Endothelin-1-Induced Microvascular ROS and Contractility in Angiotensin-II-Infused Mice Depend on COX and TP Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Animal Model and Angiotensin II Infusion
2.3. Measurement of Urinary 8-Isoprostane F2α and Thromboxane B2 (TxB2)
2.4. Protein Expression from Mesenteric Resistance Arterioles
2.5. Contractility and ROS Generation of Mesenteric Resistance Arterioles
2.6. Measurement of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase and Superoxide Dismutase (SOD) Activities in Mouse Aortas
2.7. Chemicals and Solutions
2.8. Statistical Analysis
3. Results
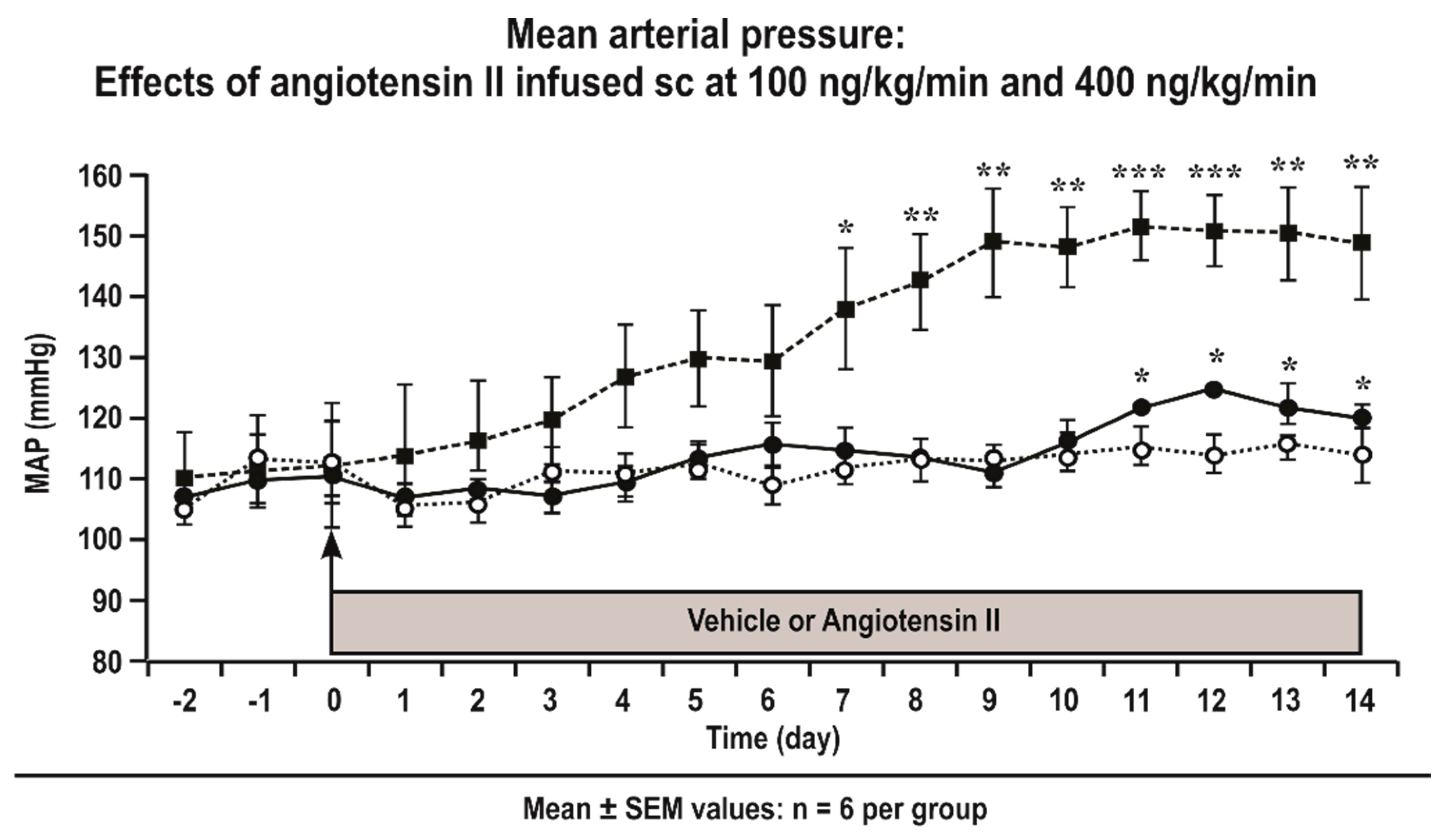
3.1. Mean Arterial Pressure (MAP) of Conscious Mice Infused with Angiotensin II
3.2. Basal Values and Urinary Biomarkers in TPR +/+ and −/− and COX1 +/+ and −/− Mice
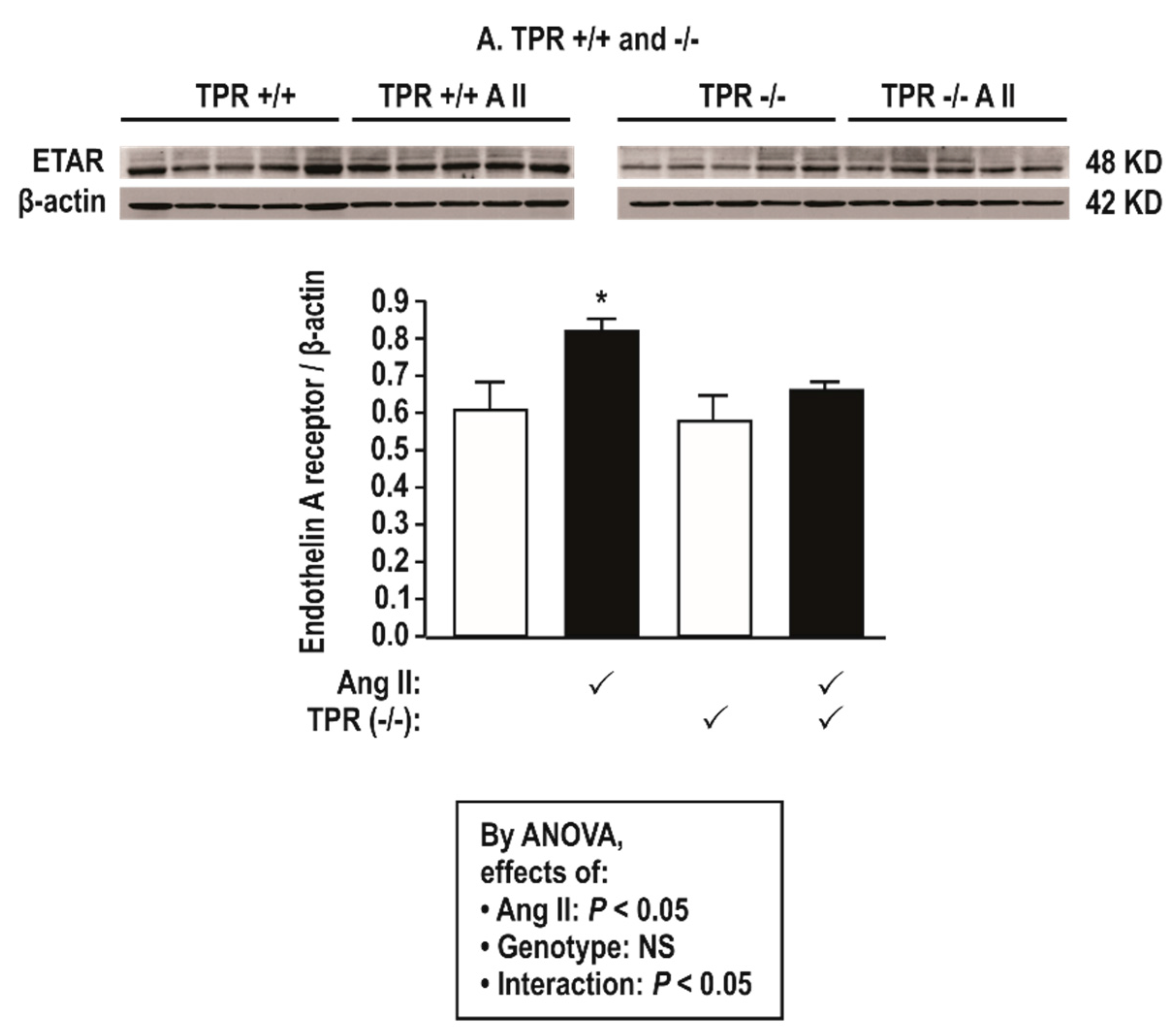
3.3. Protein Expression in Mesenteric Resistance Arterioles
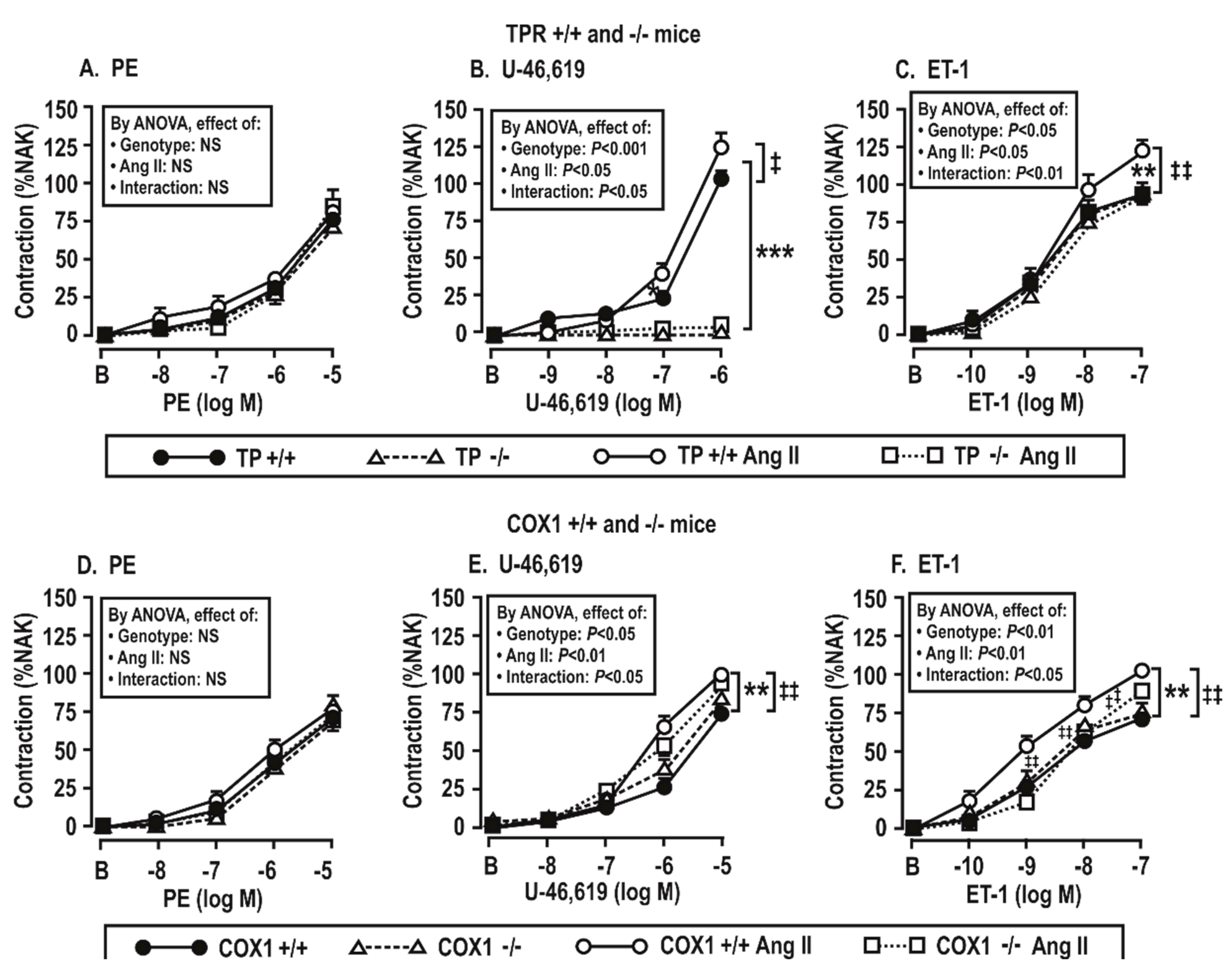
3.4. Contractile Responses of Mesenteric Resistance Arterioles to Phenylephrine (PE), U-46,619, and Endothelin 1 (ET-1)
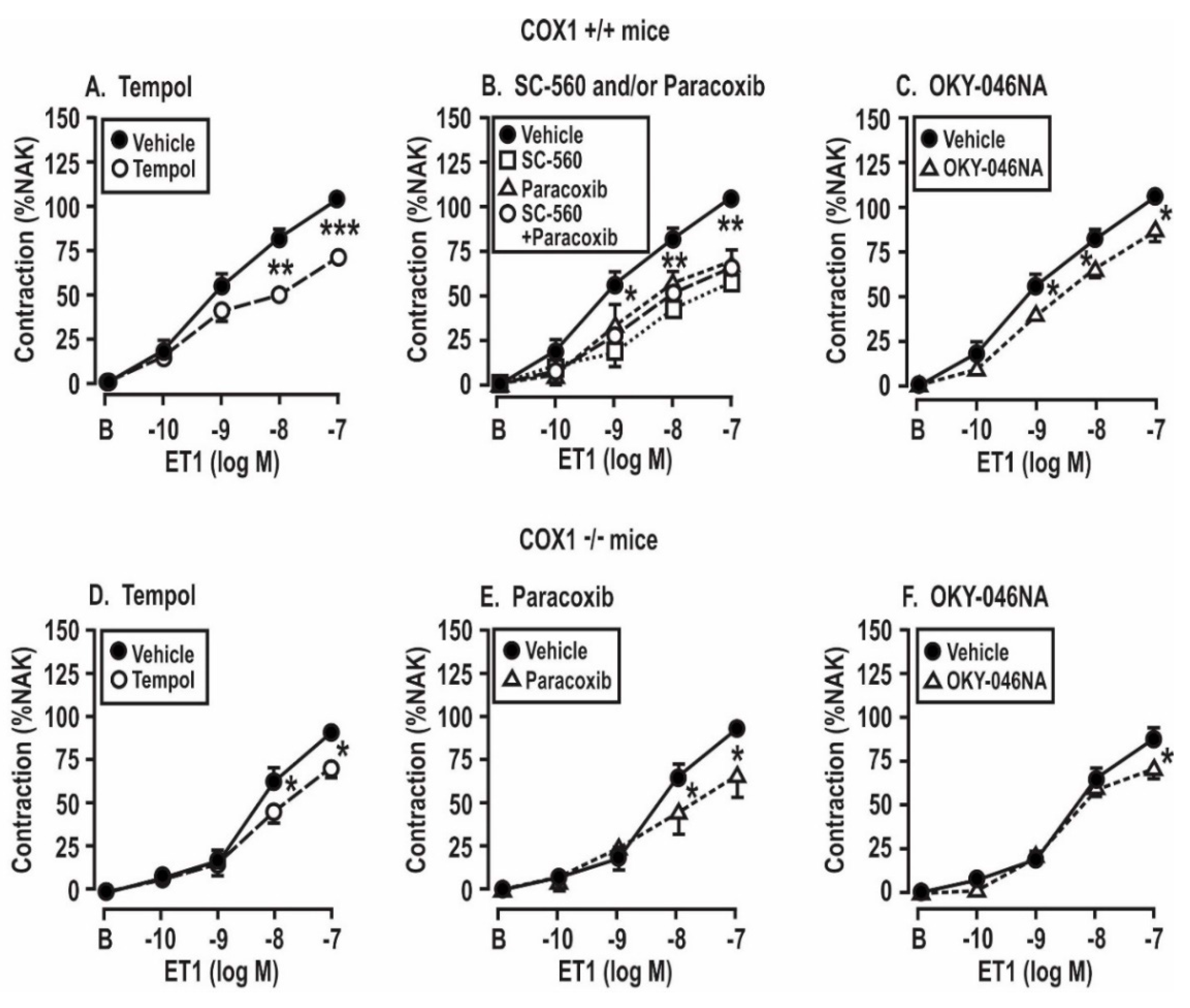
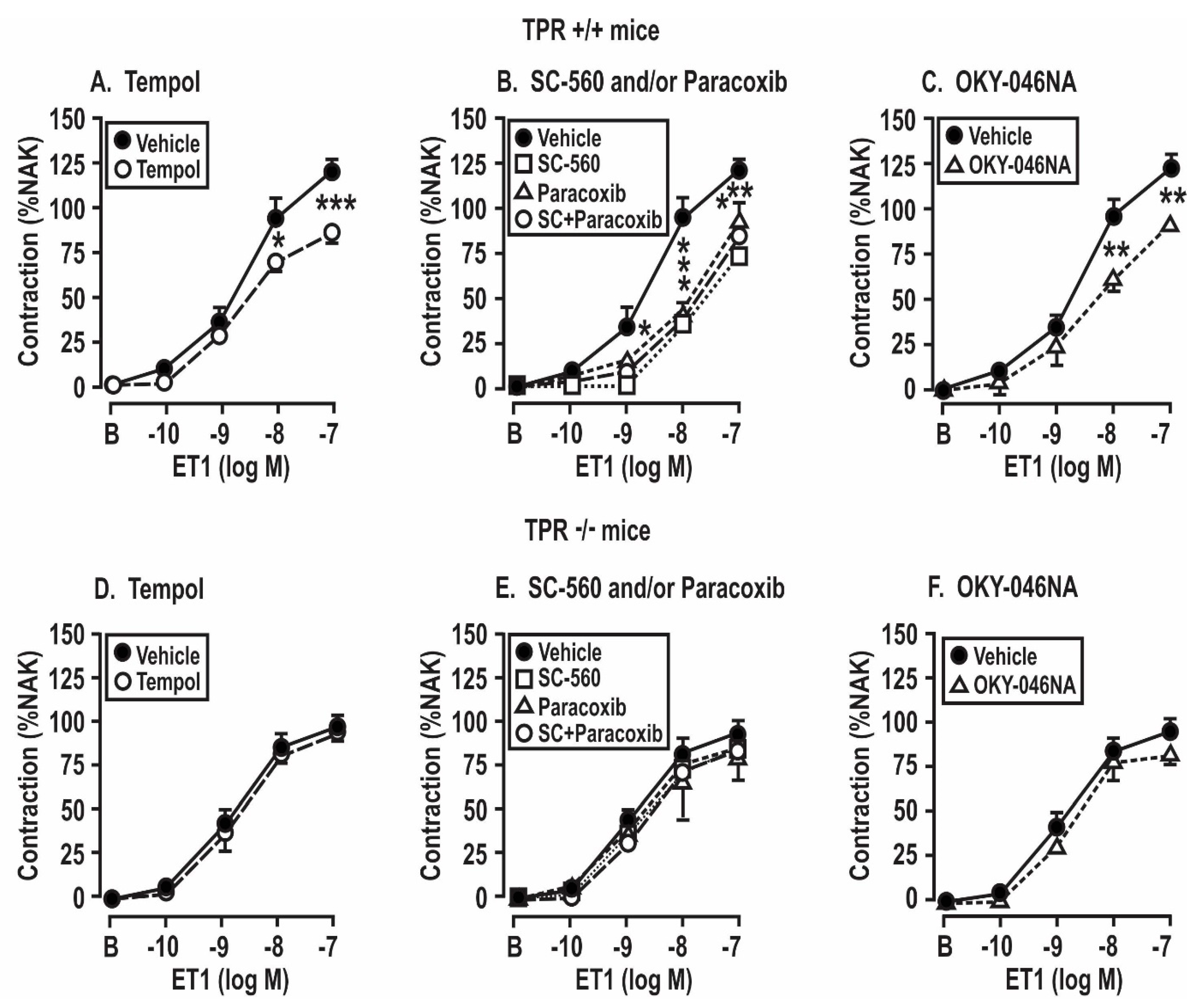
3.5. Contractile Responses of Mesenteric Resistance Arterioles to Endothelin 1: Effect of Angiotensin II Infusion and of the Blockade of ROS, COX, and TxA2 Synthase in COX1 +/+ and −/− and TPR +/+ and −/− Mice
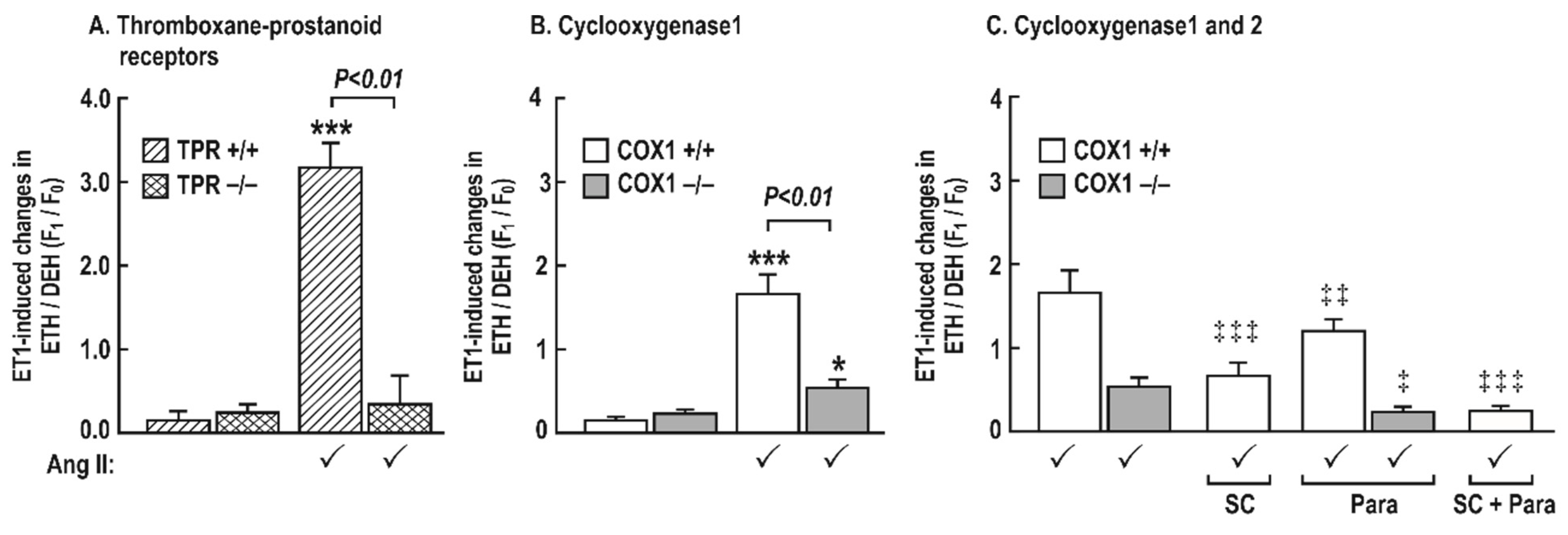
3.6. Generation of Reactive Oxygen Species (ROS) with Endothelin 1 in Mesenteric Resistance Arterioles
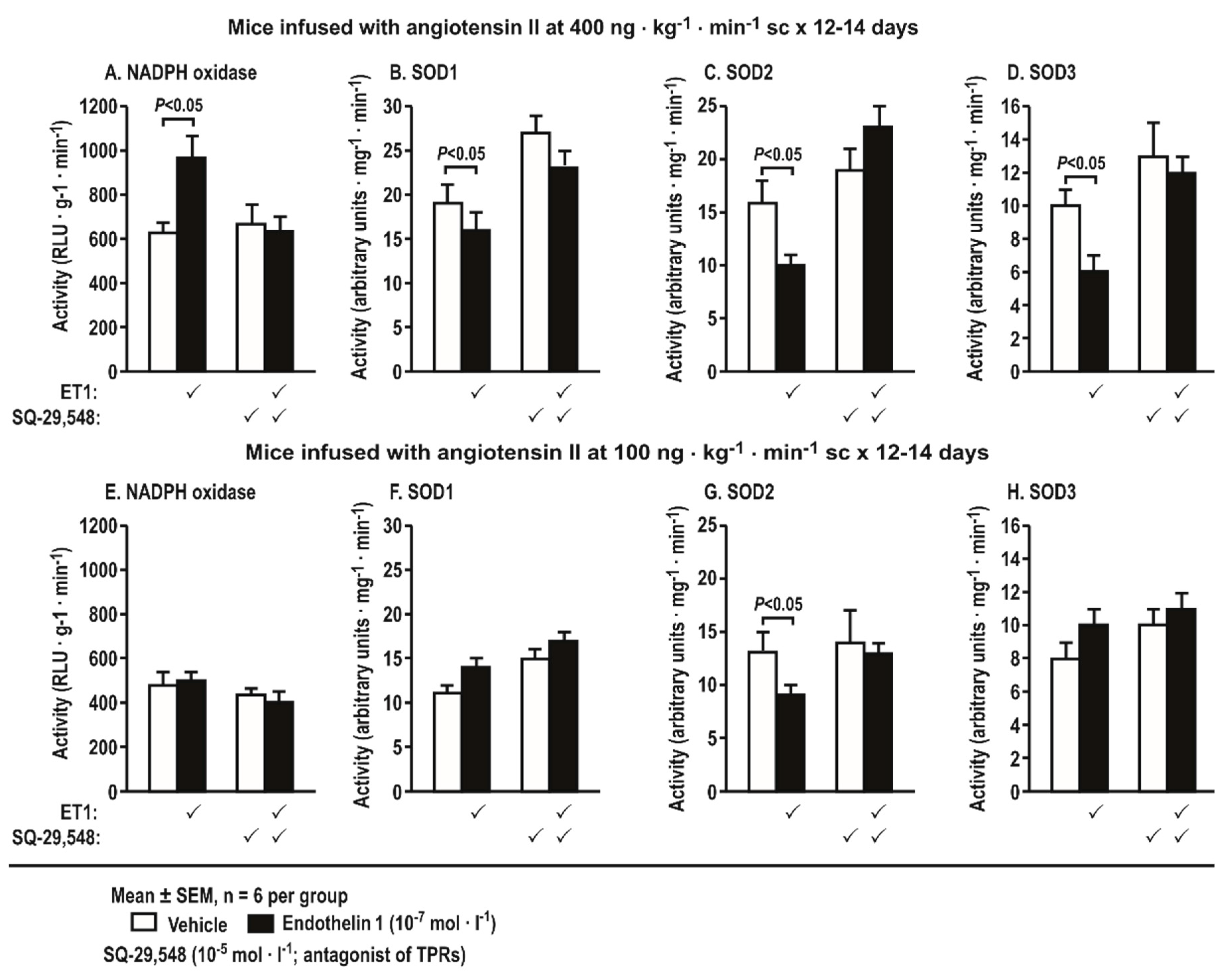
3.7. Activities of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase and Superoxide Dismutase (SOD) Isoforms in Aortas Stimulated by Endothelin 1 and Incubated with a Thromboxane Prostanoid Receptor Blocker or a Vehicle in Mice Infused with Angiotensin II at a Slow Pressor or Sub-Threshold Rate
4. Discussion
4.1. ROS are Downstream from the Activation of COX or TPRs in Mice Infused for Two Weeks with Ang II
