Relationship of the Phytochemicals from Coffee and Cocoa By-Products with their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Extracts from Coffee and Cocoa By-Products
2.3. Cell Cultures
2.3.1. Adipocyte Differentiation
2.3.2. Cell Viability Assay
2.4. Anti-Inflammatory Potential
2.4.1. Determination of Inflammatory Factors in Macrophages
2.4.2. Assessment of Adipokines and Inflammation-Triggered Lipolysis in Adipocytes
2.5. Antioxidant Potential
2.5.1. Detection of Intracellular ROS, Mitochondrial Superoxide, and Mitochondrial Membrane Potential
2.5.2. Mitochondrial Content and Activity
2.6. Anti-Adipogenic Potential
2.6.1. Determination of Cellular Lipid Accumulation
2.6.2. Assessment of Lipolysis in Adipocytes
2.6.3. Evaluation of Adipocyte Brown Differentiation
2.7. Insulin Sensitizing Potential
2.7.1. Quantification of Glucose Uptake
2.7.2. Determination of GLUT4 Translocation
2.7.3. Evaluation of Insulin Signaling Pathway Phosphorylation Pattern
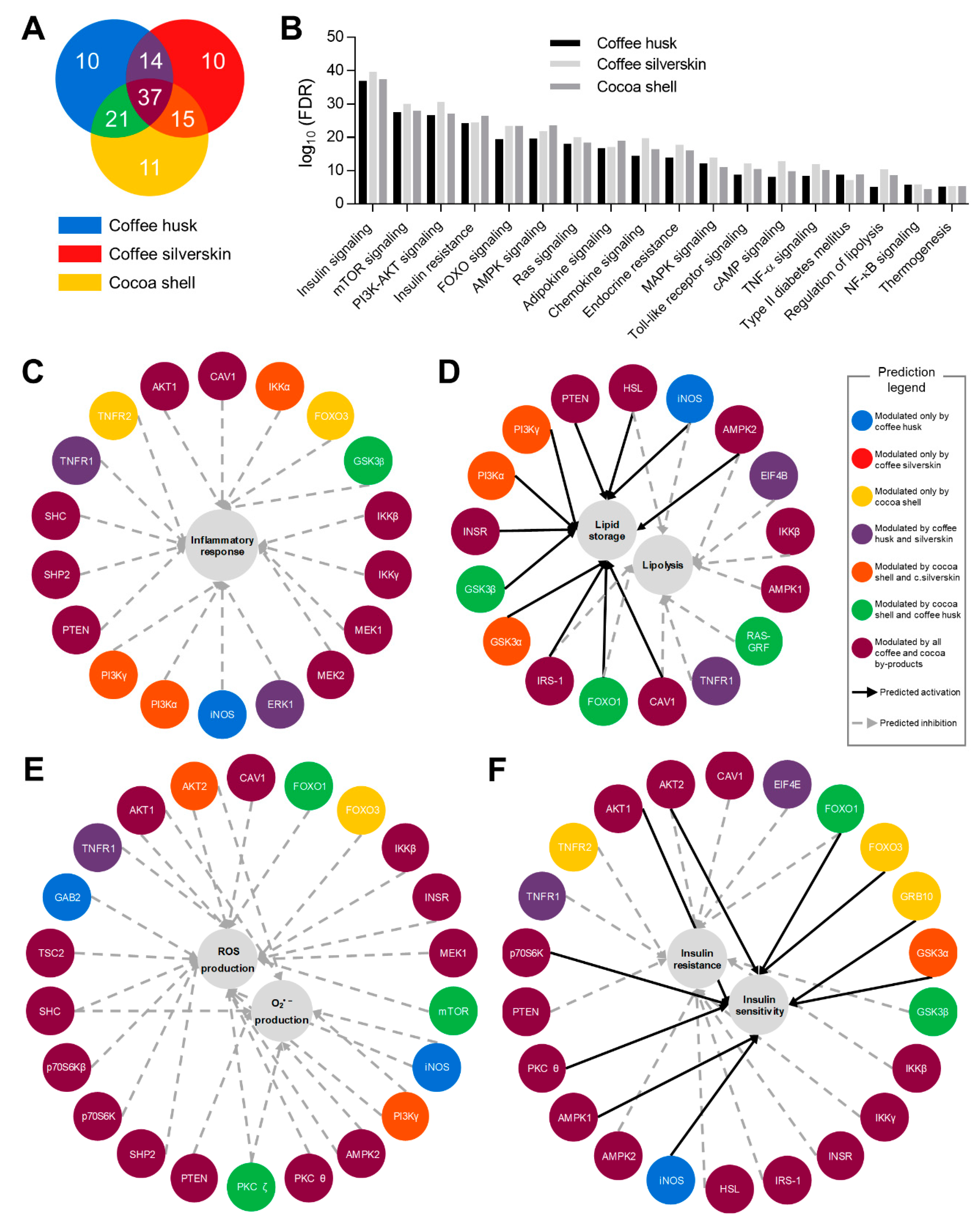
2.8. Bioinformatic Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Coffee By-Products were Mainly Composed of Chlorogenic Acid and Caffeine whereas Cocoa Shell Primarily Contained Methylxanthines
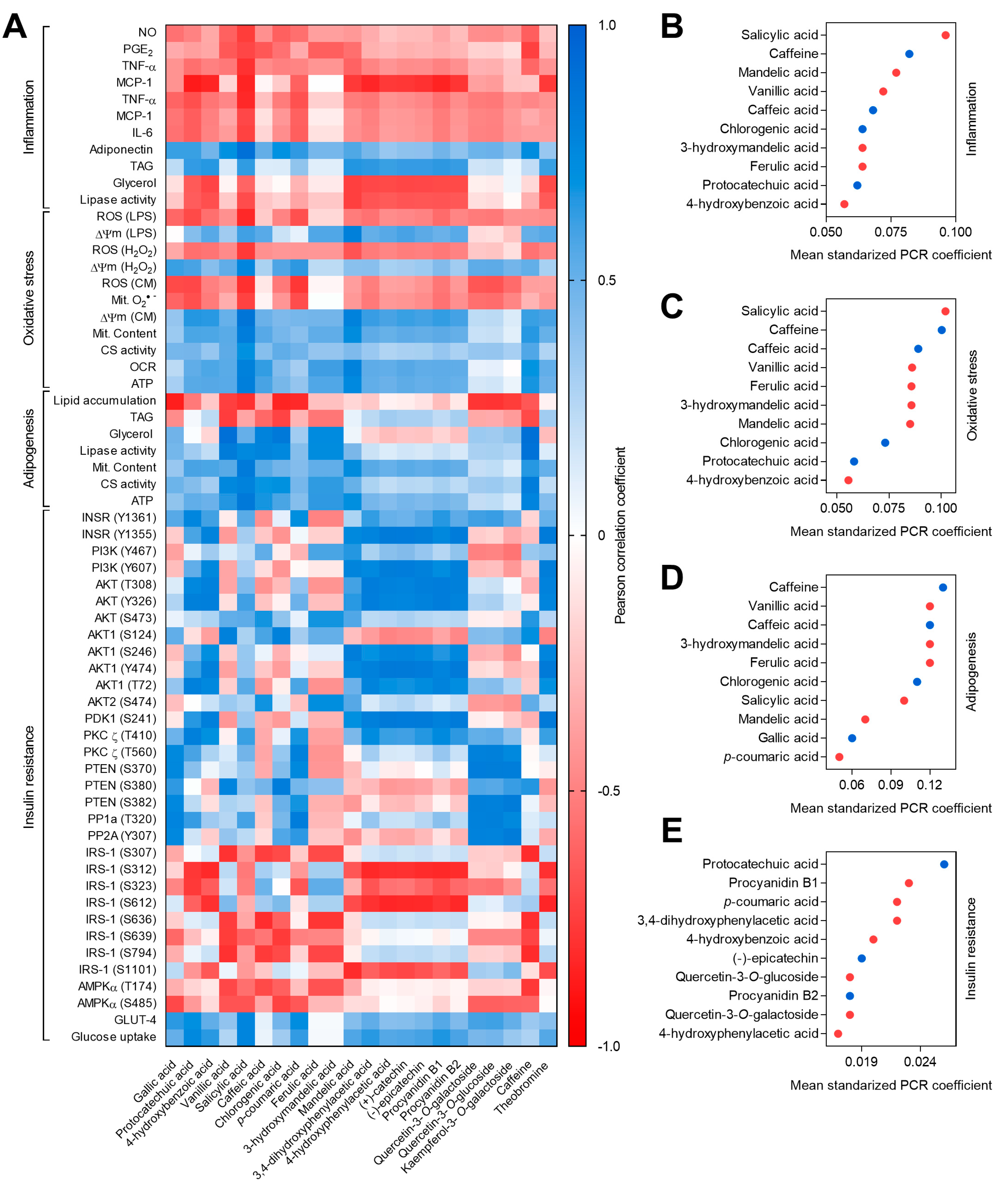
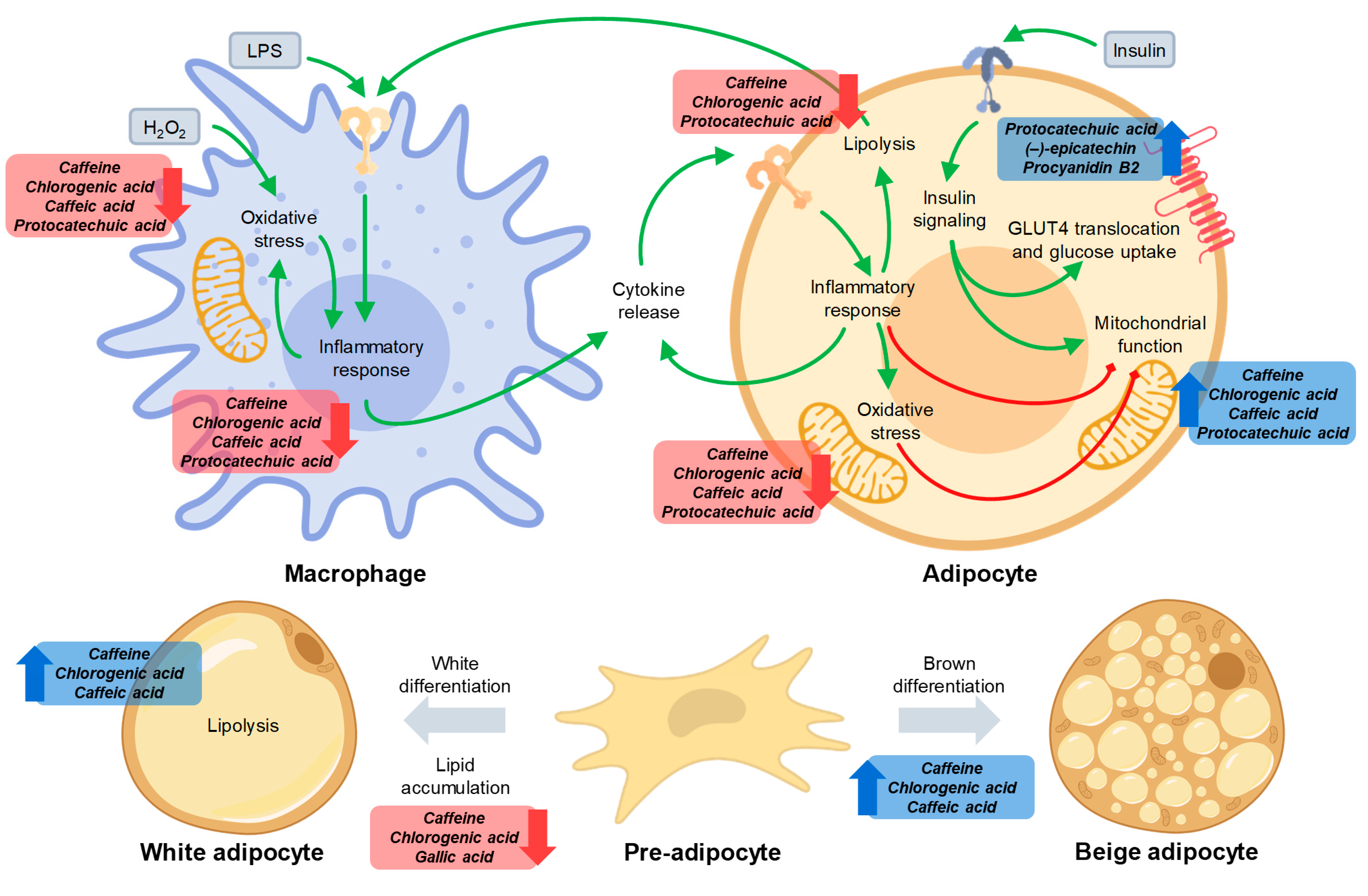
3.2. Caffeine and Phenolics in Coffee and Cocoa By-Products Reduced Inflammation in Macrophages and Adipocytes
3.3. Phenolic Compounds Reduced Oxidative Stress in Macrophages and Adipocytes and Caffeine Preserved Adipocyte Mitochondrial Function
3.4. Caffeine and Phenolics in Coffee and Cocoa By-Products Attenuated Adipogenesis and Promoted Adipocyte Browning
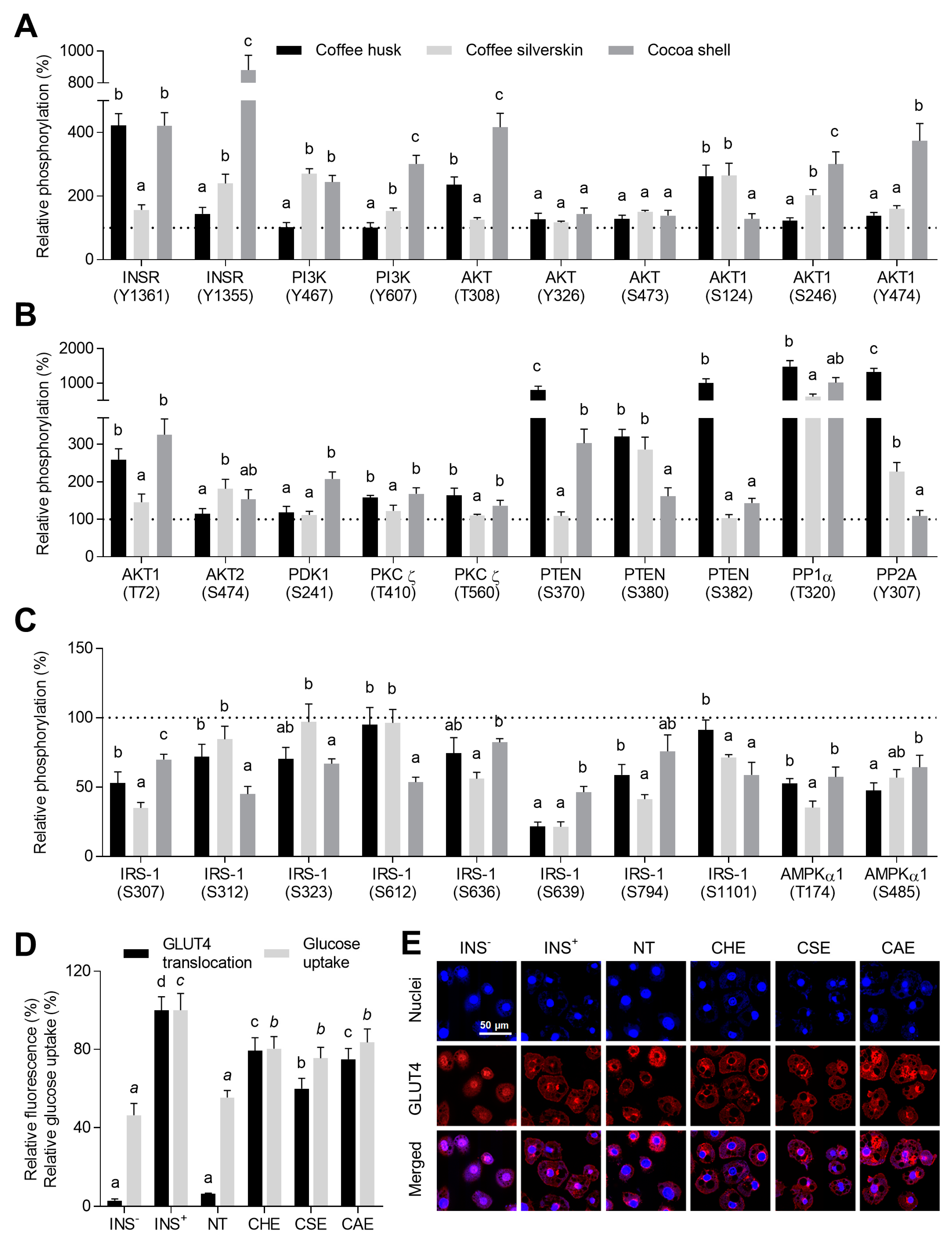
3.5. Phenolic Compounds Counteracted Insulin Resistance through the Modulation of Insulin Signaling and the Promotion of GLUT4 Translocation
3.6. Phytochemicals from Coffee and Cocoa By-Products Regulated Protein Phosphorylation thereby Preventing Inflammation, Oxidative Stress, and Insulin Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 May 2019).
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Guarner, V.; Rubio-Ruiz, M.E. Aging and Health-A Systems Biology Perspective. Interdiscipl Top Gerontol. Basel, Karger 2015, 40, 99–106. [Google Scholar]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 2015, 16, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aguilera, A.; Rull, A.; Rodríguez-Gallego, E.; Riera-Borrull, M.; Luciano-Mateo, F.; Camps, J.; Menéndez, J.A.; Joven, J. Mitochondrial dysfunction: A basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediat. Inflamm. 