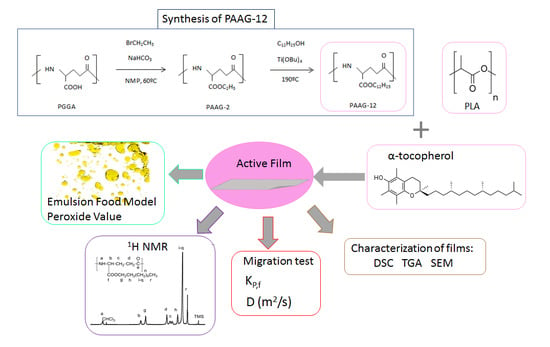
Poly (α-Dodecyl γ-Glutamate) (PAAG-12) and Polylactic Acid Films Charged with α-Tocopherol and Their Antioxidant Capacity in Food Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Films
2.3. Characterization of PAAG-12 Films
2.4. Preparation of Food Simulants
Estimation of Partition Coefficients and Diffusivities
2.5. Preparation of Oil-In-Water (O/W) Emulsions and Determination of Primary Oxidation
2.6. Statistical Analyses
3. Results and Discussion
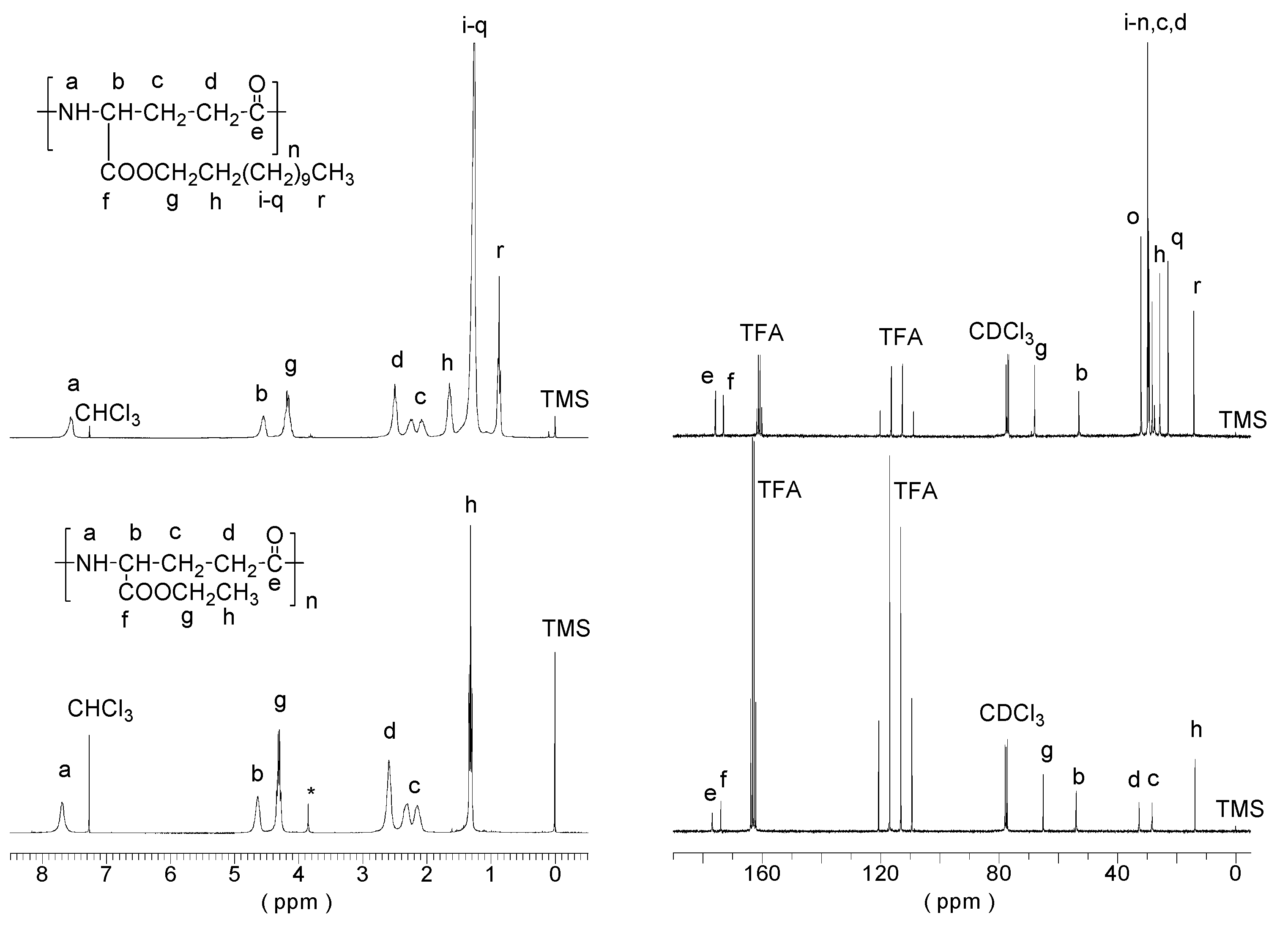
3.1. PAAG-12 Synthesis and Film Preparation
3.2. PAAG-12 Film Characterization Using TGA and DSC
3.3. Food Models
3.3.1. Food Simulant
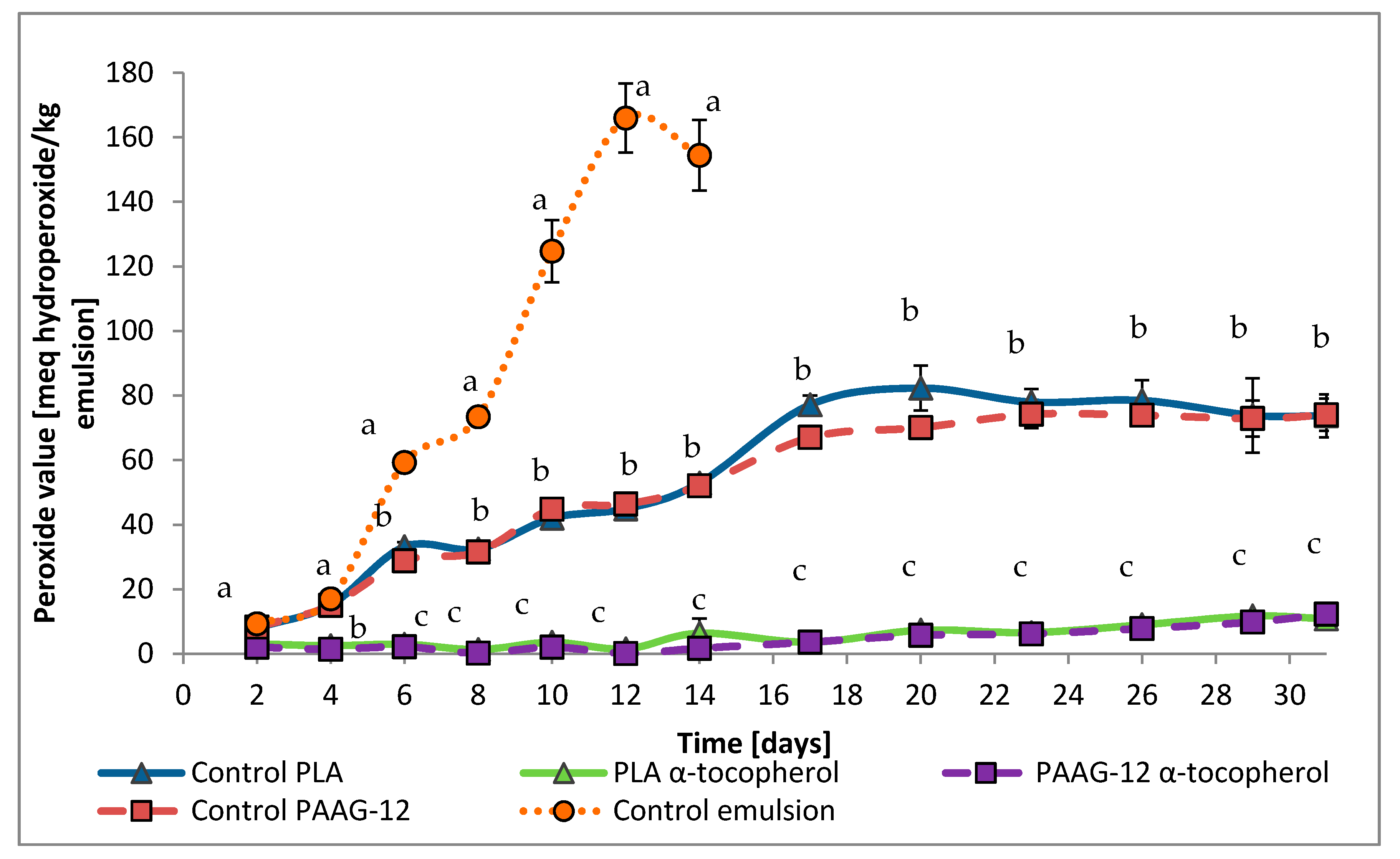
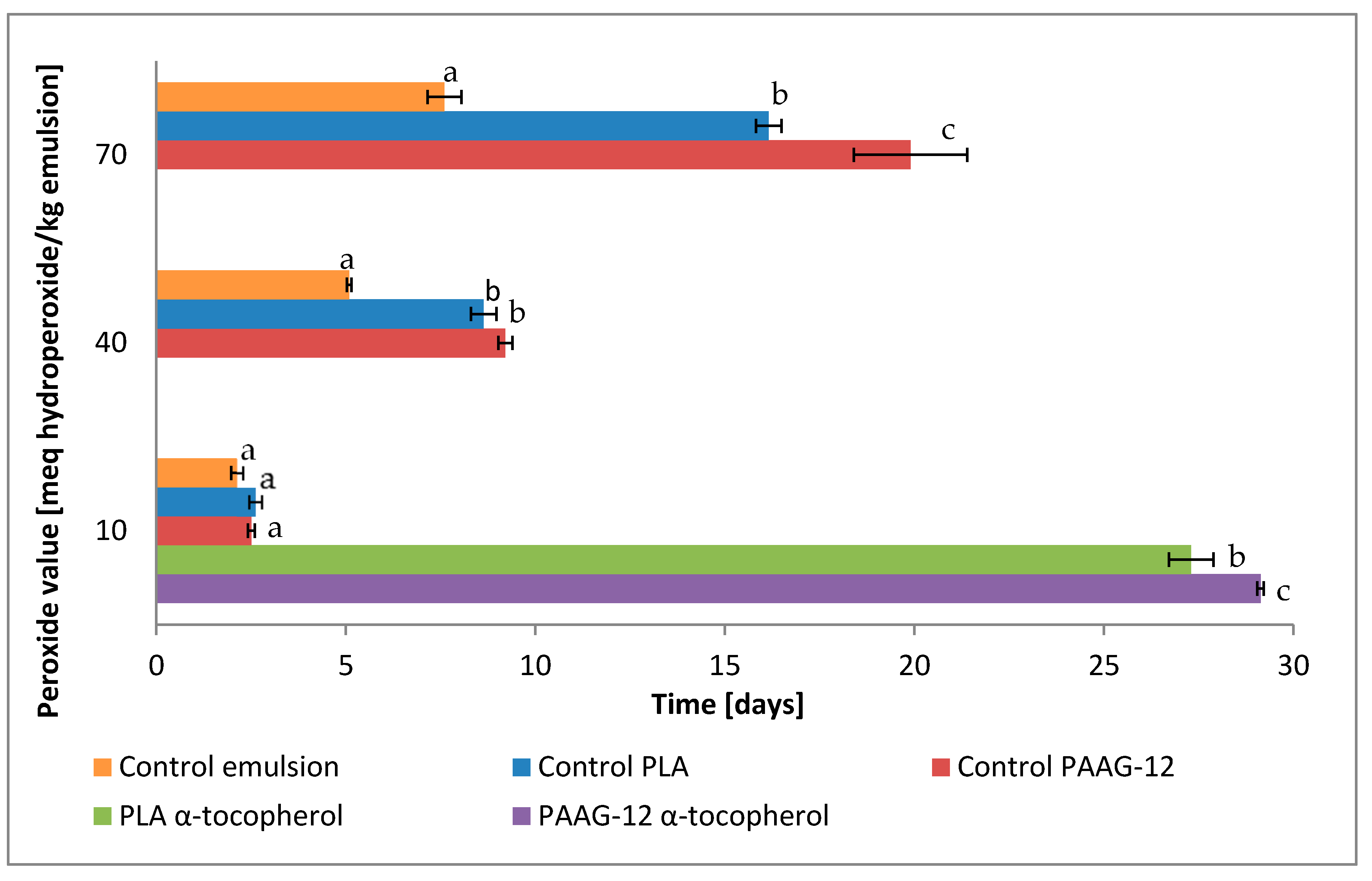
3.3.2. Primary Oxidation of O/W Emulsions Determined with Peroxide Value Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.H.; Ahn, D.U.; Eun, J.B.; Moon, S.H. Antioxidant Effect of Extracts from the Coffee Residue in Raw and Cooked Meat. Antioxidants 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.G.; Skowyra, M.; Gordon, M.H.; Azman, N.A.M.; Almajano, M.P. Effect of Leaves of Caesalpinia decapetala on Oxidative Stability of Oil-in-Water Emulsions. Antioxidants 2017, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A. Nutritional Biochemistry of the Vitamins. Nutr. Biochem. Vitam. 2003, 521, 80388 8. [Google Scholar]