4.2. Both COX1 and 2 Participate in ET-1-Induced ROS Generation in Mesenteric Resistance Arterioles from Ang-II-Infused Mice
4.3. Sources of ET-1 Stimulated ROS in Blood Vessels of Ang-II-Infused Mice
4.4. COX1 and 2 and TPRs are Absolute Requirements for Microvascular ROS Generation with ET-1 in Ang-II-Infused Mice
5. Conclusions
6. Clinical Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, M.S.; Jaimes, E.A.; Raij, L. Inhibition of oxidative stress and improvement of endothelial function by amlodipine in angiotensin II-infused rats. Am. J. Hypertens. 2004, 17, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Fukumoto, Y.; Suzuki, J.; Saji, K.; Nawata, J.; Shinozaki, T.; Kagaya, Y.; Watanabe, J.; Shimokawa, H. Diabetes mellitus accelerates left ventricular diastolic dysfunction through activation of the renin-angiotensin system in hypertensive rats. Hypertens. Res. 2009, 32, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, E.A.; Tian, R.X.; Pearse, D.; Raij, L. Up-regulation of glomerular COX-2 by angiotensin II: Role of reactive oxygen species. Kidney Int. 2005, 68, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Savoia, C.; Battistoni, A.; Calvez, V.; Cesario, V.; Montefusco, G.; Filippini, A. Microvascular alterations in hypertension and vascular aging. Curr. Hypertens. Rev. 2017, 13, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chabrashvili, T.; Wilcox, C.S. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: Roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ. Res. 2004, 94, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef]
- Araujo, M.; Wilcox, C.S. Oxidative stress in hypertension: Role of the kidney. Antioxid. Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef]
- Kawada, N.; Dennehy, K.; Solis, G.; Modlinger, P.; Hamel, R.; Kawada, J.T.; Aslam, S.; Moriyama, T.; Imai, E.; Welch, W.J.; et al. TP receptors regulate renal hemodynamics during angiotensin II slow pressor response. Am. J. Physiol. Ren. Physiol. 2004, 287, F753–F759. [Google Scholar] [CrossRef]