2013, 2013, 135698. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Nishida, J.; Ogawa, Y. A Paracrine Loop Between Adipocytes and Macrophages Aggravates Inflammatory Changes. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, Y.; Martin-Cabrejas, M.A.; González de Mejia, E. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016, 15, 405–423. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.A.; Donovan, J.L.; Waterhouse, A.L.; Williamson, G. Cocoa and health: A decade of research. Br. J. Nutr. 2008, 99, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, E.; Tamir, S.; Weinstein, A.; Weinstein, Y. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.S.; Coleman, C.; Shelton, R.; Heemstra, L.A.; Novak, C.M. Caffeine enhances activity thermogenesis and energy expenditure in rats. Clin. Exp. Pharmacol. Physiol. 2019, 46, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Fuggetta, M.P.; Zonfrillo, M.; Villivà, C.; Bonmassar, E.; Ravagnan, G. Inflammatory Microenvironment and Adipogenic Differentiation in Obesity: The Inhibitory Effect of Theobromine in a Model of Human Obesity In Vitro. Mediators Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.B.P.P. Chapter 1—State of the art in coffee processing by-products. In Handbook of Coffee Processing By-Products; Academic Press: London, UK, 2017; pp. 1–26. ISBN 9780128112908. [Google Scholar]
- Oddoye, E.O.K.; Agyente-Badu, C.K.; Gyedu-Akoto, E. Cocoa and Its By-Products: Identification and Utilization. In Chocolate in Health and Nutrition; Humana Press: Totowa, NJ, USA, 2013; pp. 23–37. [Google Scholar]
- Donovan, J.L.; Crespy, V.; Oliveira, M.; Cooper, K.A.; Gibson, B.B.; Williamson, G. (+)-Catechin is more bioavailable than (−)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radic. Res. 2006, 40, 1029–1034. [Google Scholar] [CrossRef]
- Ellam, S.; Williamson, G. Cocoa and human health. Annu. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Coffee and Health: A Review of Recent Human Research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Panak Balentić, J.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N.; Panak Balentić, J.; Ačkar, Đ.; et al. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation Between Macrophages and Adipocytes in vitro. Mol. Nutr. Food Res. 2019, 63, 1801413. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martin-Cabrejas, M. