Interaction of Potent Mitochondrial Uncouplers with Thiol-Containing Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Rat Liver Mitochondria
2.3. Mitochondrial Respiration
2.4. Membrane Potential (ΔΨ) Measurement in Isolated Mitochondria
2.5. Planar Bilayers
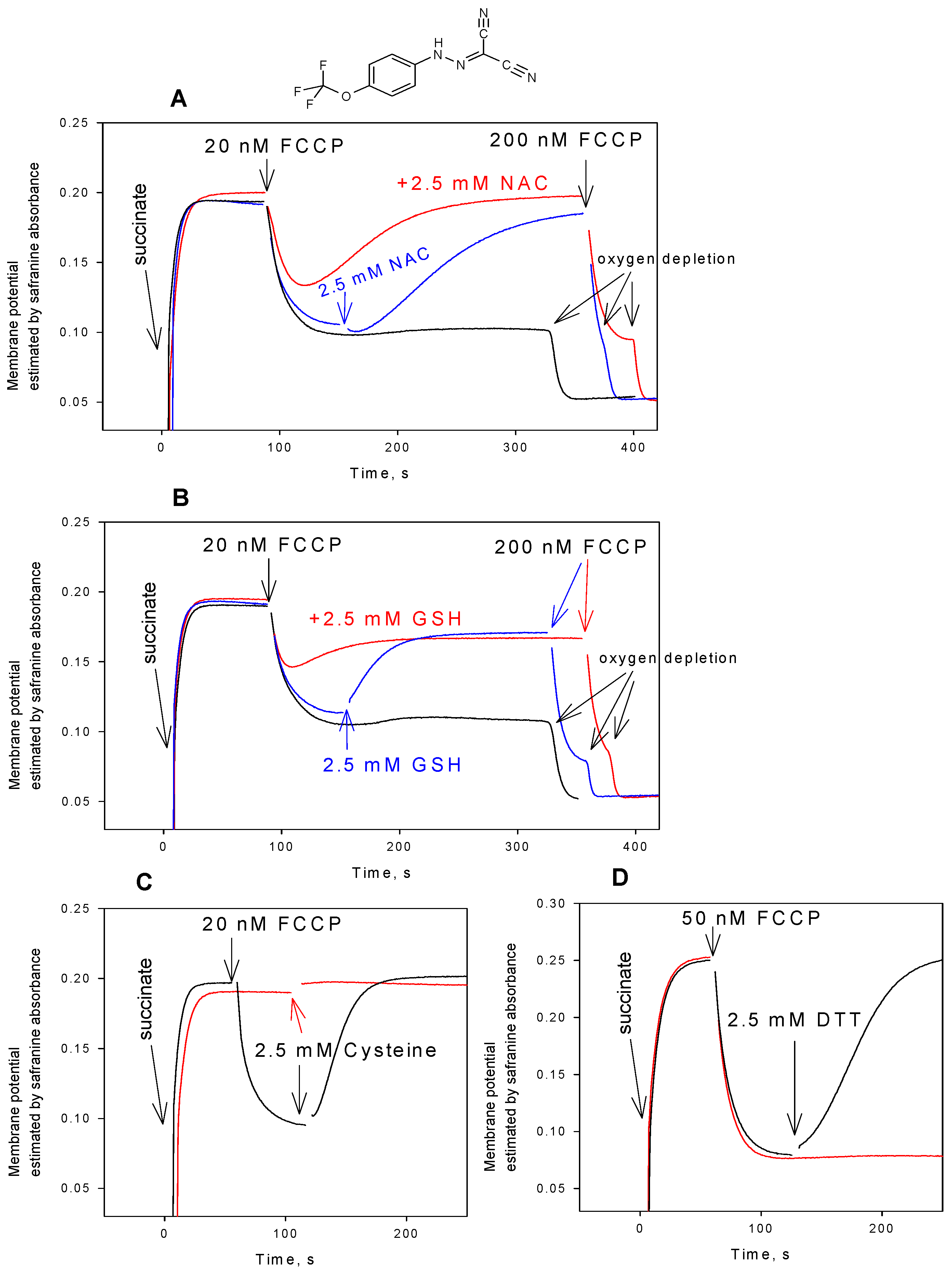
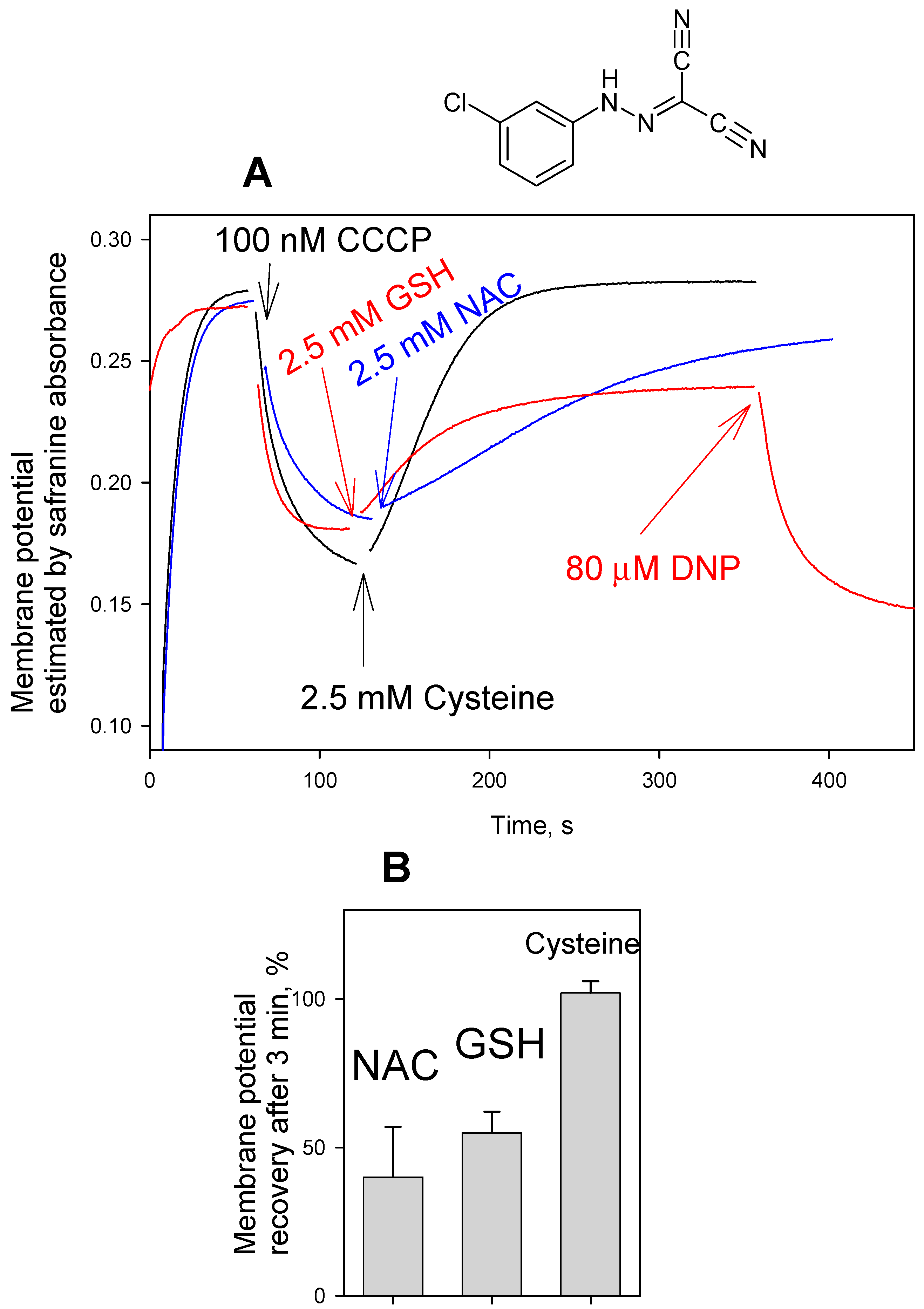
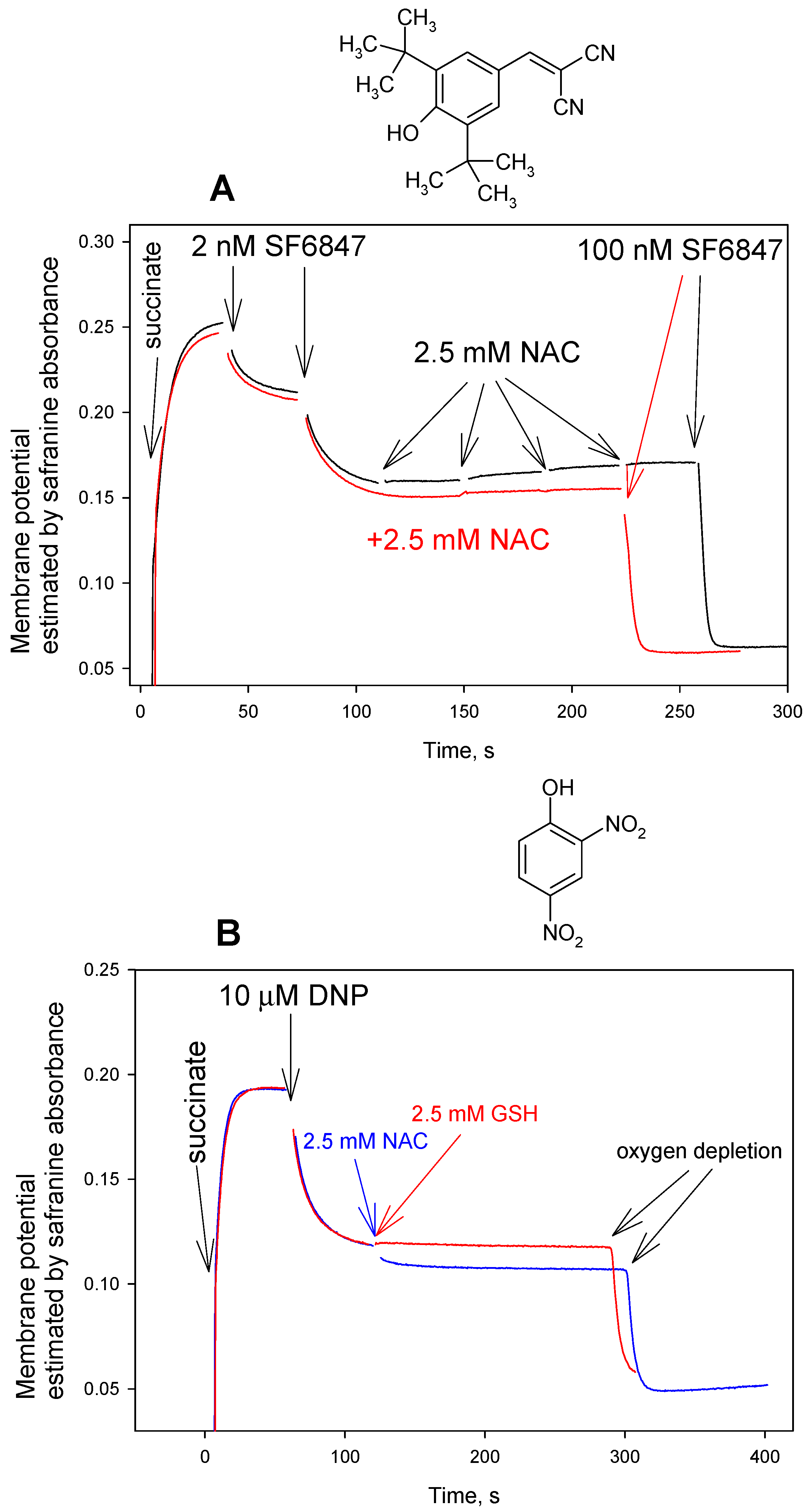
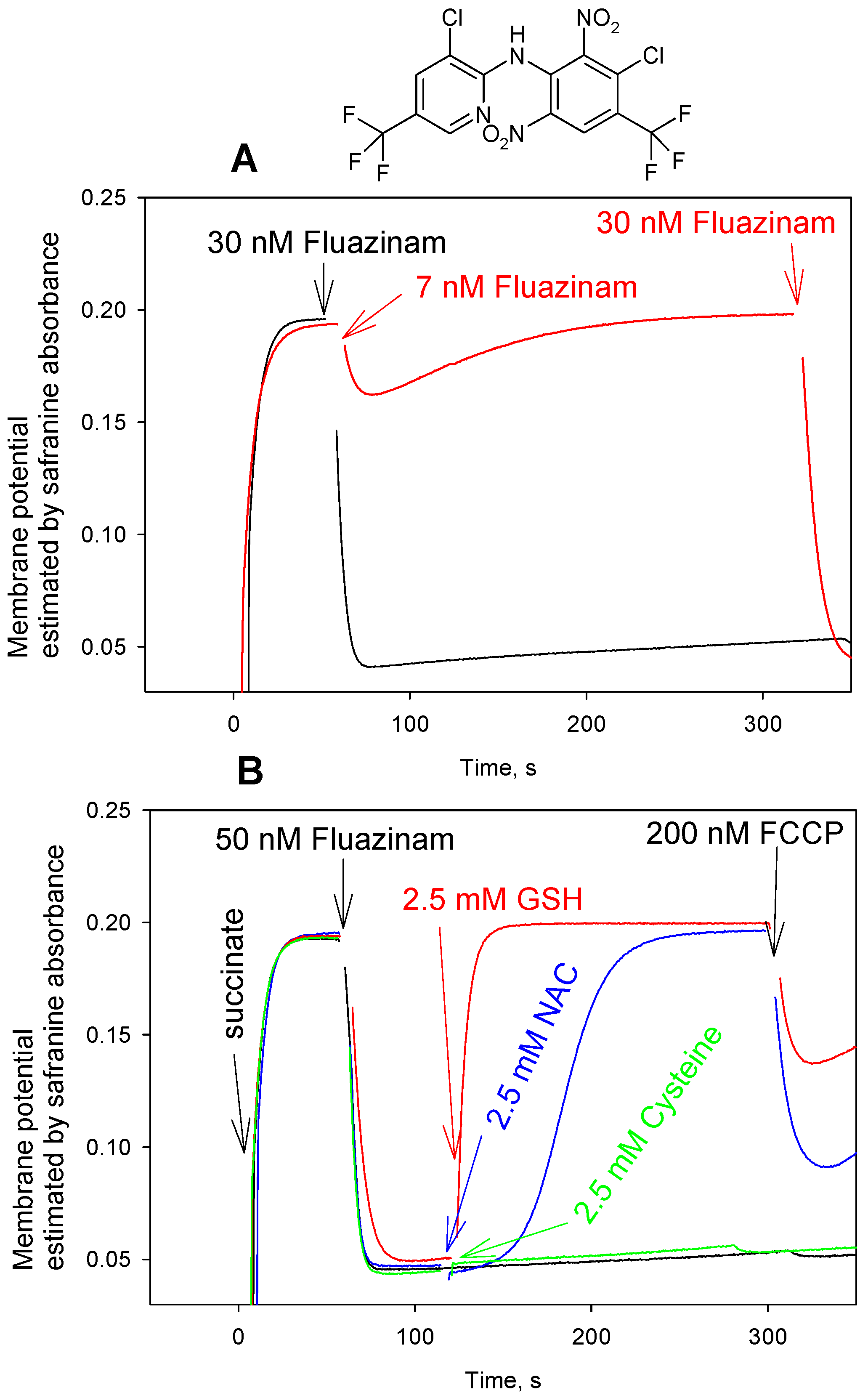
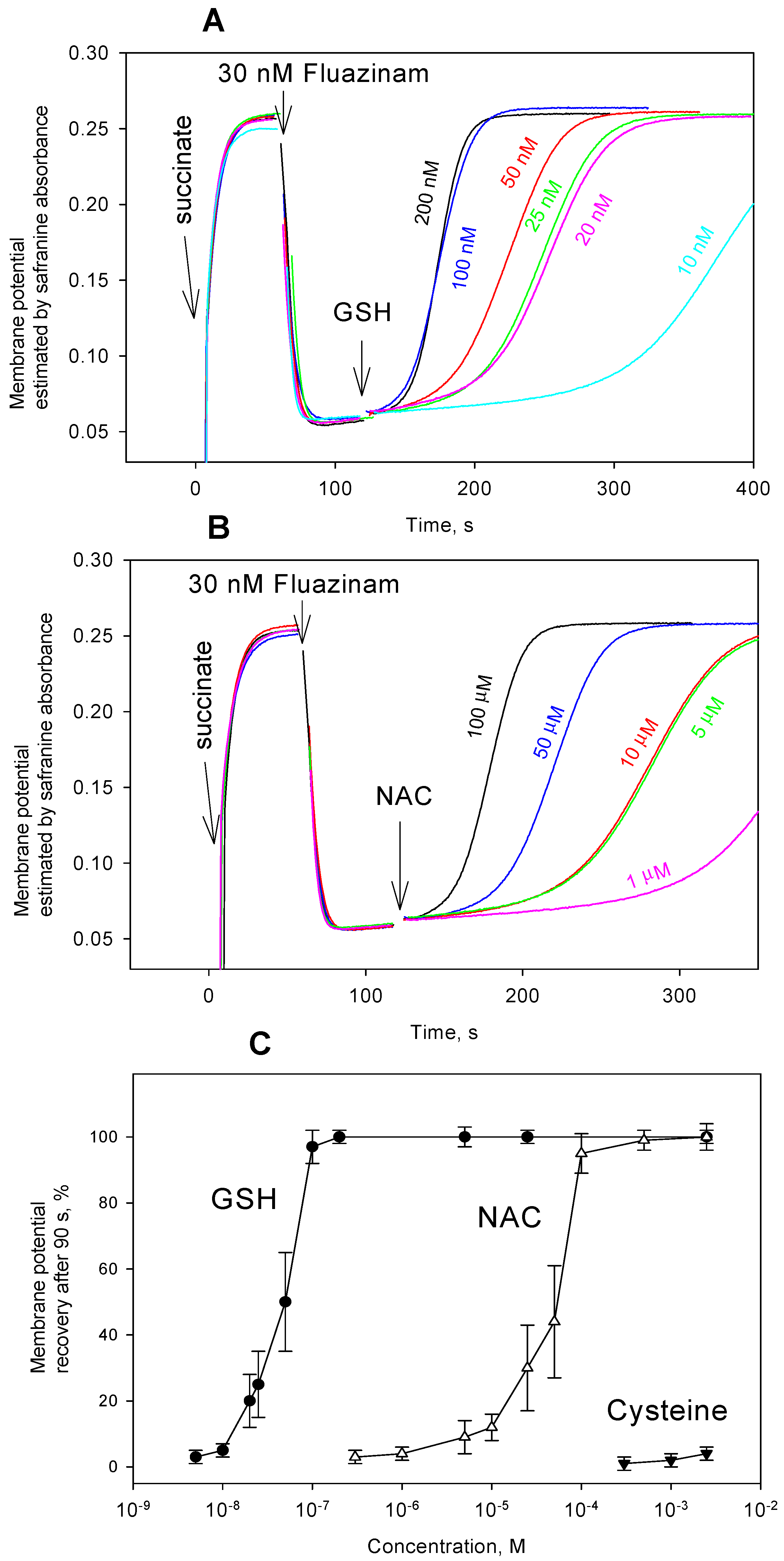
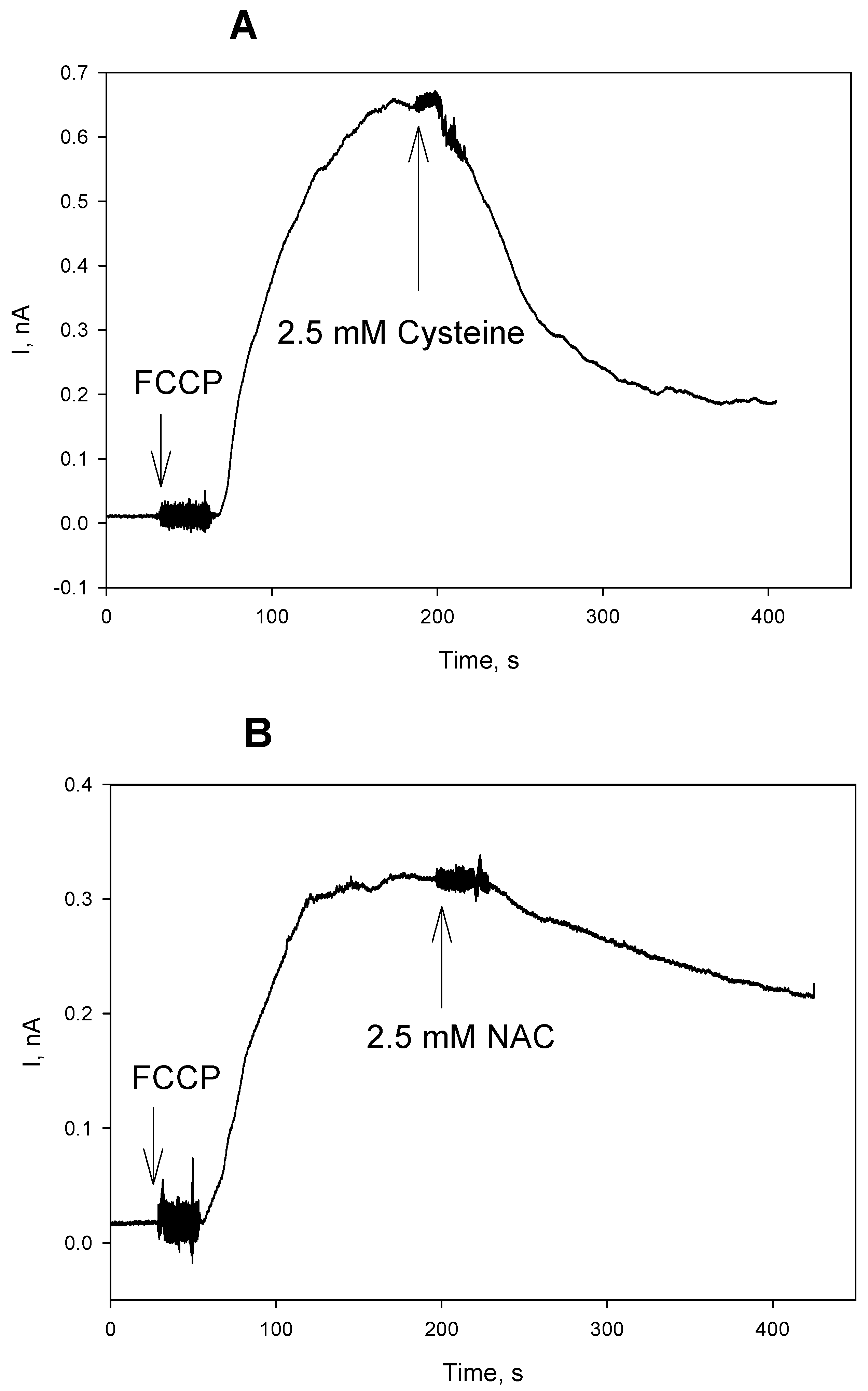
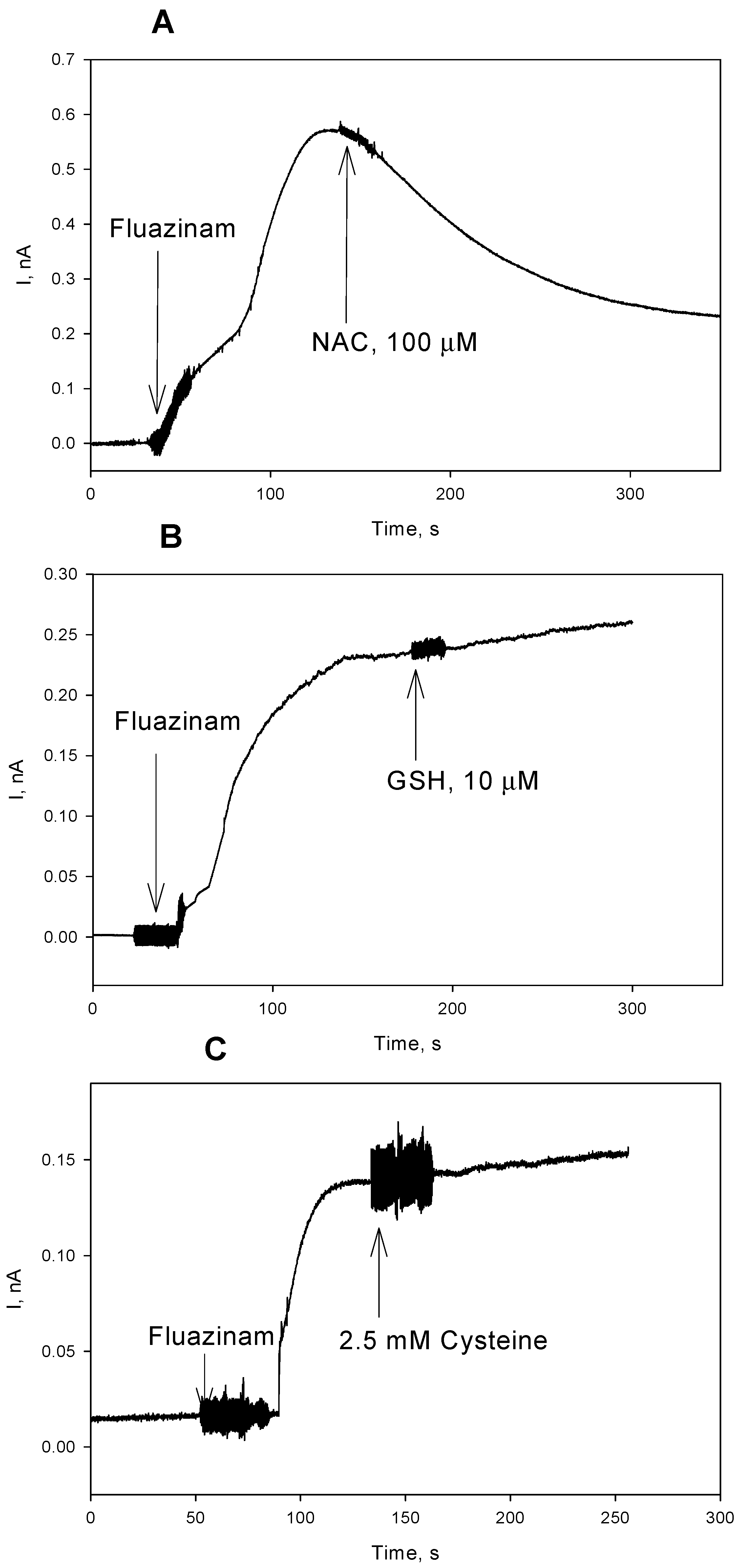
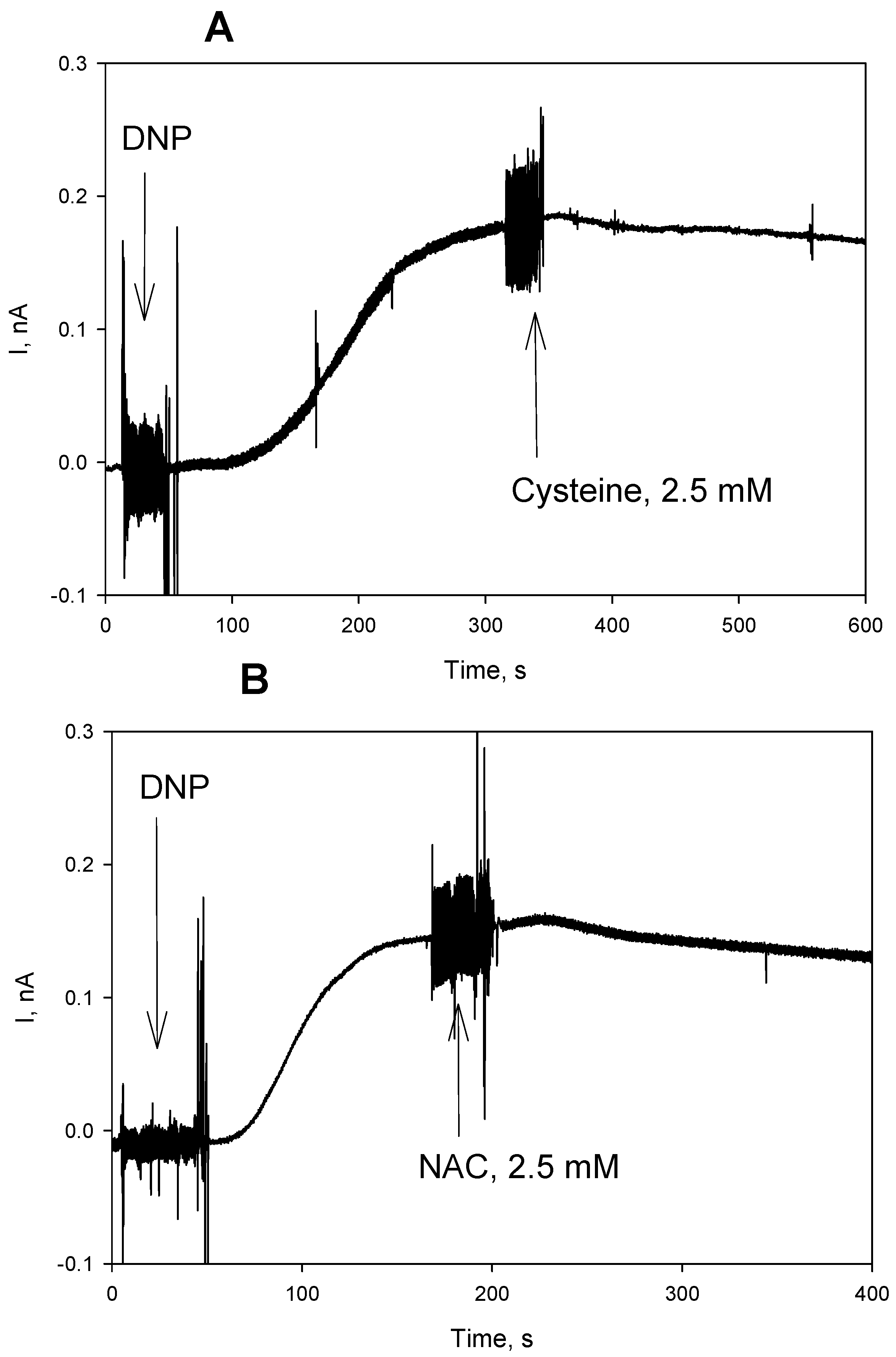
3. Results and Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Boveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv. Exp. Med. Biol. 1977, 78, 67–82. [Google Scholar] [PubMed]
- Cino, M.; Del Maestro, R.F. Generation of hydrogen peroxide by brain mitochondria: the effect of reoxygenation following postdecapitative ischemia. Arch. Biochem. Biophys. 1989, 269, 623–638. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef]
