Abstract
Dunaliella salina is a rich source of 9-cis β-carotene, which has been identified as an important biomolecule in the treatment of retinal dystrophies and other diseases. We previously showed that chlorophyll absorption of red light photons in D. salina is coupled with oxygen reduction and phytoene desaturation, and that it increases the pool size of β-carotene. Here, we show for the first time that growth under red light also controls the conversion of extant all-trans β-carotene to 9-cis β-carotene by β-carotene isomerases. Cells illuminated with red light from a light emitting diode (LED) during cultivation contained a higher 9-cis β-carotene content compared to cells illuminated with white or blue LED light. The 9-cis/all-trans β-carotene ratio in red light treated cultures reached >2.5 within 48 h, and was independent of light intensity. Illumination using red light filters that eliminated blue wavelength light also increased the 9-cis/all-trans β-carotene ratio. With norflurazon, a phytoene desaturase inhibitor which blocked downstream biosynthesis of β-carotene, extant all-trans β-carotene was converted to 9-cis β-carotene during growth with red light and the 9-cis/all-trans β-carotene ratio was ~2. With blue light under the same conditions, 9-cis β-carotene was likely destroyed at a greater rate than all-trans β-carotene (9-cis/all-trans ratio 0.5). Red light perception by the red light photoreceptor, phytochrome, may increase the pool size of anti-oxidant, specifically 9-cis β-carotene, both by upregulating phytoene synthase to increase the rate of biosynthesis of β-carotene and to reduce the rate of formation of reactive oxygen species (ROS), and by upregulating β-carotene isomerases to convert extant all-trans β-carotene to 9-cis β-carotene.
1. Introduction
Carotenoids are synthesized by photosynthetic organisms for light-harvesting and for photo-protection of the pigment-protein light-harvesting complexes and photosynthetic reaction centres in the thylakoid membrane [1,2,3,4]. Dunaliella salina, a halotolerant chlorophyte, is one of the richest sources of natural carotenoids, and accumulates up to 10% of the dry biomass as β-carotene under conditions that are sub-optimal for growth, i.e., high light intensity, sub-optimal temperatures, nutrient limitation and high salt concentrations [5,6,7,8]. Two pools of β-carotene have been identified, which may be distinguished on the basis of geometric isomer configuration, cis or trans (Z/E), and enzyme complement. Thylakoid β-carotene consists principally of all-trans β-carotene (all-trans βC), and may be constitutively expressed; the ‘accumulated’ β-carotene, which is found in globules of lipid and proline-rich, β-carotene globule protein (the βC-plastoglobuli) in the inter-thylakoid spaces of the chloroplast, appears in high concentration of both cis/trans (Z/E) configurations, ratio ~1 [5,9,10,11].
The occurrence of such high concentrations of 9-cis βC in D. salina is of great pharmaceutical interest. 9-cis βC has a higher antioxidant activity than all-trans βC, and may also be more efficient than all-trans βC in vivo [12,13]. 9-cis βC has been proposed in treatments for retinal dystrophies, chronic plaque psoriasis and atherosclerosis and as an anti-ageing therapy [14,15,16,17,18]. Importantly, a synthetic pure preparation of 9-cis βC has recently been shown to inhibit photoreceptor degeneration of eye cups from mice with a retinoid cycle genetic defect [19].
However, the mechanism and regulation of the biosynthesis of 9-cis βC in D. salina is unclear. Using different inhibitors of β-carotene biosynthesis, Shaish et al. [20] found that all the intermediates between phytoene and β-carotene in cultures maintained under low light intensity and N-starvation contained similar ratios of 9-cis/all-trans stereoisomers. They concluded that the isomerisation step must occur at or before phytoene, and that no further isomerisation was likely to occur during the further transformation of phytoene to β-carotene. On the other hand, in cultures maintained under light stress, 9-cis/all-trans βC isomerases were identified in high concentrations in plastidic globules, and were shown in vitro to catalyse conversion of all-trans βC to 9-cis βC, whilst the expression of the corresponding genes was enhanced under stress conditions [21]. The 9-cis/all-trans βC ratio has been shown to increase four-fold and the β-carotene content two-fold when the culture temperature decreased from 30 ℃ to 10 ℃ [22], and to increase with increased light intensity [21,23,24], but to be independent of light wavelength within the photosynthetically active range [7]. There have also been reports of a higher 9-cis/all-trans βC ratio in D. salina cultivated under low light intensities [25,26].