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Monagas, M.; Moreno-Arribas, M.V.; Bartolomé, B. Determination of Microbial Phenolic Acids in Human Faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011, 59, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Weiss, M.; Gonzalez de Mejia, E. Anthocyanins from Purple Corn Ameliorated Tumor Necrosis Factor-α-Induced Inflammation and Insulin Resistance in 3T3-L1 Adipocytes via Activation of Insulin Signaling and Enhanced GLUT4 Translocation. Mol. Nutr. Food Res. 2017, 61, 1700362. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Kwon, D.; Cha, H.-J.; Lee, H.; Hong, S.-H.; Park, C.; Park, S.-H.; Kim, G.-Y.; Kim, S.; Kim, H.-S.; Hwang, H.-J.; et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Lee, O.-H.; Zheng, Y.; Kang, I.-J. Erigeron annuus (L.) Pers. Extract Inhibits Reactive Oxygen Species (ROS) Production and Fat Accumulation in 3T3-L1 Cells by Activating an AMP-Dependent Kinase Signaling Pathway. Antioxidants 2019, 8, 139. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic composition, caffeine content and antioxidant capacity of coffee silverskin. Food Res. Int. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Morales-Sillero, A.M.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2017; Volume 960, pp. 327–343. [Google Scholar]
- Vane, J.R.; Botting, R.M. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998, 104, 2S–8S. [Google Scholar] [CrossRef]
- Yin, M.-J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998, 396, 77. [Google Scholar] [CrossRef]
- Bao, W.; Luo, Y.; Wang, D.; Li, J.; Wu, X.; Mei, W. Sodium salicylate modulates inflammatory responses through AMP-activated protein kinase activation in LPS-stimulated THP-1 cells. J. Cell. Biochem. 2018, 119, 850–860. [Google Scholar] [CrossRef]
- An, Y.; Liu, K.; Zhou, Y.; Liu, B. Salicylate Inhibits Macrophage-Secreted Factors Induced Adipocyte Inflammation and Changes of Adipokines in 3T3-L1 Adipocytes. Inflammation 2009, 32, 296–303. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Kim, K.-J.; Ryu, S.-J.; Lee, B.-Y. Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem. Biol. Interact. 2016, 248, 1–7. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, S.-Y.; Park, Y.-L.; Myung, D.-S.; Rew, J.-S.; Joo, Y.-E. Chlorogenic acid suppresses lipopolysaccharide-induced nitric oxide and interleukin-1β expression by inhibiting JAK2/STAT3 activation in RAW264.7 cells. Mol. Med. Rep. 2017, 16, 9224–9232. [Google Scholar] [CrossRef]
