Antioxidants: Terminology, Methods, and Future Considerations
Abstract
:1. Introduction
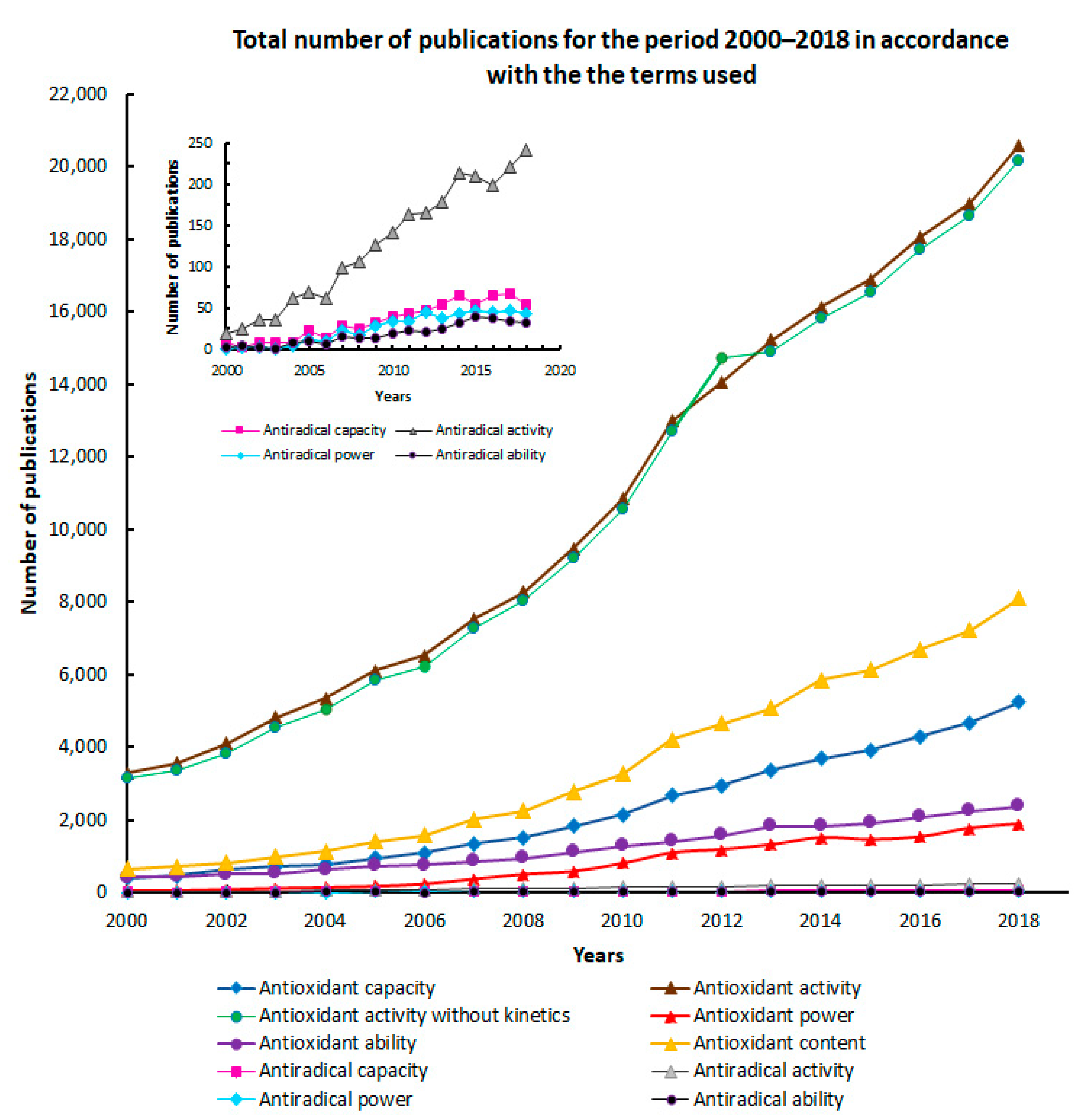
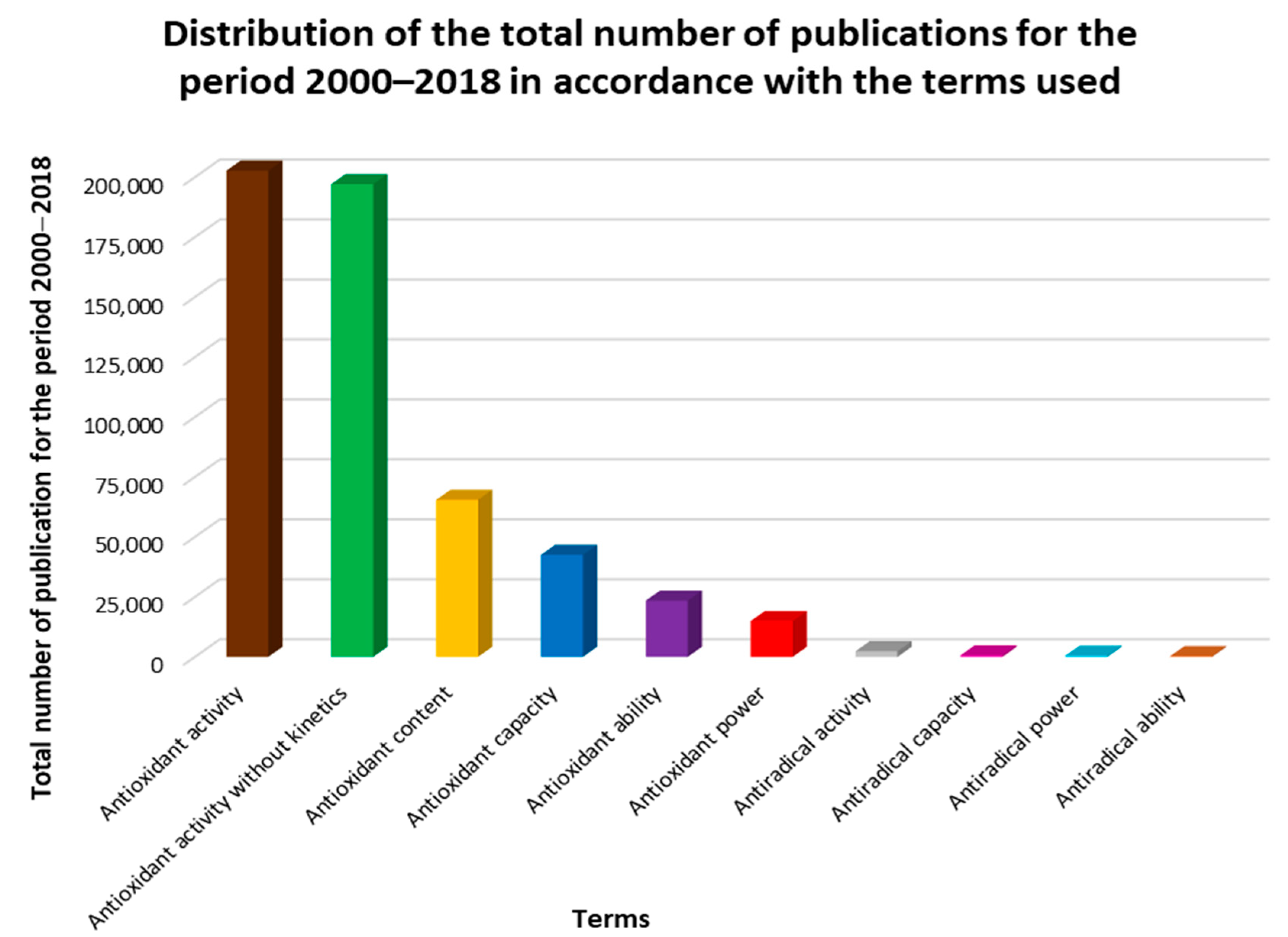
2. Terminology
3. Methods
- -
- Most fully meet the nature of the OS and the action of the body’s antioxidant defense system;
- -
- They are characterized by instrumental and operator simplicity, financial, temporal, and informative efficiency;
- -
- They are easily applied in the clinical laboratory, and on their basis, sensory systems are created for work in onsite and in situ formats.
4. Ways of Development and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- 18th ISANH Middle East Antioxidants World Congress, Beirut Antioxidants 2017. Available online: https://www. isanh-me.com (accessed on 21 June 2019).
- Montezano, A.C.; Dulak-Lis, M.; Tsiropoulou, S.; Harvey, A.; Brione, A.M.; Touyz, R.M. Oxidative Stress and Human Hypertension: Vascular Mechanisms, Biomarkers, and Novel Therapies. Can. J. Cardiol. 2015, 31, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisaha, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease. Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Brainina, K.Z.; Gerasimova, E.L.; Kazakov, J.E.; Khodos, M. Oxidative stress: Nature, contribution to pathogenesis, protection and diagnosis. In Problems of Analytical Chemistry; Budnikov, G.K., Ed.; Nauka: Moscow, Russian, 2010; pp. 132–163. [Google Scholar]
- Cortina-Puig, M.; Prieto-Simón, B.; Campàs, M.; Calas-Blanchard, C.; Marty, J.-L. Determination of the antioxidants’ ability to scavenge free radicals using biosensors. Adv. Exp. Med. Biol. 2010, 698, 222–233. [Google Scholar] [PubMed]
- Gizelli, A.; Serafinim, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef]
- Gafner, S. Scientific Journals Increasingly Skeptical of Antioxidant Research. J. Am. Bot. Counc. 2018, 117, 35. Available online: http://cms.herbalgram.org/herbalgram/issue117/hg117-resrvw-scijournals.html (accessed on 21 June 2019).
