1. Introduction
Barley is the basic raw material for brewing. Phenolic compounds identified in barley include flavonoids, phenolic acids, and proanthocyanidins (PCs) [
1,
2]. There are more than 50 PCs in barley, comprising flavan-3-ol oligomers and their polymers [
3]. The oligomers include dimers (prodelphinidin B3 and procyanidin B3), trimers, tetramers, and pentamers, while polymers are formed by oxidation and polymerization of simple flavan-3-ols [
4]. Barley PCs ranged from 25 to 250 mg/100 g of grain [
5,
6,
7,
8]. Among them, proanthocyanidin trimers, such as catechin–gallocatechin–catechin (C–GC–C), prodelphinidin B3 and procyanidin B2 [
9] are the most representative in barley. In addition, hops also contribute to the proanthocyanidin content in brewing spent grains (BSGs); in fact, according to several authors, this ingredient contains high amounts of catechin and procyanidins [
10,
11].
Furthermore, PCs showed anti-bacterial [
12], anti-viral [
13], anti-carcinogenic [
14], anti-inflammatory [
15], and cardioprotective effects [
16]. Some studies demonstrated the potential of PCs for prevention or treatment of oxidative stress-associated diseases due to their antioxidant capacity [
17]. In addition, PCs are easily extracted, affordable, and demonstrated low toxicity [
17].
During the process of brewing, many BSGs are generated from barley grains after separation of the wort, and they consist of the residues from malted barley which could contain adjuncts (non-malt sources of fermentable sugars) such as wheat, rice, or maize and hop added during mashing [
1]. Consequently, this by-product is rich in protein, fibers, arabinoxylans, and β-glucan, and also contains PCs in low concentration; thus, its reutilization could be useful for the food industry, and offers an opportunity for cereal-based baked and extruded products with acceptable sensory and nutritional characteristics [
1].
In this sense, the challenge is to increase the efficient collection of PC-rich extracts with high bioactivity by the optimization of the extraction process. Thus far, conventional solid/liquid extraction was often used, employing as an extraction solvent a mixture of acetone and water in proportions from 50/50 to 80/20 [
4,
8,
18,
19] due to the large number of OH groups in PCs. In addition, bath-ultrasound-assisted extraction is the most used extraction technique. Some authors carried out pressurized solvent extraction, which is a static solid/liquid extraction with high pressure and eventually high temperature in stainless-steel extraction cells. Nevertheless, conventional extractions using ultrasonic-assisted extraction seem to be the best choice, since it is an economical technique, can be performed at atmospheric pressure and ambient temperature, and it could be developed on an ultrasound (US) bath or even with an US probe (or sonotrode) [
20,
21].
To carry out the determination of PCs in cereal, high-performance liquid chromatography (HPLC) is the analytical technique usually applied to this aim. In many instances, this technique was coupled to a diode array detector (DAD), fluorometric detector (FLD), and mass spectrometer detector (MSD) [
8,
22,
23], or matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) analysis [
24].
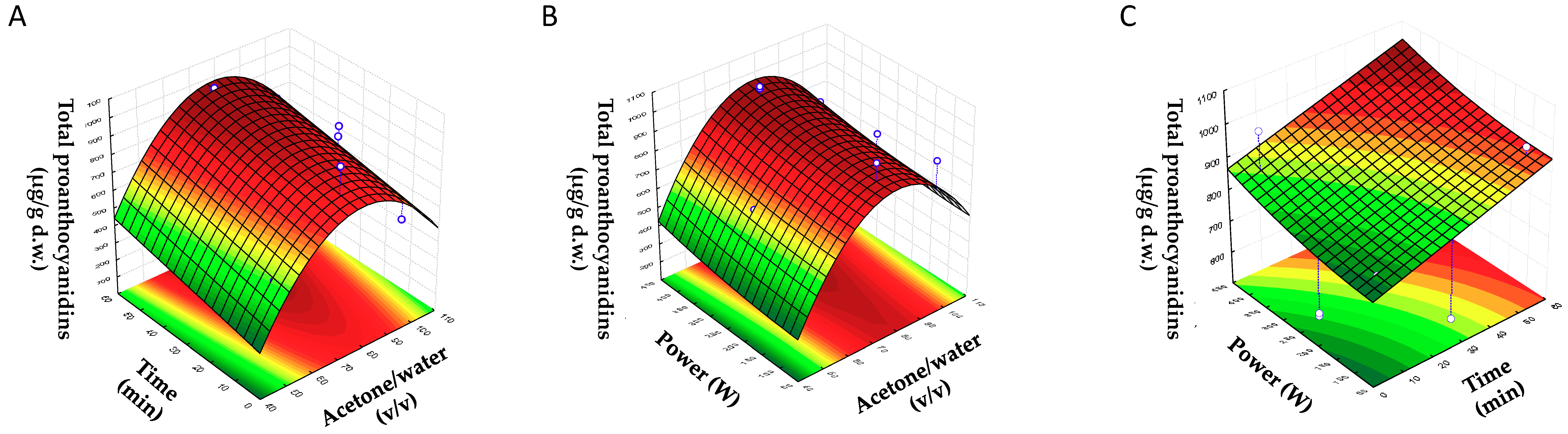
In view of the above, the objective of this work was to evaluate the recovery of proanthocyanidins from BSGs by establishing a sonotrode ultrasonic-assisted extraction method. For that purpose, response surface methodology (RSM) was performed to evaluate extraction parameters with an experimental Box–Behnken design.