- Langin, D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 2006, 53, 482–491. [Google Scholar] [CrossRef]
- Ormazabal, P.; Scazzocchio, B.; Varì, R.; Santangelo, C.; D’Archivio, M.; Silecchia, G.; Iacovelli, A.; Giovannini, C.; Masella, R. Effect of protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: Possible role for PTP1B. Int. J. Obes. 2018, 42, 2012–2021. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; Zhao, Y.; Liu, J.; Liu, C.; Zeng, X. Anti-Inflammatory Effects of p-coumaric acid in LPS-stimulated RAW264.7 cells: Involvement of NF-κB and MAPKs pathways. Med. Chem. (Los Angeles). 2016, 6, 327. [Google Scholar]
- Salmon, A.B. Beyond Diabetes: Does Obesity-Induced Oxidative Stress Drive the Aging Process? Antioxidants 2016, 5, 24. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Varì, R.; D’Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef]
- Li, J.; He, D.; Wang, B.; Zhang, L.; Li, K.; Xie, Q.; Zheng, L. Synthesis of hydroxycinnamic acid derivatives as mitochondria-targeted antioxidants and cytotoxic agents. Acta Pharm. Sin. B 2017, 7, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-H.; Yu, C.-H.; Chang, Y.-P.; Lin, Y.-T.; Huang, C.-J.; Kuo, Y.-H.; Tsai, P.-J.; Tsai, T.-H.; Yu, C.-H.; Chang, Y.-P.; et al. Protective Effect of Caffeic Acid Derivatives on tert-Butyl Hydroperoxide-Induced Oxidative Hepato-Toxicity and Mitochondrial Dysfunction in HepG2 Cells. Molecules 2017, 22, 702. [Google Scholar] [CrossRef]
- Dragicevic, N.; Delic, V.; Cao, C.; Copes, N.; Lin, X.; Mamcarz, M.; Wang, L.; Arendash, G.W.; Bradshaw, P.C. Caffeine increases mitochondrial function and blocks melatonin signaling to mitochondria in Alzheimer’s mice and cells. Neuropharmacology 2012, 63, 1368–1379. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, C.-C.; Huang, H.-C.; Wei, Y.-H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013, 280, 1039–1050. [Google Scholar] [CrossRef]
- Tarozzi, A.; Morroni, F.; Hrelia, S.; Angeloni, C.; Marchesi, A.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neurosci. Lett. 2007, 424, 36–40. [Google Scholar] [CrossRef]
- Guan, S.; Jiang, B.; Bao, Y.M.; An, L.J. Protocatechuic acid suppresses MPP+-induced mitochondrial dysfunction and apoptotic cell death in PC12 cells. Food Chem. Toxicol. 2006, 44, 1659–1666. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Miguéliz, I.; Romo-Hualde, A.; López-Yoldi, M.; Martínez, J.; Vizmanos, J.; Milagro, F.; González-Navarro, C. Phenolic Compounds Inhibit 3T3-L1 Adipogenesis Depending on the Stage of Differentiation and Their Binding Affinity to PPARγ. Molecules 2019, 24, 1045. [Google Scholar] [CrossRef]