- Kawada, N.; Imai, E.; Karber, A.; Welch, W.J.; Wilcox, C.S. A mouse model of angiotensin II slow pressor response: Role of oxidative stress. J. Am. Soc. Nephrol. 2002, 13, 2860–2868. [Google Scholar] [CrossRef]
- Wang, D.; Chabrashvili, T.; Borrego, L.; Aslam, S.; Umans, J.G. Angiotensin II infusion alters vascular function in mouse resistance vessels: Roles of O and endothelium. J. Vasc. Res. 2006, 43, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Solis, G.; Holland, S.; Luo, Z.; Carlstrom, M.; Sandberg, K.; Wellstein, A.; Welch, W.J.; Wilcox, C.S. P47phox is required for renal and afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension 2012, 59, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Griendling, K.K.; Dikalova, A.; Owens, G.K.; Taylor, W.R. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension 2005, 46, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Chabrashvili, T.; Kitiyakara, C.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J.; Patel, K.; Modlinger, P.; Mendonca, M.; Kawada, N.; Dennehy, K.; Aslam, S.; Wilcox, C.S. Roles of vasoconstrictor prostaglandins, COX-1 and -2, and AT1, AT2, and TP receptors in a rat model of early 2K,1C hypertension. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2644–H2649. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Colucci, R.; Neves, M.F.; Rugani, I.; Aydinoglu, F.; Fornai, M.; Ippolito, C.; Antonioli, L.; Duranti, E.; Solini, A.; et al. Resistance artery mechanics and composition in angiotensin II-infused mice: Effects of cyclooxygenase-1 inhibition. Eur. Heart J. 2012, 33, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Hao, C.M.; Langenbach, R.I.; Breyer, R.M.; Redha, R.; Morrow, J.D.; Breyer, M.D. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J. Clin. Investig. 2002, 110, 61–69. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Endothelin: Role in experimental hypertension. J. Cardiovasc. Pharmacol. 2000, 35, S33–S35. [Google Scholar] [CrossRef]