- Zhu, X.; Schaich, K.M.; Chen, X.; Chung, D.; Yam, K.L. Target release rate of antioxidants to extend induction period of lipid oxidation. Food Res. Int. 2012, 47, 1–5. [Google Scholar] [CrossRef]
- Graciano-Verdugo, A.Z.; Soto-Valdez, H.; Peralta, E.; Cruz-Zárate, P.; Islas-Rubio, A.R.; Sánchez-Valdes, S.; Sánchez-Escalante, A.; González-Méndez, N.; González-Ríos, H. Migration of α-tocopherol from LDPE films to corn oil and its effect on the oxidative stability. Food Res. Int. 2010, 43, 1073–1078. [Google Scholar] [CrossRef]
- Granda-Restrepo, D.M.; Soto-Valdez, H.; Peralta, E.; Troncoso-Rojas, R.; Vallejo-Cordoba, B.; Gamez-Meza, N.; Graciano-Verdugo, A.Z. Migration of α-tocopherol from an active multilayer film into whole milk powder. Food Res. Int. 2009, 42, 1396–1402. [Google Scholar] [CrossRef]
- Sun, L.N.; Lu, L.X.; Qiu, X.L.; Tang, Y.L. Development of low-density polyethylene antioxidant active films containing α-tocopherol loaded with MCM-41(Mobil Composition of Matter No. 41) mesoporous silica. Food Control 2017, 71, 193–199. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C.; Garrigós, M.D.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.F.; Rhim, J.W. Grapefruit seed extract incorporated antimicrobial LDPE and PLA films: Effect of type of polymer matrix. LWT 2016, 74, 338–345. [Google Scholar] [CrossRef]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- Manzanarez-López, F.; Soto-Valdez, H.; Auras, R.; Peralta, E. Release