- Peng, S.; Pang, Y.; Zhu, Q.; Kang, J.; Liu, M.; Wang, Z. Chlorogenic Acid Functions as a Novel Agonist of PPAR γ 2 during the Differentiation of Mouse 3T3-L1 Preadipocytes. Biomed. Res. Int. 2018, 2018, 1–14. [Google Scholar]
- Duangjai, A.; Nuengchamnong, N.; Suphrom, N.; Trisat, K.; Limpeanchob, N.; Saokaew, S. Potential of Coffee Fruit Extract and Quinic Acid on Adipogenesis and Lipolysis in 3T3-L1 Adipocytes. Kobe, J. Med. Sci. 2018, 64, E84–E92. [Google Scholar]
- Zhu, X.; Yang, L.; Xu, F.; Lin, L.; Zheng, G. Combination therapy with catechins and caffeine inhibits fat accumulation in 3T3-L1 cells. Exp. Ther. Med. 2017, 13, 688–694. [Google Scholar] [CrossRef]
- Murosaki, S.; Lee, T.R.; Muroyama, K.; Shin, E.S.; Cho, S.Y.; Yamamoto, Y.; Lee, S.J. A Combination of Caffeine, Arginine, Soy Isoflavones, and l-Carnitine Enhances Both Lipolysis and Fatty Acid Oxidation in 3T3-L1 and HepG2 Cells in Vitro and in KK Mice in Vivo. J. Nutr. 2007, 137, 2252–2257. [Google Scholar] [CrossRef] [Green Version]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Publ. Gr. 2013, 10, 24–36. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [Green Version]
- van Dam, A.D.; Nahon, K.J.; Kooijman, S.; van den Berg, S.M.; Kanhai, A.A.; Kikuchi, T.; Heemskerk, M.M.; van Harmelen, V.; Lombès, M.; van den Hoek, A.M.; et al. Salsalate activates brown adipose tissue in mice. Diabetes 2015, 64, 1544–1554. [Google Scholar] [CrossRef]
- Velickovic, K.; Wayne, D.; Leija, H.A.L.; Bloor, I.; Morris, D.E.; Law, J.; Budge, H.; Sacks, H.; Symonds, M.E.; Sottile, V. Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Sci. Rep. 2019, 9, 9104. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Matsushita, M.; Hibi, M.; Tone, H.; Takeshita, M.; Yasunaga, K.; Katsuragi, Y.; Kameya, T.; Sugie, H.; Saito, M. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am. J. Clin. Nutr. 2017, 105, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Anusree, S.S.; Nisha, V.M.; Priyanka, A.; Raghu, K.G. Insulin resistance by TNF-α is associated with mitochondrial dysfunction in 3T3-L1 adipocytes and is ameliorated by punicic acid, a PPARγ agonist. Mol. Cell. Endocrinol. 2015, 413, 120–128. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Deyoung, S.M.; Saltiel, A.R. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E166–E174. [Google Scholar] [CrossRef] [Green Version]