2. Materials and Methods
2.1. Samples
Brewers’ spent grain (BSG) samples were obtained in a micro-brewing plant after pilsner beer production (Mastrobirraio, Cesena, Italy, 44°08′00″ north (N), 12°14′00″ east €).
2.2. Chemicals
HPLC-grade water and solvents were purchased from Merck KGaA (Darmstadt, Germany). Catechin was purchased from Sigma-Aldrich (St. Louis, MO).
2.3. Experimental Design
Response surface methodology (RSM) is the most popular tool for modeling. In RSM, statistical models and polynomial equations are always combined to provide an approximate relationship between the dependent and independent variables [
25]. In the present work, a Box–Behnken design (BBD) with three factors was carried out in order to optimize the extraction parameters of proanthocyanidins in BSGs. The parameters of ultrasound-assisted extraction (US) can be divided into US parameters (ultrasound frequency, duration, acoustic power/intensity, and treatment mode) and non-US parameters (solvent type, solvent/sample ratio, particle size, temperature) [
25]. In this work, the factors investigated were acetone/water (X1), time (X2), and potency (X3), with three levels for each factor, and the response variable (Y) was the sum of the total content of proanthocyanidins (PCs). The range for the percentage of acetone/water was chosen based on the conditions previously established in other works (50, 75, and 100%) [
4,
8]; the extraction time (5, 30, and 55 min) and the US power (80, 240, and 400 W) were the same as those previously used in a study where a sonotrode US was employed to optimize these parameters for the extraction of phenolic compounds from
Psidium guajava L. leaves [
26]. The design consisted of 15 combinations including three center points (
Table 1), and the experiments were randomized to maximize the effects of unexplained variability in the observed response, due to extraneous factors.
The determination of optimal US sonotrode parameters was carried out using STATISTICA 7.0 (2002, StatSoft, Tulsa, OK).
2.4. Extraction of Proanthocyanidins from Brewers’ Spent Grains by Sonotrode Ultrasonic Extraction
The extraction was achieved with a US sonotrode UP400St (Hielscher Ultrasonics GmbH, Teltow, Germany) and, during the extraction, an ice bath was used to avoid rises in temperature. The temperature ranged between 23 and 25 °C in all extractions, and it was measured with a thermometer at the end of each extraction. The percentage of acetone/water, the extraction time, and the US power were varied according to the experimental design. After the extraction, samples were centrifuged at 1000× g for 10 min; supernatants were collected, evaporated, and reconstituted in 1 mL of methanol/water (1/1, v/v). The final extracts were filtered through 0.2-μm polytetrafluoroethylene (PTFE) syringe filters and stored at −18 °C until the analyses.
2.5. Conventional Extraction of Proanthocyanidins
The results obtained by the US sonotrode at the optimal conditions were compared with a PC extract from BSGs obtained via conventional solid/liquid extraction. The extraction methodology was carried out according to Carciochi et al. [
27]. Briefly, BSGs were subjected to mechanical agitation with a
w/
v ratio of 1/30, temperature of 80 °C, 72/28 ethanol/water (
v/
v), and an extraction time of 60 min.
2.6. Determination of Proanthocyanidins in Brewing Spent Grain Extracts by HPLC-FLD-MS Analysis
The separation of proanthocyanidins was performed on an Agilent 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA) equipped with a binary pump delivery system, a degasser, an autosampler, and FLD and MS detectors (MSD, model G1946A, Santa Clara, CA, USA). A Develosil Diol 100 Å column (250 × 4.6 mm, 5 µm particle size) purchased from Phenomenex (Torrance, CA, USA) was used for the analyses.
All solvents were HPLC-grade and were filtered in a filter disc of 0.45 μm. According to Robbins et al. [
28], the elution binary gradient consisted of CH3CN/HOAc, 98/2 (
v/v) as solvent A, and CH
3OH/H
2O/HOAc 95/3/2
v/v/v as solvent B. The analyses started with 7% of phase B from 0 to 3 min. Thus, solvent B was increased to 37.6% (from 3.1 to 57 min) and then to 100% B over the next 3 min for 7 min. After that, the initial condition was established, and they were maintained for 16 min. The injection volume was 5 μL and all the analyses were run at 35°C. Additionally, fluorescence detection was conducted with an excitation wavelength of 230 nm and an emission wavelength of 321 nm.
Moreover, identification of proanthocyanidins was carried out by HPLC-MS according to Verardo et al. [
8]. Furthermore, quantification of PCs was done employing a calibration curve of (+)-catechin done from the limit of quantitation (LOQ) to 250 μg/mL (LOQ = 0.193 µg/mL). In addition, the quantification of dimers, trimers, tetramers, pentamers, and the polymers was done using the correction factors suggested by Robbins et al. [
28].
4. Conclusions
HPLC-FLD-MS was used for the determination of proanthocyanidins in brewers spent grains for the first time. A Box–Behnken experimental design was used in order to optimize the sonotrode ultrasound-assisted extraction parameters to obtain the maximum proanthocyanidin content from BSG. According to the model, the most important effect on the response came from the quadratic term of acetone/water ratio, followed by the linear term of acetone/water, the linear term of the time of extraction, and the quadratic term of US power. The highest value of proanthocyanidins was obtained at 80% acetone/water (v/v), 55 min, and 400 W. Finally, it was proven that sonotrode ultrasonic extraction is a more effective technique than conventional extraction method, providing a higher recovery of proanthocyanidins from BSG.
To conclude, BSGs represent a good raw material that could be used for the extraction of bioactive compounds or could be reused for the production of functional flours. In this way, further work will be done in order to validate this hypothesis.