of α-Tocopherol from Poly(lactic acid) films, and its effect on the oxidative stability of soybean oil. J. Food Eng. 2011, 104, 508–517. [Google Scholar] [CrossRef]
- Gonçalves, C.M.; Tomé, L.C.; Garcia, H.; Brandão, L.; Mendes, A.M.; Marrucho, I.M. Effect of natural and synthetic antioxidants incorporation on the gas permeation properties of poly(lactic acid) films. J. Food Eng. 2013, 116, 562–571. [Google Scholar] [CrossRef]
- Marcos, B.; Sárraga, C.; Castellari, M.; Kappen, F.; Schennink, G.; Arnau, J. Development of biodegradable films with antioxidant properties based on polyesters containing α-tocopherol and olive leaf extract for food packaging applications. Food Packag. Shelf Life 2014, 1, 140–150. [Google Scholar] [CrossRef]
- Martelli, S.M.; Motta, C.; Caon, T.; Alberton, J.; Bellettini, I.C.; Prado, A.C.P.D.; Barreto, P.L.M.; Soldi, V. Edible carboxymethyl cellulose films containing natural antioxidant and surfactants: α-tocopherol stability, in vitro release and film properties. LWT 2017, 77, 21–29. [Google Scholar] [CrossRef]
- Tolentino, A.; Alla, A.; De Ilarduya, A.M.; Muñoz-Guerra, S. Complexes of polyglutamic acid and long-chain alkanoylcholines: Nanoparticle formation and drug release. Int. J. Biol. Macromol. 2014, 66, 346–353. [Google Scholar] [CrossRef]