- Liu, S.S. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci. Rep. 1997, 17, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Ishige, K.; Tsai, C.; Maher, P. Tyrphostins protect neuronal cells from oxidative stress. J. Biol. Chem. 2002, 277, 36204–36215. [Google Scholar] [CrossRef] [PubMed]
- Starkov, A.A.; Fiskum, G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J. Neurochem. 2003, 86, 1101–1107. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Springer, J.E.; Hall, E.D.; Scheff, S.W. Mitochondrial uncoupling as a therapeutic target following neuronal injury. J. Bioenerg. Biomembr. 2004, 36, 353–356. [Google Scholar] [CrossRef]
- Geisler, J.G.; Marosi, K.; Halpern, J.; Mattson, M.P. DNP, Mitochondrial Uncoupling and Neuroprotection: A Little Dab’ll Do Ya. Alzheimers Dement. 2017, 13, 582–591. [Google Scholar] [CrossRef]
- Childress, E.S.; Alexopoulos, S.J.; Hoehn, K.L.; Santos, W.L. Small molecule mitochondrial uncouplers and their therapeutic potential. J. Med. Chem. 2018, 61, 4641–4655. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.P.; Southworth, R.; Medina, R.A.; Davidson, S.M.; Duchen, M.R.; Shattock, M.J. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc. Res. 2006, 72, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Goh, J.-Y.; Xiao, L.; Xian, H.; Lim, K.-L.; Liou, Y.-C. Reactive oxygen species trigger Parkin/PINK1 pathway-dependent mitophagy by inducing mitochondrial recruitment of Parkin. J. Biol. Chem. 2017, 292, 16697–16708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Shin, S.; Jeong, S.; Kim, S.; Song, K.-S.; Park, J.-H.; Heo, J.-Y.; Seo, K.-S.; Park, S.-K.; Kweon, G.-R.; et al. Docosahexaenoic acid induces the degradation of HPV E6/E7 oncoproteins by activating the ubiquitin–proteasome system. Cell Death Dis. 2014, 5, e1524. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H.; Han, Y.H. Intracellular glutathione levels are involved in carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone-induced apoptosis in As4.1 juxtaglomerular cells. Int. J. Mol. Med. 2011, 27, 575–581. [Google Scholar] [CrossRef]
- Lee, J.E.; Kang, J.S.; Shin, I.C.; Lee, S.J.; Hyun, D.H.; Lee, K.S.; Koh, H.C. Fluazinam-induced apoptosis of SH-SY5Y cells is mediated by p53 and Bcl-2 family proteins. NeuroToxicology 2011, 32, 702–710. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Seol, J.W.; Kim, S.J.; Lee, Y.J.; Kang, H.S.; Kim, I.S.; Kim, N.S.; Park, S.Y. Reactive oxygen species regulate Bax translocation and mitochondrial transmembrane potential, a possible mechanism for enhanced TRAIL-induced apoptosis by CCCP. Oncol. Rep. 2007, 18, 71–76. [Google Scholar] [CrossRef]
- Drobnica, L.; Sturdik, E. The reaction of carbonyl cyanide phenylhydrazones with thiols. Biochim. Biophys. Acta 1979, 585, 462–476. [Google Scholar] [CrossRef]
- Toninello, A.; Siliprandi, N. Restoration of membrane potential in mitochondria deenergized with carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP). Biochim. Biophys. Acta 1982, 682, 289–292. [Google Scholar] [CrossRef]
- Mlejnek, P.; Dolezel, P. Loss of mitochondrial transmembrane potential and glutathione depletion are not sufficient to account for induction of apoptosis by carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone in human leukemia K562 cells. Chem. Biol. Interact. 2015, 239, 110. [Google Scholar] [CrossRef] [PubMed]