- De Meyts, P. The Insulin Receptor and Its Signal. Transduction Network. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Scazzocchio, B.; Varì, R.; Filesi, C.; Del Gaudio, I.; D’Archivio, M.; Santangelo, C.; Iacovelli, A.; Galvano, F.; Pluchinotta, F.R.; Giovannini, C.; et al. Protocatechuic acid activates key components of insulin signaling pathway mimicking insulin activity. Mol. Nutr. Food Res. 2015, 59, 1472–1481. [Google Scholar] [CrossRef]
- Vishnu Prasad, C.N.; Anjana, T.; Banerji, A.; Gopalakrishnapillai, A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010, 584, 531–536. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Stafeev, I.S.; Vorotnikov, A.V.; Ratner, E.I.; Menshikov, M.Y.; Parfyonova, Y.V. Latent Inflammation and Insulin Resistance in Adipose Tissue. Int. J. Endocrinol. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of all compounds are available from the authors for possible research projects in cooperation. |
| Compound | Concentration (µg/g Extract) | Chemical Structure | ||
|---|---|---|---|---|
| Coffee Husk | Coffee Silverskin | Cocoa Shell | ||
| Hydroxybenzoic acids |  | |||
| Gallic acid | 87.0 ± 5.5c | 16.9 ± 1.2a | 19.2 ± 0.4b | |
| Protocatechuic acid | 488.4 ± 26.2b | 44.1 ± 3.4a | 761.5 ± 47.6c |  |
| 4-hydroxybenzoic acid | 13.4 ± 1.3b | 3.4 ± 0.3a | 70.1 ± 9.3c |  |
| Vanillic acid | 22.90 ± 9.62a | 29.6 ± 0.6a | N.D. |  |
| Salicylic acid | 3.1 ± 0.1b | 2.3 ± 0.2a | 3.3 ± 0.2b |  |
| Hydroxycinnamic acids |  | |||
| Caffeic acid | 57.9 ± 2.0b | 538.0 ± 54.3c | 1.9 ± 0.2a | |
| Chlorogenic acid | 3456.8 ± 70.6b | 2791.7 ± 97.3a | N.D. |  |
| p-coumaric acid | 8.7 ± 0.2c | 0.9 ± 0.1a | 4.2 ± 0.6b |  |
| Ferulic acid | N.D. | 3.8 ± 0.2 | N.D. |  |
| Mandelic acids |  | |||
| 3-hydroxymandelic acid | N.D. | 4.4 ± 0.5 | N.D. | |
| Mandelic acid | N.D. | 5.1 ± 0.2a | 11.18 ± 1.2b |  |
| Phenylacetic acids |  | |||
| 3,4-dihydroxyphenylacetic acid | 5.6 ± 2.0a | N.D. | 25.9 ± 2.4b | |
| 4-hydroxyphenylacetic acid | N.D. | N.D. | 48.5 ± 4.3 |  |
| Flavan-3-ols: monomers |  | |||
| (+)-catechin | 1.7 ± 0.2a | 10.2 ± 1.1b | 200.8 ± 16.0c | |
| (−)-epicatechin | 18.0 ± 2.0a | N.D. | 222.1 ± 13.8b |  |
| Flavan-3-ols: dimers |  | |||
| Procyanidin B1 | 22.3 ± 2.6a | N.D. | 83.6 ± 7.8b | |
| Procyanidin B2 | 11.6 ± 1.8a | N.D. | 219.9 ± 11.4b |  |
