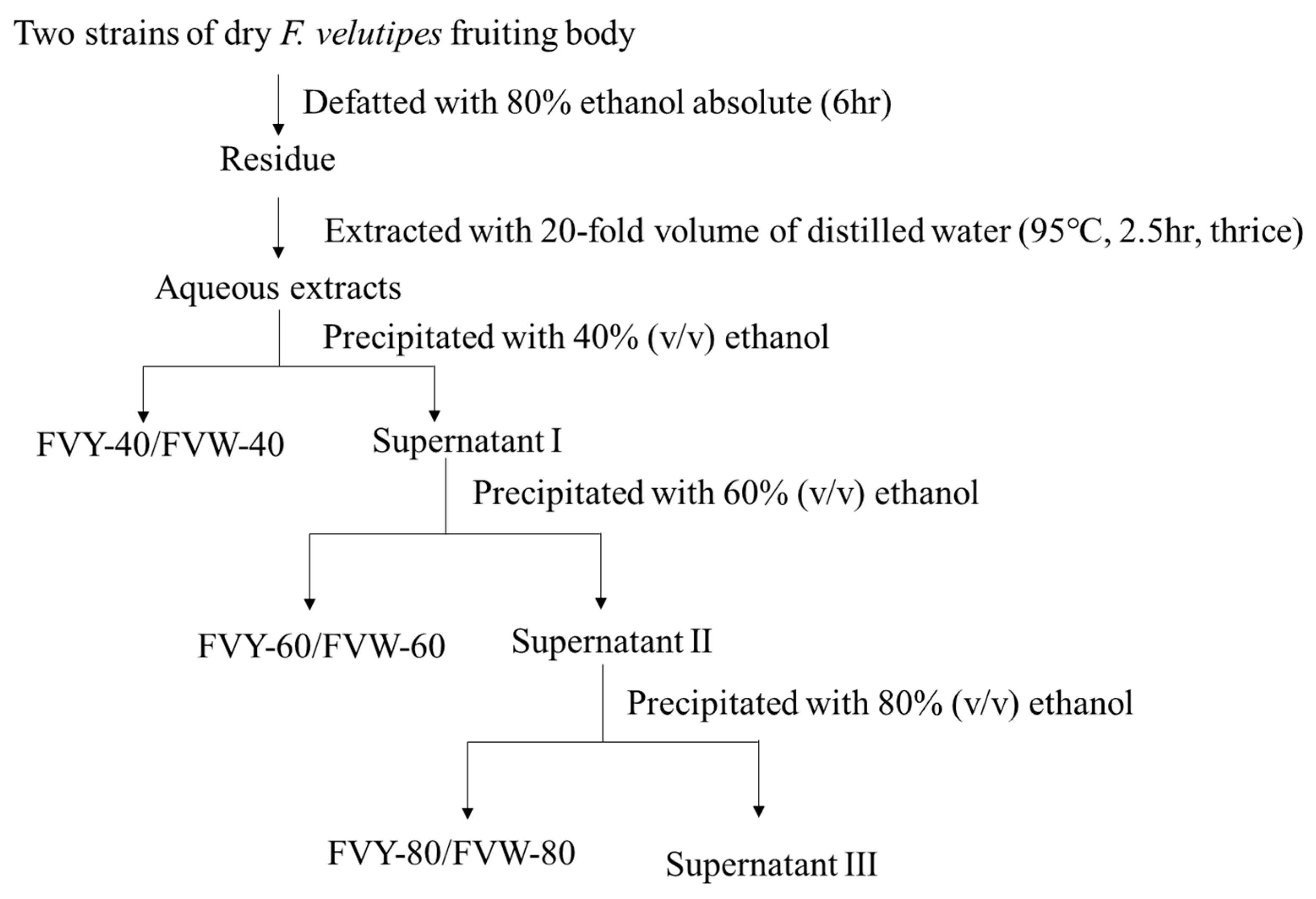
2.2. Preparation of Extract Fraction from F. Velutipes
2.3. Determination of Total Sugar, Uronic Acid, β-Glucan, and Protein Contents
2.4. Determination of Weight Average Molecular Weight
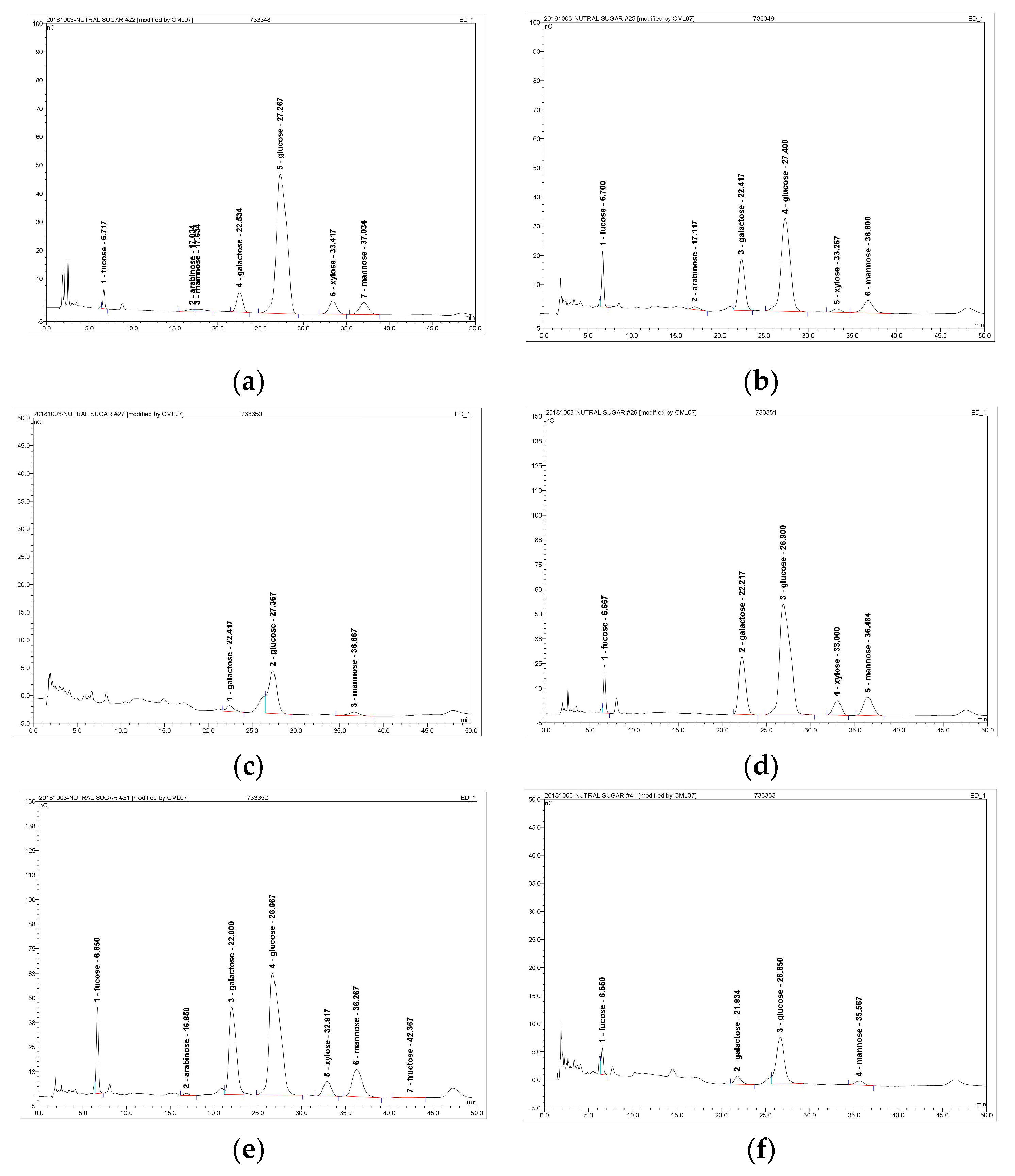
2.5. Monosaccharide Components Analysis
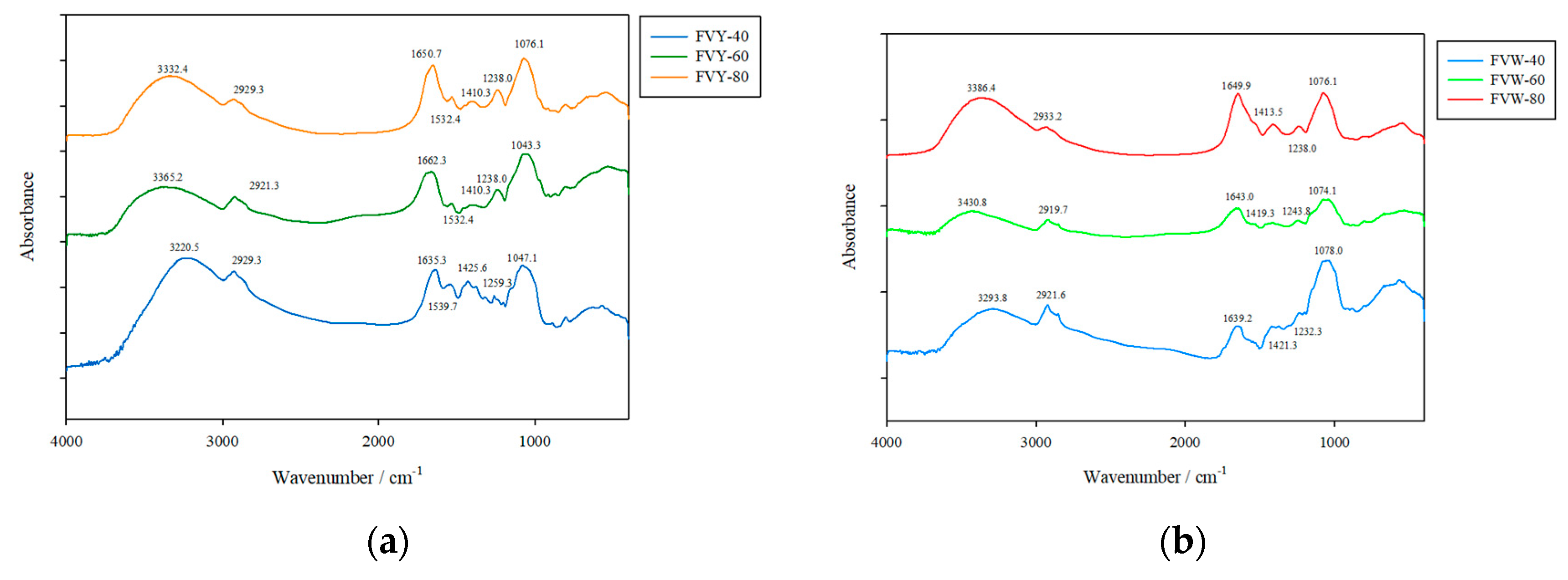
2.6. FT-IR Spectroscopic Analysis
2.7. Determination of Antioxidant Activities in Vitro
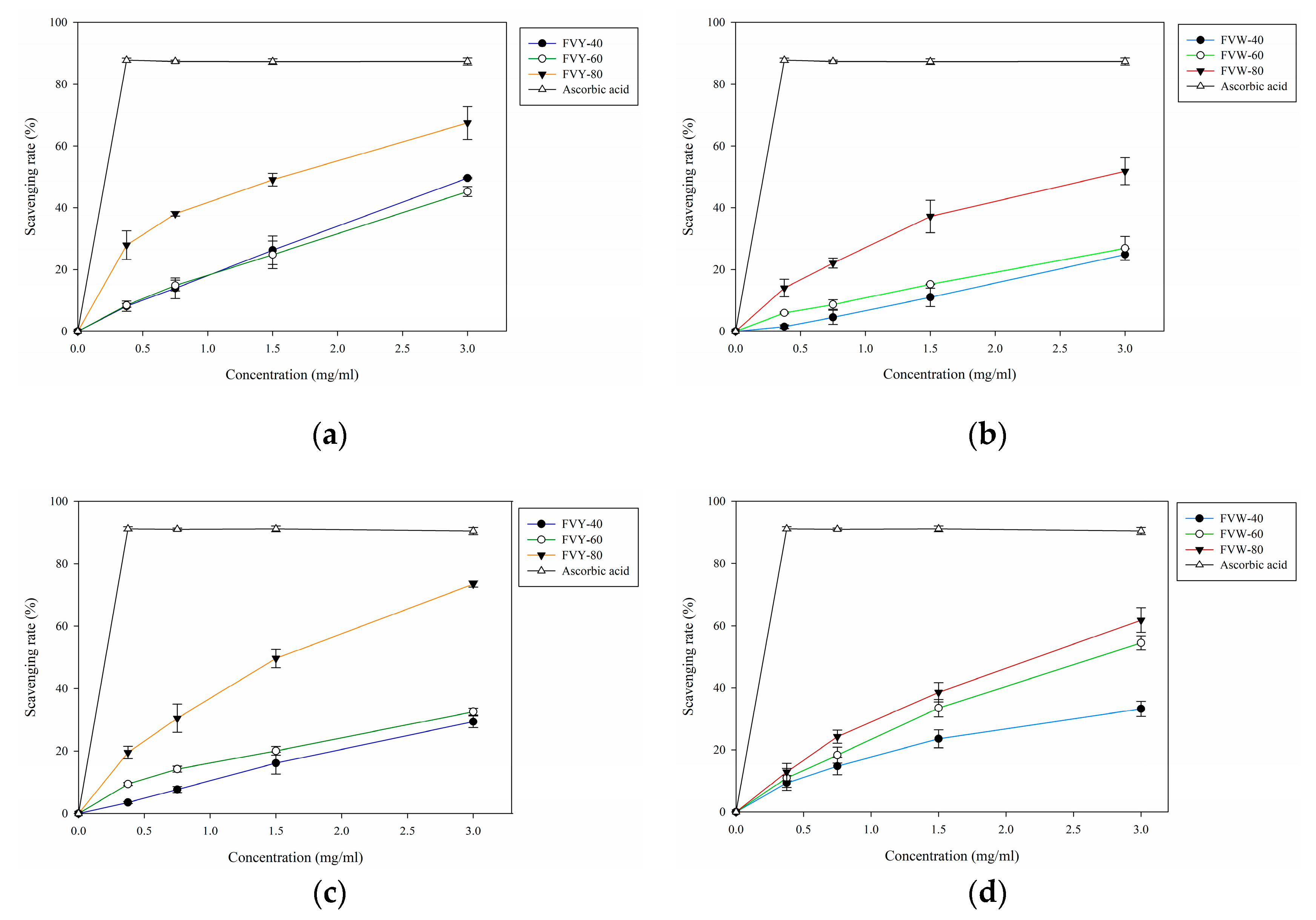
2.7.1. DPPH Radical Scavenging Assay
2.7.2. ABTS Radical Scavenging Assay
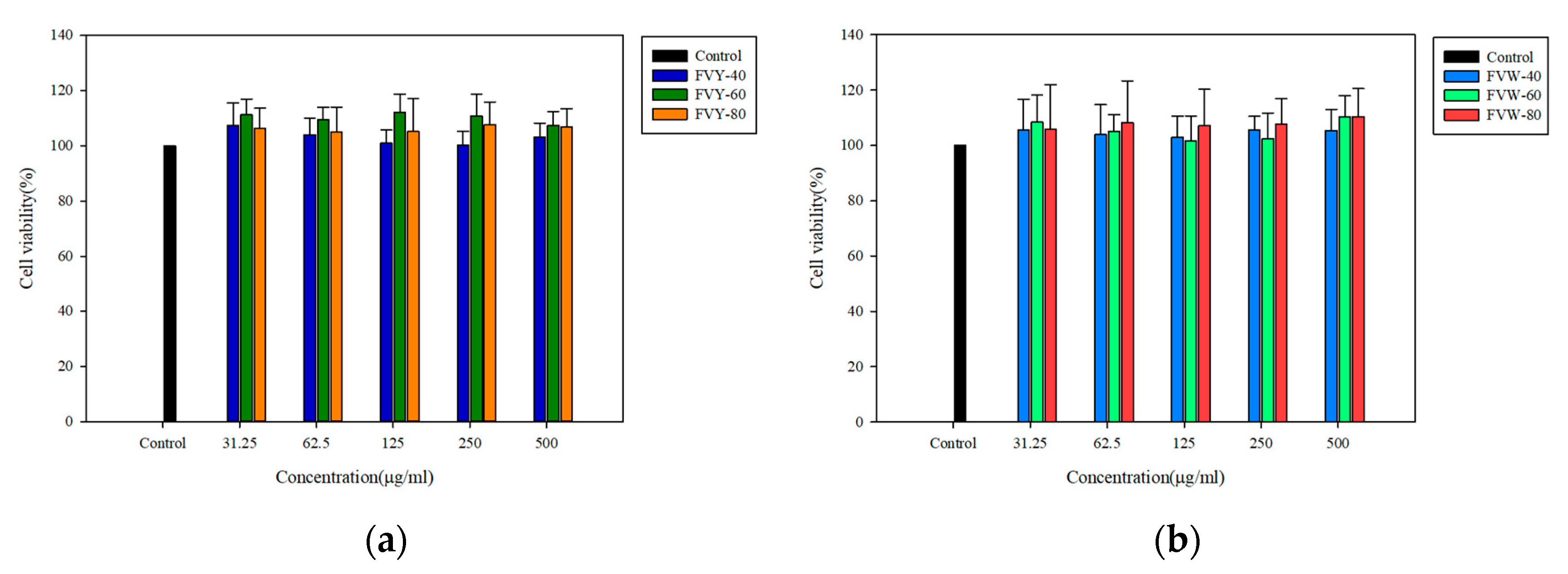
2.8. Cell Viability Assay
2.9. Intracellular ROS Content
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of FVYs and FVWs
3.2. Monosaccharide Compositions analysis of FVYs and FVWs
3.3. FTIR Spectra of FVYs and FVWs
3.4. DPPH and ABTS Radical Scavenging Assay
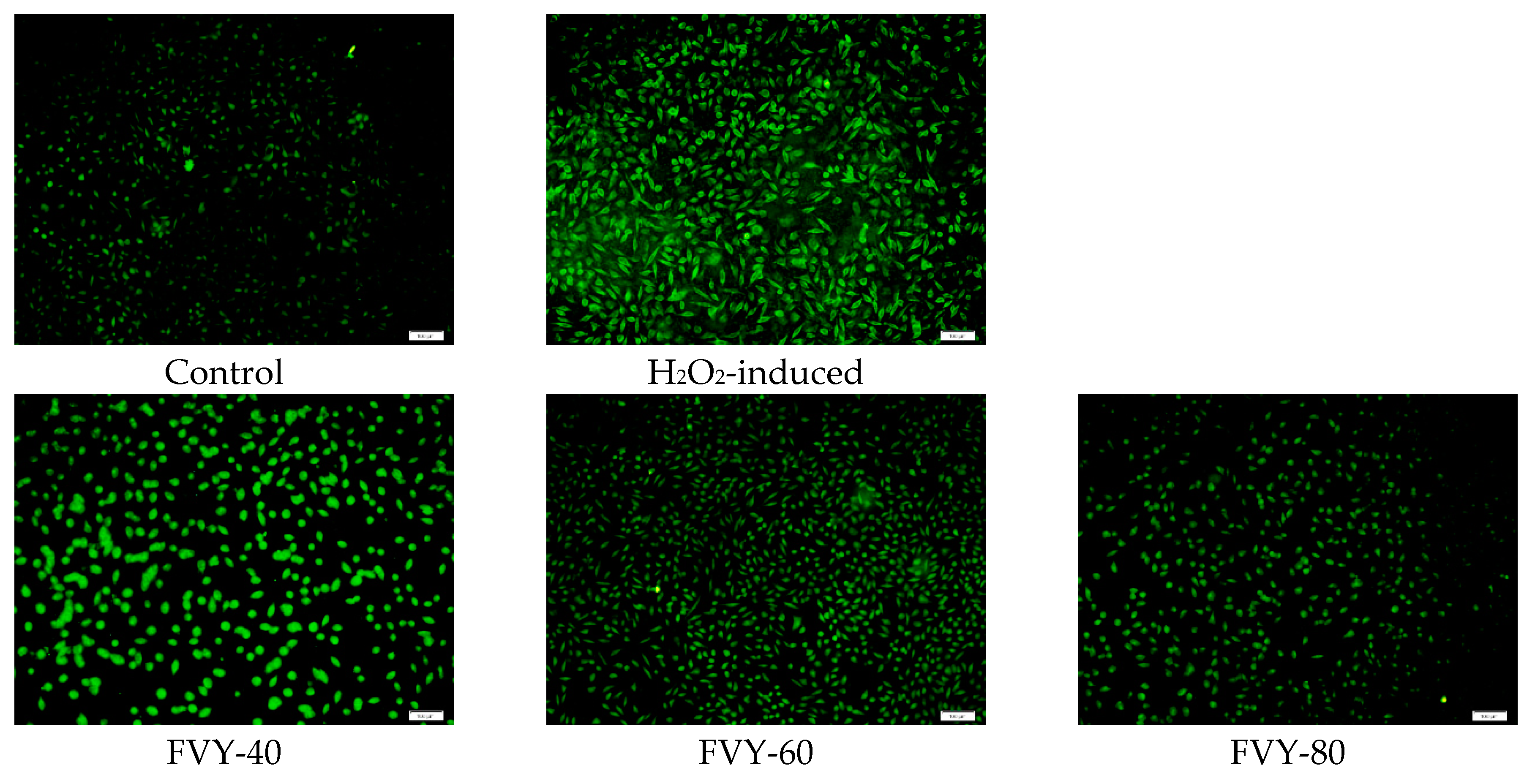
3.5. Effect of FVYs and FVWs on Cell Viability and ROS Production of H2O2-Induced L929 Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.-H.; Zhang, J.-S.; Feng, T.; Deng, J.; Lin, C.-C.; Fan, H.; Yu, W.-J.; Bao, H.-Y.; Jia, W. Structural elucidation of a polysaccharide from Flammulina velutipes and its immunomodulation activities on mouse B lymphocytes. Sci. Rep. 2018, 8, 3120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-Q.; Xiao, N.-N.; Deng, Y.-L.; He, P.-F.; Sun, P.-L. Purification and structural investigation of a water-soluble polysaccharide from Flammulina velutipes. Carbohydr. Polym. 2012, 87, 2279–2283. [Google Scholar] [CrossRef]