- Portilla-Arias, J.A.; Muñoz-Guerra, S.; Ma Barradas-Dermitz, D.; Guadalupe Aguilar-Uscanga, M. El ácido Poli(γ-glutámico): Producción y Aplicaciones Biomédicas. BioTecnología 2012, 16, 21–36. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1979; ISBN 9780198534112. [Google Scholar]
- Samsudin, H.; Auras, R.; Mishra, D.; Dolan, K.; Burgess, G.; Rubino, M.; Selke, S.; Soto-Valdez, H. Migration of antioxidants from polylactic acid films: A parameter estimation approach and an overview of the current mass transfer models. Food Res. Int. 2018, 103, 515–528. [Google Scholar] [CrossRef]
- Chen, X.; Lee, D.S.; Zhu, X.; Yam, K.L. Release Kinetics of Tocopherol and Quercetin from Binary Antioxidant Controlled-Release Packaging Films. J. Agric. Food Chem. 2012, 60, 3492–3497. [Google Scholar] [CrossRef]
- Gallego, G.; Hakkarainen, M.; Almajano, M.P. Stability of O/W emulsions packed with PLA film with incorporated rosemary and thyme. Eur. Food Res. Technol. 2017, 243, 1249–1259. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M.; Almajano, M.P. Antioxidant Properties of Three Aromatic Herbs (Rosemary, Thyme and Lavender) in Oil-in-Water Emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- Polymer stabilization: Some recent developments. Plast. Addit. Compd. 2001, 3, 28–30. [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Desobry, S. Release of synthetic phenolic antioxidants from extruded poly lactic acid (PLA) film. Food Control 2012, 28, 445–455. [Google Scholar] [CrossRef]