- Ortiz, M.C.; Sanabria, E.; Manriquez, M.C.; Romero, J.C.; Juncos, L.A. Role of endothelin and isoprostanes in slow pressor responses to angiotensin II. Hypertension 2001, 37, 505–510. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, X.; Ackerman, W.E.T.; Belury, M.A.; Koster, C.; Rovin, B.H.; Landon, M.B.; Kniss, D.A. Differentiation-dependent regulation of the cyclooxygenase cascade during adipogenesis suggests a complex role for prostaglandins. Diabetes Obes. Metab. 2006, 8, 83–93. [Google Scholar] [CrossRef]
- Francois, H.; Athirakul, K.; Mao, L.; Rockman, H.; Coffman, T.M. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertens 2004, 43, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J.; Chabrashvili, T.; Solis, G.; Chen, Y.; Gill, P.; Aslam, S.; Wang, X.; Ji, H.; Sandberg, K.; Jose, P.; et al. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension (Dallas) 2006, 48, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Kawada, N.; Solis, G.; Ivey, N.; Connors, S.; Dennehy, K.; Modlinger, P.; Hamel, R.; Kawada, J.T.; Imai, E.; Langenbach, R.; et al. Cyclooxygenase-1-deficient mice have high sleep-to-wake blood pressure ratios and renal vasoconstriction. Hypertension (Dallas) 2005, 45, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.W.; Mannon, R.B.; Mannon, P.J.; Latour, A.; Oliver, J.A.; Hoffman, M.; Smithies, O.; Koller, B.H.; Coffman, T.M. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J. Clin. Investig. 1998, 102, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J.; Peng, B.; Takeuchi, K.; Abe, K.; Wilcox, C.S. Salt loading enhances rat renal TxA2/PGH2 receptor expression and TGF response to U-46,619. Am. J. Physiol. 1997, 273, F976–F983. [Google Scholar] [CrossRef] [PubMed]