- Allamaneni, S.S.R.; Agarwal, A.; Nallella, K.P.; Sharma, R.K.; Thomas, A.J.J.; Sikka, S.C. Characterization of oxidative stress status by evaluation of reactive oxygen species levels in whole semen and isolated spermatozoa. Fertil. Steril. 2005, 83, 800–803. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Allamaneni, S. Oxidative Stress and Human Reproduction. In Oxidative Stress Disease and Cancer; Singh, K.K., Ed.; Imperial College Press: London, UK, 2006; pp. 687–703. [Google Scholar]
- Kancheva, V.D.; Kasaikina, O.T. Bio-Antioxidants–A Chemical Base of Their Antioxidant Activity and Beneficial Effect on Human Health. Curr. Med. Chem. 2013, 20, 4784–4805. [Google Scholar] [CrossRef]
- Morin, D. New Research on Antioxidants; Marín, D., García, P., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2008. [Google Scholar]
- Cadenas, E.; Packer, L. (Eds.) Handbook of Antioxidants, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [PubMed]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoǧlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Badarinath, A.V.; Rao, K.M.; Chetty, C.M.S.; Ramkanth, S.; Rajan, T.V.S. Review on In-vitro Antioxidant Methods: Comparisions, Correlations and Considerations. Inter. J. PharmTech Res. 2010, 2, 1276–1285. [Google Scholar]
- Nile, S.H.; Khobragade, C.N.; Park, S.W. Optimized and Comparative Antioxidant Assays and Its Applications in Herbal and Synthetic Drug Analysis as an Antioxidants. Mini-Rev. Med. Chem. 2012, 12, 1007–1014. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
- Khodos, M.Y.; Kazakov, Y.E.; Vidrevich, M.B.; Brainina, K.Z. Monitoring of oxidative stress in biological objects. J. Ural Med. Acad. Sci. 2017, 14, 262–274. (In Russian) [Google Scholar]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical Methods for Total Antioxidant Capacity and its Main Contributors Determination: A review. Open Chem. 2015, 13, 824–856. [Google Scholar] [CrossRef]
- Romeroa, M.P.R.; Britoa, R.E.; Melladoa, J.M.R.; González-Rodríguez, J.; Montoyac, M.R.; Rodríguez-Amaroa, R. Exploring the relation between composition of extracts of healthy foods and their antioxidant capacities determined by electrochemical and spectrophotometrical methods. LWT–Food Sci. Technol. 2018, 95, 157–166. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Céspedes, F.; Capdevila, J.; Mínguez, S.; Jiménez-Jorquera, C.; Valle, M. Determination of total polyphenol index in wines employing a voltammetric electronic tongue. Anal. Chim. Acta 2012, 732, 172–179. [Google Scholar] [Green Version]
- Arteaga, J.F.; Ruiz-Montoya, M.; Palma, A.; Alonso-Garrido, G.; Pintado, S.; Rodríguez-Mellado, J.M. Comparison of the Simple Cyclic Voltammetry (CV) and DPPH Assays for the Determination of Antioxidant Capacity of Active Principles. Molecules 2012, 17, 5126–5138. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric study of antioxidant activity: Development and prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, Y.; Khodos, M.; Vidrevich, M.; Brainina, K. Potentiometry as a Tool for Monitoring of Antioxidant Activity and Oxidative Stress Estimation in Medicine. Crit. Rev. Anal. Chem. 2019, 49, 150–159. [Google Scholar] [CrossRef]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: Clinical utility in male factor infertility. Reprod. Biomed. Online 2017, 34, 48–57. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Varzakova, D.P.; Gerasimova, E.L. Chronoamperometric method for determination of integral antioxidant activity. J. Anal. Chem. 2012, 67, 364–369. [Google Scholar] [CrossRef]
- Tessutti, L.S.; Macedo, D.V.; Kubota, L.T.; Alves, A.A. Measuring the antioxidant capacity of blood plasma using potentiometry. Anal. Biochem. 2013, 441, 109–114. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Markina, M.G.; Stozhko, N.Y. Optimized Potentiometric Assay for Non-invasive Investigation of Skin Antioxidant Activity. Electroanalysis 2018, 30, 2405–2412. [Google Scholar] [CrossRef]
- Brainina, K.; Tarasov, A.; Khamzina, E.; Kazakov, Y.; Stozhko, N. Disposable Potentiometric Sensory System for Skin Antioxidant Activity Evaluation. Sensors 2019, 19, 2586. [Google Scholar] [CrossRef]
- Brainina, K.; Galperin, L.; Gerasimova, E.; Khodos, M. Noninvasive Potentiometric Method of Determination of Skin Oxidant/Antioxidant Activity. IEEE Sens. J. 2012, 12, 527–532. [Google Scholar] [CrossRef]
- Joël, P.; Mouna-Messaouda, K.; Jean-Paul, C.B.; Jean-Olivier, D.; Smail, M. Electrochemical Methodology for Evaluating Skin Oxidative Stress Status (SOSS). Diseases 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. New antiradical capacity assay with the use potentiometric method. Anal. Chim. Acta 2019, 1046, 69–76. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants 2019, 8, 297. https://doi.org/10.3390/antiox8080297
Brainina K, Stozhko N, Vidrevich M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants. 2019; 8(8):297. https://doi.org/10.3390/antiox8080297
Chicago/Turabian StyleBrainina, Khiena, Natalia Stozhko, and Marina Vidrevich. 2019. "Antioxidants: Terminology, Methods, and Future Considerations" Antioxidants 8, no. 8: 297. https://doi.org/10.3390/antiox8080297