- Muller, J.; Quesada, A.C.; González-Martínez, C.; Chiralt, A. Justine Antimicrobial properties and release of cinnamaldehyde in bilayer films based on polylactic acid (PLA) and starch. Eur. Polym. J. 2017, 96, 316–325. [Google Scholar] [CrossRef]
- Mascheroni, E.; Guillard, V.; Nalin, F.; Mora, L.; Piergiovanni, L. Diffusivity of propolis compounds in Polylactic acid polymer for the development of anti-microbial packaging films. J. Food Eng. 2010, 98, 294–301. [Google Scholar] [CrossRef]
- Codex Alimentarius Codex Standard for Named Vegetable Oils. Codex-Stan 1999, 210, 1–13.
- Samsudin, H.; Soto-Valdez, H.; Auras, R. Poly(lactic acid) film incorporated with marigold flower extract (Tagetes erecta) intended for fatty-food application. Food Control 2014, 46, 55–66. [Google Scholar] [CrossRef]
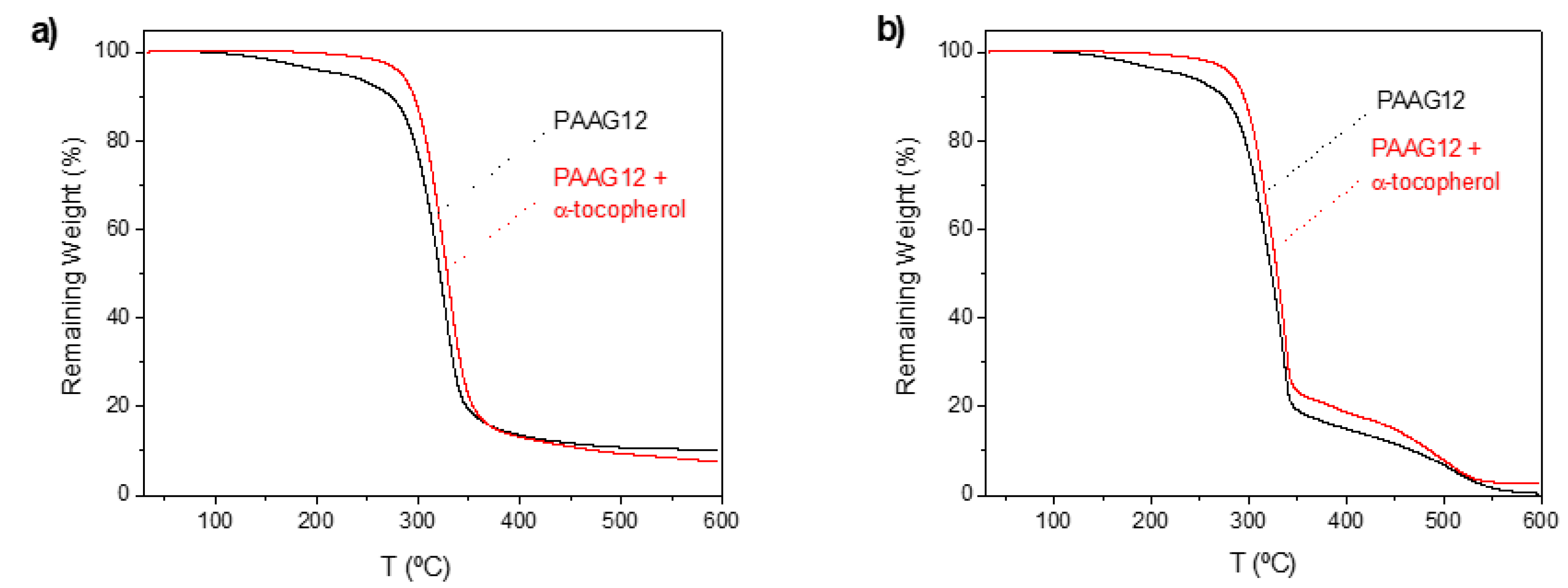
| Sample | Atmosphere | TGA a | ||
|---|---|---|---|---|
| oTd (°C) | maxTd (°C) | Rw (%) | ||
| PAAG-12 | N2 | 226.4 | 325.6 | 10.2 |
| Air | 229.1 | 337.4 | 0.4 | |
| PAAG-12 + 5% α-tocopherol | N2 | 284.1 | 328.9 | 7.5 |
| Air | 279.4 | 338.7 | 2.6 | |
| α-tocopherol | N2 | 275.8 | 365.7 | 0 |
| Air | 291.1 | 341.1 | 0.2 | |
| Polymer | Simulant | KP,f | D (m2/s) |
|---|---|---|---|
| PAAG-12 | EtOH 50% | 1.05 | 1.12 × 10−11 |
| EtOH 95% | 0.37 | 5.58 × 10−10 | |