- Leung, M.; Fung, K.; Choy, Y. The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacology 1997, 35, 255–263. [Google Scholar] [CrossRef]
- Liu, X.B.; Feng, B.; Li, J.; Yan, C.; Yang, Z.L. Genetic diversity and breeding history of Winter Mushroom (Flammulina velutipes) in China uncovered by genomic SSR markers. Gene 2016, 591, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Boil. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Pitz Hda, S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.; Affonso, R.C.; Fanan, S.; Trevisan, A.C.; Ribeiro-do-Valle, R.M.; Maraschin, M. In Vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxidative Med. Cell. Longev. 2016, 2016, 3403586. [Google Scholar] [CrossRef]
- Liu, Y.M.; Qi, Z.M.; Wang, S.Y.; Liu, S.X.; Li, X.; Wang, H.J.; Xia, X.C. An overview on natural polysaccharides with antioxidant properties. Curr. Med. Chem. 2013, 20, 2899–2913. [Google Scholar] [CrossRef]
- Jhan, M.-H.; Yeh, C.-H.; Tsai, C.-C.; Kao, C.-T.; Chang, C.-K.; Hsieh, C.-W. Enhancing the Antioxidant Ability of Trametes versicolor Polysaccharopeptides by an Enzymatic Hydrolysis Process. Molecules 2016, 21, 1215. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Sung, T.-J.; Hu, Y.-N.; Lu, H.-Y.; Yang, L.-C.; Cheng, K.-C.; Lai, P.-S.; Hsieh, C.-W.; Sung, T.-J. Chemical analysis, moisture-preserving, and antioxidant activities of polysaccharides from Pholiota nameko by fractional precipitation. Int. J. Boil. Macromol. 2019, 131, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hoo, P.C.-X.; Tan, L.T.