- Schnackenberg, C.G.; Wilcox, C.S. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension (Dallas) 1999, 33, 424–428. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Z.; Kohan, D.; Wellstein, A.; Jose, P.A.; Welch, W.J.; Wilcox, C.S.; Wang, D. Thromboxane prostanoid receptors enhance contractions, endothelin-1, and oxidative stress in microvessels from mice with chronic kidney disease. Hypertension 2015, 65, 1055–1063. [Google Scholar] [CrossRef]
- Wang, D.; Iversen, J.; Strandgaard, S. Contractility and endothelium-dependent relaxation of resistance vessels in polycystic kidney disease rats. J. Vasc. Res. 1999, 36, 502–509. [Google Scholar] [CrossRef]
- Li, L.; Feng, D.; Luo, Z.; Welch, W.J.; Wilcox, C.S.; Lai, E.Y. Remodeling of Afferent Arterioles from Mice with Oxidative Stress Does Not Account for Increased Contractility but Does Limit Excessive Wall Stress. Hypertension 2015, 66, 550–556. [Google Scholar] [CrossRef]
- Callera, G.E.; Tostes, R.C.; Yogi, A.; Montezano, A.C.; Touyz, R.M. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin. Sci. (London) 2006, 110, 243–253. [Google Scholar] [CrossRef]
- Feng, D.; Yang, C.; Geurts, A.M.; Kurth, T.; Liang, M.; Lazar, J.; Mattson, D.L.; O’Connor, P.M.; Cowley, A.W., Jr. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab. 2012, 15, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.S.; Wilcox, C.S. NADPH oxidases in the kidney. Antioxid. Redox Signal. 2006, 8, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Folger, W.H.; Lawson, D.; Wilcox, C.S.; Mehta, J.L. Response of rat thoracic aortic rings to thromboxane mimetic U-46,619: Roles of endothelium-derived relaxing factor and thromboxane A2 release. J. Pharmacol. Exp. Ther. 1991, 258, 669–675. [Google Scholar] [PubMed]
- Wilcox, C.S.; Folger, W.H.; Welch, W.J. Renal vasoconstriction with U-46,619; role of arachidonate metabolites. J. Am. Soc. Nephrol. 1994, 5, 1120–1124. [Google Scholar] [PubMed]