Recently, we showed that growth of D. salina under high intensity red light was associated with carotenoid accumulation and a high rate of oxygen uptake [1]. We proposed a mechanism for carotenoid synthesis under red light, which involved absorption of red light photons by chlorophyll to reduce plastoquinone in photosystem II, coupled with phytoene desaturation by a plastoquinol:oxygen oxidoreductase, with oxygen as electron acceptor. Partitioning of electrons between photosynthesis and carotenoid biosynthesis would depend on both red photon flux intensity and phytoene synthase upregulation by the red light photoreceptor, phytochrome.
In this paper, the effects of red, white and blue light on the β-carotene isomeric composition in D. salina were investigated. Isomerisation between all-trans and 9-cis βC in D. salina was regulated by light wavelength but not light intensity, with red light shifting the equilibrium in the direction of 9-cis βC production. In blue light, 9-cis βC was more rapidly destroyed than all-trans βC.
2. Materials and Methods
2.1. Strains and Cultivation
D. salina strain CCAP 19/41 (PLY DF15) was isolated from a salt pond in Israel and obtained from the Marine Biological Association (MBA, Plymouth, UK). Algae were cultured in Modified Johnsons Medium [27] in an ALGEM Environmental Modeling Labscale Photobioreactor (Algenuity, Bedfordshire, UK) and growth was monitored as described previously [1]. For initial experiments described by Figure 1, D. salina cells were grown under 12/12 light/dark (L/D) with 200 µmol photons m−2 s−1 supplied by white light emitting diode (LED) light (Figure A1a) to exponential growth phase, then dark-adapted for 36 h. After dark adaptation, they were transferred to continuous white, blue or red LED light at light intensities of 200, 500, or 1000 μmol photons m−2 s−1 for 48 h. Samples were taken at 0, 24 and 48 h for carotenoids analysis. For experiments with norflurazon described by Figure 5, cultures were grown for 24 h under white LED light then norflurazon as added to cultures to a working concentration of 5 µM and maintained for a further 48 h under red, blue or a mix of red and blue LED light at 200 µmol m−2 s−1 or kept in the dark. Red filters (Lee filter 26 Bright red, 27 Medium red, and 787 Marius red (Figure A1b–d)) when used, were purchased from Lee Filters Andover (Hampshire, UK) and placed over the LED lights. The cultures were shaken for 10 min at 100 rpm every hour before taking samples to monitor cell growth in order to minimise sheer stress to the cells which have no cell wall.
2.2. Carotenoids Analysis
The composition of pigments was analysed by High-Performance Liquid Chromatography with Diode-Array Detection (HPLC-DAD) (Agilent Technologies 1200 series, Agilent, Santa Clara, United States). Biomass was harvested and extracted for HPLC analysis as described previously [1], and analysed at least in triplicate. A carotene standard for all-trans βC was obtained from Sigma-Aldrich Inc. (Merck KGaA, Darmstadt, Germany); a carotene standard for 9-cis βC was obtained from Dynamic Extractions (Tredegar, Gwent, UK). The all-trans and 9-cis βC contents were quantified from their absorption at 450 nm.
2.3. Statistical Analysis
Each experiment was carried out at least in triplicate. The collected data were analyzed in R by one way analysis of variance (ANOVA) with posterior Dunnett’s test and Turkey multiple pairwise-comparisons. A p < 0.05 value was considered significant.
3. Results
9-cis βC and all-trans βC were the major carotenoids that accumulated in D. salina biomass after 48 h exposure to red or blue LED light, but the relative pool sizes of each depended on the concentration of red and blue photons of light received. Under blue light, the contents of both 9-cis- and all-trans βC per cell increased with time (Figure 1a,b), and the ratio of cis/trans βC isomers remained approximately the same at all light intensities (Figure 1c). The concentration of 9-cis βC was ~half as much as all-trans βC. Under red light, by contrast, the concentration of 9-cis βC and total pool of carotenoids increased massively compared to that in blue in all light intensities and the content of 9-cis βC was ~twice as much as all-trans βC (Figure 1a,b). With increasing light intensity, the relative pool sizes of the isomers changed; that of all-trans βC decreased and that of 9-cis βC increased. Furthermore 9-cis βC increased with time to >60% of total β-carotene under red light (Figure 1d). HPLC profiles of the carotenoid extracts showed 9-cis βC and all-trans βC were the major carotenoids that accumulated in D. salina biomass, and that the ratios of the two isomers were different under different wavelengths (Figure 1e).