| Flavonols |  | |||
| Quercetin-3-O-galactoside | 54.7 ± 0.5b | N.D. | 9.3 ± 0.4a | |
| Quercetin-3-O-glucoside | 57.4 ± 3.7b | N.D. | 11.12 ± 0.77a |  |
| Kaempferol-3-O-galactoside | 122.6 ± 3.6 | N.D. | N.D. |  |
| Alkaloids |  | |||
| Caffeine | 9815.5 ± 15.4b | 19,219.2 ± 37.6c | 2433.5 ± 7.8a | |
| Theobromine | N.D. | N.D. | 10,035.0 ± 4.5 |  |
| Biomarkers | Coffee Husk | Coffee Silverskin | Cocoa Shell | |||
|---|---|---|---|---|---|---|
| EC30 | EC50 | EC30 | EC50 | EC30 | EC50 | |
| RAW264.7 macrophages | ||||||
| NO release | 31.5 ± 5.0a | 73.4 ± 8.9a | 41.8 ± 4.4b | 97.5 ± 6.5b | 40.3 ± 9.8ab | 94.0 ± 16.5ab |
| PGE2 release | 134.0 ± 33.7b | 312.6 ± 48.4b | 58.0 ± 6.4a | 135.2 ± 9.4a | 118.4 ± 13.1b | 276.4 ± 19.2b |
| TNF-α release | 158.8 ± 43.2a | 370.4 ± 61.7a | 146.5 ± 29.5a | 341.8 ± 42.8a | 106.7 ± 35.7a | 498.9 ± 50.3b |
| MCP-1 release | 87.2 ± 12.1b | 203.5 ± 17.7b | 173.9 ± 19.7c | 420.3 ± 25.0a | 32.6 ± 4.7a | 76.1 ± 8.6a |
| 3T3-L1 adipocytes | ||||||
| TNF-α release | 59.8 ± 15.3a | 139.5 ± 21.9a | 138.0 ± 16.5c | 321.8 ± 24.1c | 87.8 ± 10.4b | 204.9 ± 15.2b |
| MCP-1 release | <31.0 | 80.0 ± 16.6a | <31.0 | 179.3 ± 36.8b | <31.0 | 132.5 ± 18.8b |
| IL-6 release | 88.2 ± 7.6a | >500 | 233.5 ± 86.7c | >500 | 112.5 ± 19.8b | >500 |
| Adiponectin release | 96.6 ± 9.7a | 225.4 ± 14.3a | 95.7 ± 9.8a | 232.9 ± 16.5a | 115.2 ± 17.2a | 268.7 ± 25.1b |
| Triglyceride content | <31.0 | 60.3 ± 10.0b | <31.0 | 32.6 ± 5.5a | <31.0 | <31.0 |
| Glycerol release | 77.5 ± 30.2a | 265.7 ± 42.2c | 68.5 ± 23.4a | 187.2 ± 25.7b | <31.0 | 26.7 ± 2.8a |
| Lipase activity | 107.1 ± 20.5b | 249.8 ± 29.7c | 70.5 ± 8.2a | 148.0 ± 12.3b | <31.0 | 30.2 ± 4.4a |
| Biomarkers | Coffee Husk | Coffee Silverskin | Cocoa Shell | |||
|---|---|---|---|---|---|---|
| EC30 | EC50 | EC30 | EC50 | EC30 | EC50 | |
| RAW264.7 macrophages | ||||||
| ROS (LPS) | <31.0 | 48.2 ± 2.4b | <31.0 | 69.5 ± 6.6c | <31.0 | 34.7 ± 2.7a |
| ΔΨm (LPS) | >500 | >500 | 167.7 ± 22.6a | 391.3 ± 33.0a | 143.5 ± 36.9a | 334.9 ± 52.8a |
| ROS (H2O2) | 51.7 ± 9.3 | 120.7 ± 13.5c | <31.0 | 71.7 ± 3.2b | <31.0 | 46.6 ± 3.5a |
| ΔΨm (H2O2) | <31.0 | 33.6 ± 3.7a | 32.4 ± 3.5a | 75.6 ± 6.6b | 41.9 ± 8.6a | 97.8 ± 12.4c |
| 3T3-L1 adipocytes | ||||||
| ROS | 54.8 ± 10.5a | 128.1 ± 15.2a | 150.2 ± 39.7c | 350.6 ± 56.9b | 82.4 ± 16.1b | 192.3 ± 23.4a |
| Mitochondrial O2• ‒ | 86.4 ± 24.5a | 201.6 ± 35.0a | 207.8 ± 57.2b | >500 | 94.8 ± 28.5a | 221.2 ± 40.4a |