- Heytler, P.G. Uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry 1963, 2, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.J.; Sanadi, D.R. Activation of myosin adenosine triphosphatase by carbonyl cyanide p-chlorophenylhydrazone. J. Biol. Chem. 1964, 239, 452–454. [Google Scholar] [PubMed]
- De Kiewiet, D.Y.; Hall, D.O.; Jenner, E.L. Effect of carbonylcyanide m-chlorophenylhydrazone on the photochemical reactions of isolated chloroplasts. Biochim. Biophys. Acta 1965, 109, 284–292. [Google Scholar] [CrossRef]
- Guo, Z.; Miyoshi, H.; Komyoji, T.; Haga, T.; Fujita, T. Uncoupling activity of a newly developed fungicide, fluazinam [3-chloro-N-(3-chloro-2,6-dinitro-4-trifluoromethylphenyl)-5-trifluoromethyl-2-pyridinamine]. Biochim. Biophys. Acta 1991, 1056, 89–92. [Google Scholar] [CrossRef]
- Brandt, U.; Schubert, J.; Geck, P.; von Jagow, G. Uncoupling activity and physicochemical properties of derivatives of fluazinam. Biochim. Biophys. Acta 1992, 1101, 41–47. [Google Scholar] [CrossRef]
- Hollingworth, R.M.; Gadelhak, G.G. Mechanisms of action and toxicity of new pesticides that disrupt oxidative phosphorylation. Rev. Toxicol. 1998, 2, 253–266. [Google Scholar]
- Johnson, D.; Lardy, H. Isolation of liver or kidney mitochondria. Methods Enzymol. 1967, 10, 94–96. [Google Scholar]
- Antonenko, Y.N.; Khailova, L.S.; Rokitskaya, T.I.; Nosikova, E.S.; Nazarov, P.A.; Luzina, O.A.; Salakhutdinov, N.F.; Kotova, E.A. Mechanism of action of an old antibiotic revisited: role of calcium ions in protonophoric activity of usnic acid. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 310–316. [Google Scholar] [CrossRef]
- Akerman, K.E.; Wikstrom, M.K. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976, 68, 191–197. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Methods for the formation of single bimolecular lipid membranes in aqueous solution. J. Phys. Chem. 1963, 67, 534–535. [Google Scholar] [CrossRef]
- Sulo, P.; Sturdik, E.; Liptaj, T.; Jakubik, T.; Antalik, M. Structure characterization of reaction products from phenylhydrazono-propandinitriles and thiols. Collect. Czechoslovak Chem. Commun. 1985, 50, 375–382. [Google Scholar] [CrossRef]