- Funk, C.D.; FitzGerald, G.A. COX-2 inhibitors and cardiovascular risk. J. Cardiovasc. Pharmacol. 2007, 50, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Valentin, F.; Field, M.C.; Tippins, J.R. The mechanism of oxidative stress stabilization of the thromboxane receptor in COS-7 cells. J. Biol. Chem. 2004, 279, 8316–8324. [Google Scholar] [CrossRef]
- Ansar, S.; Eftekhari, S.; Waldsee, R.; Nilsson, E.; Nilsson, O.; Saveland, H.; Edvinsson, L. MAPK signaling pathway regulates cerebrovascular receptor expression in human cerebral arteries. BMC Neurosci. 2013, 14, 12. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, B.; Maitland, K.A.; Bayat, H.; Gu, J.; Nadler, J.L.; Corda, S.; Lavielle, G.; Verbeuren, T.J.; Zuccollo, A.; et al. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes 2006, 55, 110–119. [Google Scholar] [CrossRef]
- Tajiri, Y.; Inoguchi, T.; Umeda, F.; Nawata, H. Reduction of urinary albumin excretion by thromboxane synthetase inhibitor, OKY-046, through modulating renal prostaglandins in patients with diabetic nephropathy. Diabetes Res. Clin. Pract. 1990, 10, 231–239. [Google Scholar] [CrossRef]
- Nakamura, A.; Nagaya, N.; Obata, H.; Sakai, K.; Sakai, Y.; Yoshikawa, M.; Hamada, K.; Matsumoto, K.; Kimura, H. Oral administration of a novel long-acting prostacyclin agonist with thromboxane synthase inhibitory activity for pulmonary arterial hypertension. Circ. J. 2013, 77, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Cardozo, J.; Welch, W.J. AT1 and TxA2/PGH2 receptors maintain hypertension throughout 2K,1C Goldblatt hypertension in the rat. Am. J. Physiol. 1996, 271, R891–R896. [Google Scholar] [CrossRef] [PubMed]

| Variable | Vehicle | Ang II Infusion | By ANOVA, Effect of | ||||
|---|---|---|---|---|---|---|---|
| TPR +/+ | TPR −/− | TPR +/+ | TPR −/− | TPR Genotype | Ang II | Interaction | |
| MAP (mmHg) | 92 ± 3 | 94 ± 2 | 108 ± 4 * | 97 ± 3 | NS | P < 0.05 | P < 0.05 |
| Body weight (g) | 27.5 ± 0.5 | 27.0 ± 0.7 | 26.5 ± 0.7 | 27.4 ± 0.8 | NS | NS | NS |