| ΔΨm | 140.9 ± 14.8c | 336.0 ± 12.5b | 58.0 ± 6.3a | 135.3 ± 9.2a | 94.8 ± 18.5b | 161.5 ± 22.9a |
| Mitochondrial content | 40.2 ± 7.6 | 96.0 ± 8.6b | <31.0 | 43.2 ± 6.8a | <31.0 | 74.9 ± 12.1b |
| CS activity | <31.0 | <31.0 | <31.0 | <31.0 | <31.0 | <31.0 |
| OCR | 399.0 ± 66.1b | >500 | 125.01 ± 23.5a | 291.8 ± 34.0a | 158.11 ± 24.6a | 368.8 ± 35.9b |
| ATP content | 80.7 ± 11.3b | 188.2 ± 16.5c | <31.0 | 59.4 ± 7.6a | 39.4 ± 8.3a | 91.9 ± 14.3b |
| Biomarkers | Coffee Husk | Coffee Silverskin | Cocoa Shell | |||
|---|---|---|---|---|---|---|
| EC30 | EC50 | EC30 | EC50 | EC30 | EC50 | |
| Lipid accumulation | 132.7 ± 18.1a | 309.6 ± 23.8a | 220.8 ± 35.1b | 515.3 ± 46.1b | 388.1 ± 67.4c | 905.5 ± 88.4c |
| Triglyceride content | 64.9 ± 12.4a | 151.5 ± 30.5b | 49.6 ± 17.5a | 115.7 ± 22.3a | 353.1 ± 64.5b | 823.8 ± 146.7c |
| Glycerol release | <31.0 | <31.0 | <31.0 | <31.0 | 36.8 ± 8.4 | 85.9 ± 4.3 |
| Lipase activity | 357.3 ± 73.8b | 833.6 ± 106.7b | 210.9 ± 33.4a | 492.1 ± 48.6a | 422.2 ± 57.6b | 985.2 ± 142.9b |
| Mitochondrial content | >500 | >500 | >500 | >500 | >500 | >500 |
| CS activity | 227.6 ± 43.1b | 531.1 ± 62.4b | 140.9 ± 22.8a | 328.9 ± 33.2a | 247.4 ± 51.4b | 577.2 ± 74.3b |
| ATP content | >500 | >500 | 251.8 ± 24.0a | 587.5 ± 35.2a | 312.4 ± 44.6b | 728.9 ± 65.1b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the Phytochemicals from Coffee and Cocoa By-Products with their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro. Antioxidants 2019, 8, 279. https://doi.org/10.3390/antiox8080279
Rebollo-Hernanz M, Zhang Q, Aguilera Y, Martín-Cabrejas MA, Gonzalez de Mejia E. Relationship of the Phytochemicals from Coffee and Cocoa By-Products with their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro. Antioxidants. 2019; 8(8):279. https://doi.org/10.3390/antiox8080279
Chicago/Turabian StyleRebollo-Hernanz, Miguel, Qiaozhi Zhang, Yolanda Aguilera, Maria A. Martín-Cabrejas, and Elvira Gonzalez de Mejia. 2019. "Relationship of the Phytochemicals from Coffee and Cocoa By-Products with their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro" Antioxidants 8, no. 8: 279. https://doi.org/10.3390/antiox8080279
APA StyleRebollo-Hernanz, M., Zhang, Q., Aguilera, Y., Martín-Cabrejas, M. A., & Gonzalez de Mejia, E. (2019). Relationship of the Phytochemicals from Coffee and Cocoa By-Products with their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro. Antioxidants, 8(8), 279. https://doi.org/10.3390/antiox8080279