- Keen, J.H.; Habig, W.H.; Jakoby, W.B. Mechanism for the several activities of the glutathione S-transferases. J. Biol. Chem. 1976, 251, 6183–6188. [Google Scholar] [PubMed]
- Inoue, K.; Ohe, T.; Mori, K.; Sagara, T.; Ishii, Y.; Chiba, M. Aromatic substitution reaction of 2-chloropyridines catalyzed by microsomal glutathione S-transferase 1. Drug. Metab. Dispos. 2009, 37, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.D.; Greenhow, D.T.; Adams, D. Metabolism-related assays and their application to agrochemical research: Reactivity of pesticides with glutathione and glutathione transferases. Pestic. Sci. 1998, 54, 385–393. [Google Scholar] [CrossRef]
- Weinander, R.; Anderson, C.; Morgenstern, R. Identification of N-acetylcysteine as a new substrate for rat liver microsomal glutathione transferase. A study of thiol ligands. J. Biol. Chem. 1994, 269, 71–76. [Google Scholar]
- Kraus, P. Resolution, purification and some properties of three glutathione transferases from rat liver mitochondria. Hoppe Seylers Z. Physiol. Chem. 1980, 361, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Redox-optimized ROS balance: A unifying hypothesis. Biochim. Biophys. Acta 2010, 1797, 865–877. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khailova, L.S.; Firsov, A.M.; Kotova, E.A.; Antonenko, Y.N. Interaction of Potent Mitochondrial Uncouplers with Thiol-Containing Antioxidants. Antioxidants 2019, 8, 194. https://doi.org/10.3390/antiox8060194
Khailova LS, Firsov AM, Kotova EA, Antonenko YN. Interaction of Potent Mitochondrial Uncouplers with Thiol-Containing Antioxidants. Antioxidants. 2019; 8(6):194. https://doi.org/10.3390/antiox8060194
Chicago/Turabian StyleKhailova, Ljudmila S., Alexander M. Firsov, Elena A. Kotova, and Yuri N. Antonenko. 2019. "Interaction of Potent Mitochondrial Uncouplers with Thiol-Containing Antioxidants" Antioxidants 8, no. 6: 194. https://doi.org/10.3390/antiox8060194
APA StyleKhailova, L. S., Firsov, A. M., Kotova, E. A., & Antonenko, Y. N. (2019). Interaction of Potent Mitochondrial Uncouplers with Thiol-Containing Antioxidants. Antioxidants, 8(6), 194. https://doi.org/10.3390/antiox8060194