| PLA | EtOH 50% | 0.94 | 1.11 × 10−10 |
| EtOH 95% | 0.36 | 1.12 × 10−10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villasante, J.; Codina, E.; Hidalgo, G.I.; Martínez de Ilarduya, A.; Muñoz-Guerra, S.; Almajano, M.P. Poly (α-Dodecyl γ-Glutamate) (PAAG-12) and Polylactic Acid Films Charged with α-Tocopherol and Their Antioxidant Capacity in Food Models. Antioxidants 2019, 8, 284. https://doi.org/10.3390/antiox8080284
Villasante J, Codina E, Hidalgo GI, Martínez de Ilarduya A, Muñoz-Guerra S, Almajano MP. Poly (α-Dodecyl γ-Glutamate) (PAAG-12) and Polylactic Acid Films Charged with α-Tocopherol and Their Antioxidant Capacity in Food Models. Antioxidants. 2019; 8(8):284. https://doi.org/10.3390/antiox8080284
Chicago/Turabian StyleVillasante, Juliana, Eva Codina, Gádor Indra Hidalgo, Antxon Martínez de Ilarduya, Sebastián Muñoz-Guerra, and María Pilar Almajano. 2019. "Poly (α-Dodecyl γ-Glutamate) (PAAG-12) and Polylactic Acid Films Charged with α-Tocopherol and Their Antioxidant Capacity in Food Models" Antioxidants 8, no. 8: 284. https://doi.org/10.3390/antiox8080284
APA StyleVillasante, J., Codina, E., Hidalgo, G. I., Martínez de Ilarduya, A., Muñoz-Guerra, S., & Almajano, M. P. (2019). Poly (α-Dodecyl γ-Glutamate) (PAAG-12) and Polylactic Acid Films Charged with α-Tocopherol and Their Antioxidant Capacity in Food Models. Antioxidants, 8(8), 284. https://doi.org/10.3390/antiox8080284