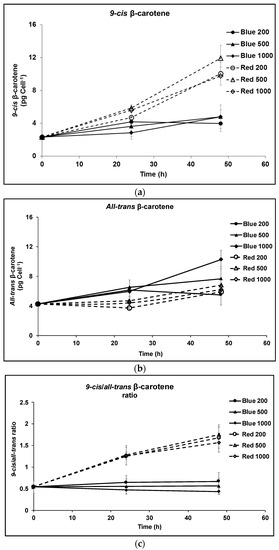
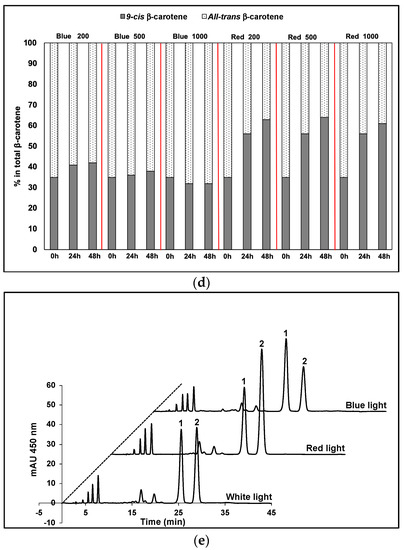
Figure 1.
Cultivation of D. salina under continuous blue or red LED light at three different light intensities of 200, 500 and 1000 µmol m−2 s−1 for 48 h. (a) Cellular content of 9-cis βC, (b) cellular content of all-trans βC; (c) 9-cis/all-trans βC ratio. (d) Percentage of 9-cis and all-trans βC in total βC. (e) HPLC profiles at 450 nm of carotenoid extracts from D. salina cultivated under continuous white light, red light or blue light, each at 1000 µmol m−2 s−1 for 48 h. Peak 1: all-trans β-carotene; peak 2: 9-cis β-carotene. Biomass was collected at 48 h illumination and carotenoids extracted for HPLC analysis. Each culture condition was set up at least in triplicate. mAU: milli-absorbance unit.
To test the effect of blue light exposure on carotene isomers that had accumulated in red light and vice versa, dark-adapted cultures of D. salina were cultivated in red or blue LED high intensity light for 24 h (T0), and then cultivated for a further 24 h in red, blue, or a mixture of red and blue LED light (1:1) with the same light intensity, or the dark. As before, red-shifted cells maintained in red light produced the greatest amount of carotenoids with ~twice as much as 9-cis βC as all-trans βC (Figure 2). On the other hand, 9-cis βC decreased when red-shifted cells were transferred to blue light (Figure 2), to the same level as for blue-shifted cells maintained continuously in blue (Figure 3); the pool size of carotenoids for both conditions was about the same and the concentration of 9-cis βC was ~half as much as all-trans βC. Conversely, blue-shifted cells when transferred to red LED produced more carotenoids (28% greater content), principally as 9-cis βC (Figure 3).
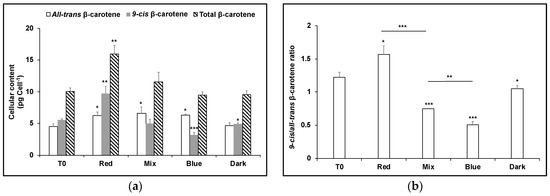
Figure 2.
(a) Cellular content of 9-cis βC and all-trans βC and (b) 9-cis/all-trans βC ratio in D. salina cultures exposed to continuous red LED light at 1000 µmol m−2 s−1 for 24 h followed by 24 h under either red light, a mix of 1:1 red and blue light, blue light at the same light intensity of 1000 µmol m−2 s−1 or dark. Each culture condition was set up at least in triplicate. Results were analysed by one way analysis of variance (ANOVA) with posterior Dunnett’s test compared to T0 and Tukey multiple pairwise-comparisons. Asterisks represent different levels of significance (*** 0 < p ≤ 0.001, ** 0.001 < p ≤ 0.01, * 0.01 < p ≤ 0.05).
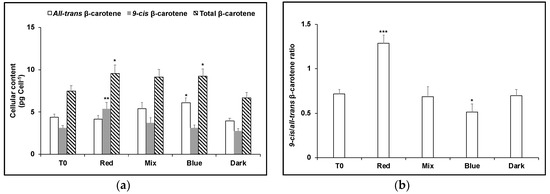
Figure 3.