-H.; Pusparajah, P.; Khan, T.M.; Lee, L.-H.; Goh, B.-H.; Chan, K.-G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Lv, G.Y.; He, W.Q.; Shi, L.G.; Pan, H.J.; Fan, L.F. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-F.; Liu, N.-X.; Mao, X.-X.; Li, Y.; Li, C.-T. Activation Effects of Polysaccharides of Flammulina velutipes Mycorrhizae on the T Lymphocyte Immune Function. J. Immunol. Res. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cui, F.; Gao, X.; Zhang, J.; Zheng, L.; Jia, L. Purification, characterization, antioxidant activity and anti-aging of exopolysaccharides by Flammulina velutipes SF-06. Antonie Van Leeuwenhoek 2015, 107, 73–82. [Google Scholar] [CrossRef]
- Chang, C.-K.; Ho, W.-J.; Chang, S.-L.; Yeh, C.-H.; Liang, Z.-C.; Hsu, T.-H.; Hsieh, C.-W. Fractionation, characterization and antioxidant activity of exopolysaccharide from fermentation broth of a Xylaria nigripes. Bioact. Carbohydrates Diet. Fibre 2018, 16, 37–42. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Alsop, R.; Vlachogiannis, G. Determination of the molecular weight of clinical dextran by gel permeation chromatography on TSK PW type columns. J. Chromatogr. A 1982, 246, 227–240. [Google Scholar] [CrossRef]
- Wang, H.-T.; Yang, L.-C.; Yu, H.-C.; Chen, M.-L.; Wang, H.-J.; Lu, T.-J. Characteristics of fucose-containing polysaccharides from submerged fermentation of Agaricus blazei Murill. J. Food Drug Anal. 2018, 26, 678–687. [Google Scholar] [CrossRef]