| Heart weight/Bwt (g) | 0.99 ± 0.05 | 1.09 ± 0.10 | 0.89 ± 0.08 | 0.95 ± 0.12 | NS | NS | NS |
| Aorta (mg) | 0.15 ± 0.01 | 0.16 ± 0.007 | 0.16 ± 0.005 | 0.15 ± 0.006 | NS | NS | NS |
| MRA lumen (µm) | 139 ± 12 | 135 ± 6 | 121 ± 8 | 134 ± 5 | NS | NS | NS |
| MRA media (µm) | 42 ± 5 | 43 ± 7 | 51 ± 5 * | 48 ± 6 | NS | P < 0.05 | NS |
| MRA M/L ratio | 0.30 ± 0.06 | 0.31 ± 0.09 | 0.42 ± 0.08 * | 0.35 ± 0.07 † | NS | P < 0.05 | P < 0.05 |
| Urinary 8-Isoprostane F2α (ng/mg creatinine) | 1.43 ± 0.18 | 1.79 ± 0.11 | 2.23 ± 0.13 * | 1.66 ± 0.07 † | NS | P < 0.05 | P < 0.05 |
| Urinary TxB2 (ng/mg creatinine) | 1.18 ± 0.07 | 0.92 ± 0.12 | 1.80 ± 0.13 * | 1.18 ± 0.0 † | P < 0.05 | P < 0.05 | NS |
| Variable | Vehicle | Ang II Infusion | By ANOVA, Effect of | ||||
|---|---|---|---|---|---|---|---|
| COX1 +/+ | COX1 −/− | COX1 +/+ | COX1 −/− | COX1 Genotype | Ang II | Interaction | |
| MAP (mmHg) | 88 ± 3 | 94 ± 3 | 124 ± 4 * | 112 ± 3† | P < 0.05 | P < 0.05 | P < 0.05 |
| Body weight (BW, g) | 26.5 ± 0.6 | 28.0 ± 0.8 | 27.5 ± 0.6 | 29.4 ± 0.9 | NS | NS | NS |
| Heart weight (g) | 0.94 ± 0.05 | 0.98 ± 0.11 | 1.05 ± 0.07 | 0.99 ± 0.12 | NS | NS | NS |
| Aorta (g) | 0.15 ± 0.01 | 0.16 ± 0.007 | 0.16 ± 0.005 | 0.15 ± 0.006 | NS | NS | NS |
| MRA lumen (µm) | 142 ± 9 | 135 ± 5 | 123 ± 7 | 132 ± 5 | NS | NS | NS |
| MRA media (µm) | 43 ± 4 | 41 ± 5 | 55 ± 6 * | 47 ± 5 | NS | P < 0.05 | NS |
| MRA M/L ratio | 0.30 ± 0.07 | 0.31 ± 0.04 | 0.44 ± 0.05 * | 0.36 ± 0.06 | NS | P < 0.05 | P < 0.05 |
| Urinary 8-Isoprostane F2α (ng·mg creatinine-1) | 1.44 ± 0.10 | 1.45 ± 0.22 | 2.11 ± 0.19 * | 1.79 ± 0.21† | NS | P < 0.05 | P < 0.05 |
| Urinary TxB2 (ng·mg creatinine-1) | 1.0 ± 0.05 | 0.99 ± 0.06 | 1.32 ± 0.07 * | 1.03 ± 0.06 † | NS | P < 0.05 | NS |
| Added to the Bath | Vehicle Infusion | Ang II Infusion | By ANOVA, Effect of | ||||
| TPR +/+ | TPR −/− | TPR +/+ | TPR −/− | TPR Genotype | Ang II Infusion | Interaction | |