- Erbing, B.; Jansson, P.-E.; Widmalm, G.; Nimmich, W. Structure of the capsular polysaccharide from the Klebsiella K8 reference strain 1015. Carbohydr. Res. 1995, 273, 197–205. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Dong, Y.-R.; Cheng, S.-J.; Qi, G.-H.; Yang, Z.-P.; Yin, S.-Y.; Chen, G.-T. Antimicrobial and antioxidant activities of Flammulina velutipes polysacchrides and polysacchride-iron(III) complex. Carbohydr. Polym. 2017, 161, 26–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Boil. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Cai, B.; Wan, P.; Chen, H.; Chen, D.; Chen, X.; Sun, H.; Pan, J. Composition characterization of oyster polysaccharides from Crassostrea hongkongensis and their protective effect against H2O2-induced oxidative damage in IEC-6 cells. Int. J. Boil. Macromol. 2019, 124, 246–254. [Google Scholar] [CrossRef]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Na Mahasarakham, C.P.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar] [CrossRef]
- Kaplum, V.; Ramos, A.C.; Consolaro, M.E.L.; Fernandez, M.A.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Silva, S.D.O.; De Mello, J.C.P.; Nakamura, C.V. Proanthocyanidin Polymer-Rich Fraction of Stryphnodendron adstringens Promotes in Vitro and in Vivo Cancer Cell Death via Oxidative Stress. Front. Pharmacol. 2018, 9, 694. [Google Scholar] [CrossRef]
- Nurulita, N.A.; Kusuma, A.M.; Darsini, D.; Delvia, W.; Yulianti, V.T. The Cytoprotective and Cell Recovery Properties of Apple Extracts on H2O2 induced-NIH3T3 Cells: An Anti Aging Candidate. Indones. J. Cancer Chemoprevention 2018, 9, 78–85. [Google Scholar] [CrossRef]
- Li, J.-E.; Wang, W.-J.; Zheng, G.-D.; Li, L.-Y. Physicochemical properties and antioxidant activities of polysaccharides from Gynura procumbens leaves by fractional precipitation. Int. J. Boil. Macromol. 2017, 95, 719–724. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Huang, W.-W.; Zhao, H.-T.; Wang, J.; Xu, R.-B.; Hu, X.-L.; Shen, S.-Y.; Qin, D. Characterization and Bioactivity of Polysaccharides Obtained from Pine Cones of Pinus koraiensis by Graded Ethanol Precipitation. Molecules 2013, 18, 9933–9948. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, S.; Shen, S.; Zhang, L.; Yang, R.; Zhou, Y.; Ding, C. Extraction, antioxidant and antimicrobial activities of Epimedium acuminatum Franch. polysaccharide. Carbohydr. Polym. 2013, 96, 101–108. [Google Scholar] [CrossRef]
- Koh, G.Y.; Chou, G.; Liu, Z. Purification of a water extract of Chinese sweet tea plant (Rubus suavissimus S. Lee) by alcohol precipitation. J. Agric. Food Chem. 2009, 57, 5000–5006. [Google Scholar] [CrossRef]
- Huang, Q.-L.; Siu, K.-C.; Wang, W.-Q.; Cheung, Y.-C.; Wu, J.-Y. Fractionation, characterization and antioxidant activity of exopolysaccharides from fermentation broth of a Cordyceps sinensis fungus. Process. Biochem. 2013, 48, 380–386. [Google Scholar] [CrossRef]
- Yang, W.; Pei, F.; Shi, Y.; Zhao, L.; Fang, Y.; Hu, Q. Purification, characterization and anti-proliferation activity of polysaccharides from Flammulina velutipes. Carbohydr. Polym. 2012, 88, 474–480. [Google Scholar] [CrossRef]