| PSS (%) | 94 ± 6 | 96 ± 3 | 122 ± 5 *,† | 93 ± 7 † | NS | P < 0.05 | P < 0.05 |
| Tempol (%) | 90 ± 5 | 91 ± 4 | 86 ± 5 # | 92 ± 5 | NS | NS | NS |
| SC-560 (%) | 89 ± 7 | 90 ± 5 | 74 ± 7 # | 86 ± 7 | NS | NS | NS |
| Paracoxib (%) | 91 ± 4 | 92 ± 3 | 94 ± 10 # | 82 ± 6 | NS | NS | NS |
| SC + Paracoxib (%) | 86 ± 7 | 88 ± 5 | 85 ± 7 # | 87 ± 2 | NS | NS | NS |
| OKY-046NA (%) | 88 ± 6 | 92 ± 4 | 90 ± 2 # | 94 ± 3 | NS | NS | NS |
| Added to the Bath | Vehicle Infusion | Ang II Infusion | By ANOVA, Effect of | ||||
| COX1 +/+ | COX1 −/− | COX1 +/+ | COX1 −/− | COX1 Genotype | Ang II Infusion | Interaction | |
| PSS (%) | 75 ± 3 | 76 ± 7 | 104 ± 3 *,† | 88 ± 4 † | NS | P < 0.05 | P < 0.05 |
| Tempol (%) | 70 ± 4 | 71 ± 5 | 70 ± 3 # | 72 ± 6 | NS | NS | NS |
| SC-560 (%) | 69 ± 5 | 59 ± 9 # | |||||
| Paracoxib (%) | 72 ± 6 | 70 ± 4 | 76 ± 6 # | 67 ± 9 | NS | NS | NS |
| SC + Paracoxib (%) | 68 ± 5 | 64 ± 4 # | |||||
| OKY-046NA (%) | 72 ± 6 | 73 ± 6 | 86 ± 6 *,# | 76 ± 6 | NS | P < 0.05 | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilcox, C.S.; Wang, C.; Wang, D. Endothelin-1-Induced Microvascular ROS and Contractility in Angiotensin-II-Infused Mice Depend on COX and TP Receptors. Antioxidants 2019, 8, 193. https://doi.org/10.3390/antiox8060193
Wilcox CS, Wang C, Wang D. Endothelin-1-Induced Microvascular ROS and Contractility in Angiotensin-II-Infused Mice Depend on COX and TP Receptors. Antioxidants. 2019; 8(6):193. https://doi.org/10.3390/antiox8060193
Chicago/Turabian StyleWilcox, Christopher S., Cheng Wang, and Dan Wang. 2019. "Endothelin-1-Induced Microvascular ROS and Contractility in Angiotensin-II-Infused Mice Depend on COX and TP Receptors" Antioxidants 8, no. 6: 193. https://doi.org/10.3390/antiox8060193
APA StyleWilcox, C. S., Wang, C., & Wang, D. (2019). Endothelin-1-Induced Microvascular ROS and Contractility in Angiotensin-II-Infused Mice Depend on COX and TP Receptors. Antioxidants, 8(6), 193. https://doi.org/10.3390/antiox8060193