- Chen, G.-T.; Fu, Y.-X.; Yang, W.-J.; Hu, Q.-H.; Zhao, L.-Y. Effects of polysaccharides from the base of Flammulina Velutipes stipe on growth of murine RAW264.7, B16F10 and L929 cells. Int. J. Boil. Macromol. 2018, 107, 2150–2156. [Google Scholar] [CrossRef]
- Li, H.-J.; Chen, H.-Y.; Fan, L.-L.; Jiao, Z.-H.; Chen, Q.-H.; Jiao, Y.-C. In Vitro Antioxidant Activities and in Vivo Anti-Hypoxic Activity of the Edible Mushroom Agaricus bisporus (Lange) Sing. Chaidam. Molecules 2015, 20, 17775–17788. [Google Scholar] [CrossRef]
- Shang, H.; Wang, M.; Li, R.; Duan, M.; Wu, H.; Zhou, H. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from Astragalus cicer L. Sci. Rep. 2018, 8, 3359. [Google Scholar] [CrossRef]
- Fan, Y.; He, X.; Zhou, S.; Luo, A.; He, T.; Chun, Z. Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int. J. Boil. Macromol. 2009, 45, 169–173. [Google Scholar] [CrossRef]
- Li, J.; Chi, Z.; Yu, L.; Jiang, F.; Liu, C. Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int. J. Boil. Macromol. 2017, 105, 1544–1553. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Zhang, S.; Zhu, W.; Tang, J.; Liu, J.; Chen, P.; Zhang, D.; Ye, W.; Zheng, Y. Polysaccharides from Panax japonicus C.A. Meyer and their antioxidant activities. Carbohydr. Polym. 2014, 101, 386–391. [Google Scholar] [CrossRef]
- Chai, Z.; Huang, W.; Zhao, X.; Wu, H.; Zeng, X.; Li, C. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of polysaccharide from Cynanchum auriculatum Royle ex Wight. Int. J. Boil. Macromol. 2018, 119, 1068–1076. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Wu, M.; Luo, X.; Xu, X.; Wei, W.; Yu, M.; Jiang, N.; Ye, L.; Yang, Z.; Fei, X. Antioxidant and immunomodulatory activities of a polysaccharide from Flammulina velutipes. J. Tradit. Chin. Med. 2014, 34, 733–740. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, C.; Gao, X.; Cui, F.; Zhang, J.; Jia, M.; Jia, S.; Jia, L. Enzymatic and acidic degradation effect on intracellular polysaccharide of Flammulina velutipes SF-08. Int. J. Boil. Macromol. 2015, 73, 236–244. [Google Scholar] [CrossRef]

| Sample | Total Sugar Content (%) | Uronic Acid Content (%) | β-Glucan Content (%) | Protein Content (%) |
|---|---|---|---|---|
| FVY-40 | 83.13 ± 1.29 Aa | 2.89 ± 0.36 Aa | 44.56 ± 1.77 Aa | 15.52 ± 3.14 Cb |
| FVY-60 | 75.94 ± 5.03 ABa | 2.10 ± 017 Bb | 18.45 ± 0.49 Cb | 17.15 ± 3.45 Cb |
| FVY-80 | 41.10 ± 2.09 Db | 1.71 ± 0.31 Bb | 16.68 ± 1.62 Cb | 35.85 ± 2.91 Aa |
| FVW-40 | 77.99 ± 0.87 ABd | 2.02 ± 0.05 Bd | 44.18 ± 0.27 Ad | 14.16 ± 1.38 Ce |
| FVW-60 | 60.54 ± 3.64 Ce | 1.81 ± 0.06 Be | 26.73 ± 2.11 Be | 23.60 ± 0.99 Bd |
| FVW-80 | 40.87 ± 5.21 Df | 1.68 ± 0.14 Be | 13.55 ± 0.34 Df | 25.95 ± 0.25 Bd |
| 40% | 60% | 80% | |
|---|---|---|---|
| Yellow strain | 2377.04 kDa | 18.32 kDa | 3.84 kDa |
| White strain | 2782.00 kDa | 19.09 kDa | 2.76 kDa |
| Monosaccharide Composition | Yellow Strain | White Strain | |||||
|---|---|---|---|---|---|---|---|
| 40% | 60% | 80% | 40% | 60% | 80% | ||
| Glucose | 87.90 | 54.50 | 75.00 | 66.49 | 58.02 | 64.22 | |
| Fructose | - | - | - | - | 0.25 | - | |
| Molar | Mannose | 5.55 | 11.76 | 14.29 | 11.89 | 14.80 | 9.88 |
| Percentage | Rhamnose | 0.81 | - | - | - | - | - |
| (%) | Fucose | 1.36 | 11.90 | - | 3.73 | 7.24 | 13.55 |
| Galactose | 2.59 | 21.03 | 10.71 | 15.37 | 18.79 | 12.35 | |
| Arabinose | 0.29 | 0.82 | - | - | 0.10 | - | |
| Xylose | 1.49 | - | - | 2.53 | 0.81 | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.-N.; Sung, T.-J.; Chou, C.-H.; Liu, K.-L.; Hsieh, L.-P.; Hsieh, C.-W. Characterization and Antioxidant Activities of Yellow Strain Flammulina velutipes (Jinhua Mushroom) Polysaccharides and Their Effects on ROS Content in L929 Cell. Antioxidants 2019, 8, 298. https://doi.org/10.3390/antiox8080298
Hu Y-N, Sung T-J, Chou C-H, Liu K-L, Hsieh L-P, Hsieh C-W. Characterization and Antioxidant Activities of Yellow Strain Flammulina velutipes (Jinhua Mushroom) Polysaccharides and Their Effects on ROS Content in L929 Cell. Antioxidants. 2019; 8(8):298. https://doi.org/10.3390/antiox8080298
Chicago/Turabian StyleHu, Yu-Ning, Tzu-Jung Sung, Chun-Hsu Chou, Kai-Lun Liu, Liang-Po Hsieh, and Chang-Wei Hsieh. 2019. "Characterization and Antioxidant Activities of Yellow Strain Flammulina velutipes (Jinhua Mushroom) Polysaccharides and Their Effects on ROS Content in L929 Cell" Antioxidants 8, no. 8: 298. https://doi.org/10.3390/antiox8080298
APA StyleHu, Y.-N., Sung, T.-J., Chou, C.-H., Liu, K.-L., Hsieh, L.-P., & Hsieh, C.-W. (2019). Characterization and Antioxidant Activities of Yellow Strain Flammulina velutipes (Jinhua Mushroom) Polysaccharides and Their Effects on ROS Content in L929 Cell. Antioxidants, 8(8), 298. https://doi.org/10.3390/antiox8080298