(a) Cellular content of 9-cis βC and all-trans βC and (b) 9-cis/all-trans βC ratio in D. salina cultures exposed to continuous blue LED light at 1000 µmol m−2 s−1 for 24 h followed by 24 h under either red light, a mix of 1:1 red and blue light, blue light at the same light intensity of 1000 µmol m−2 s−1 or dark. Each culture condition was set up at least in triplicate. Results were analysed by one way ANOVA with posterior Dunnett’s test compared to T0 and Tukey multiple pairwise-comparisons. Asterisks represent different levels of significance (*** 0 < p ≤ 0.001, ** 0.001 < p ≤ 0.01, * 0.01 < p ≤ 0.05).
Since red light increased the net content of 9-cis βC, the effects of red light/dark cycles of increasing red light duration during cultivation were tested. Increasing red light duration increased the total amount of β-carotene, in particular the amount of 9-cis βC (Figure 4). With a red light/dark cycle of 10 min/110 min, the ratio of 9-cis/all-trans βC was 1.1, but in a red light/dark cycle of 30 min/30 min, this increased to 2.2, similar to that in continuous red (2.3). However, in continuous red light, the total pool size β-carotene was nearly 25% greater.
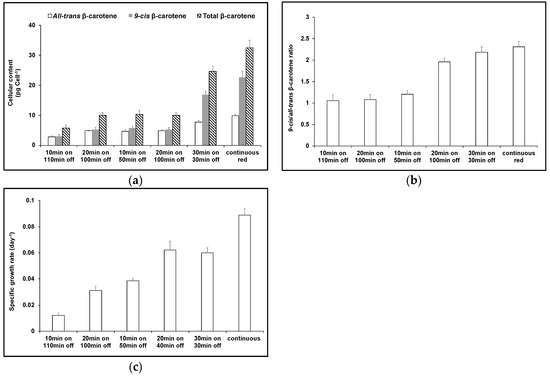
Figure 4.
Effect of cultivating D. salina under different red light/dark cycles. (a) Cellular content of 9-cis βC, all-trans βC and total βC and (b) 9-cis/all-trans βC ratio and (c) specific growth rate of D. salina cultures grown under different light/dark cycles of red LED light supplied at 500 µmol m−2 s−1. Cultures of D. salina were grown to a cell density of ~0.2 million cells mL−1 under white LED light and then transferred into red LED light growth cycles of different duration. Carotenoids were analysed after 6 days growth.
The accumulation of carotenoids under red light has previously been shown to involve upregulation of phytoene synthase to increase the pool size of phytoene in D. salina cultures [1]. In order to test the effect of blue and red light on the β-carotene isomer composition, but without interference of de novo synthesis of β-carotene from phytoene, norflurazon, a phytoene desaturase inhibitor, was applied to the D. salina cultures (Figure 5). After 48 h without light, the total pool size of carotenoids was the same as that at the outset of the experiment (T0) before light treatment i.e., norflurazon blocked any further downstream synthesis of β-carotene. Under these conditions, the β-carotene isomer composition, 9-cis/all-trans βC, was 1.1, the same as that recorded for growth in a red light/dark cycle of 10 min/110 min. Both red and blue light treatments lowered the total pool size of total β-carotene, blue more than red: ~31–32% total β-carotene was destroyed under red light and under the 1:1 red/ blue light mix, and ~41% under blue light. Carotenoids absorb photons in the range 400–550 nm, exactly overlapping the emission spectrum of the blue LED (440–500 nm) therefore the greater loss in blue light compared to red was to be anticipated. Furthermore, although both all-trans βC and 9-cis βC were destroyed under blue light, the loss of 9-cis βC was very much greater: only ~40% of the content of 9-cis βC recorded in dark-treated cultures remained, compared to 78% for all-trans βC. Since 9-cis βC has a higher antioxidant activity than all-trans βC, this result might also be anticipated. Somewhat surprisingly, however, loss of 9-cis βC under red light compared to blue was much smaller and the ratio of 9-cis/all-trans βC was 3-fold greater than under blue light. Since the emission spectrum of the red LED (625–680 nm) emits photons that are not absorbed by β-carotene, these data imply isomerisation of extant all-trans βC to 9-cis βC to increase the content of 9-cis βC at the expense of all-trans βC during growth.
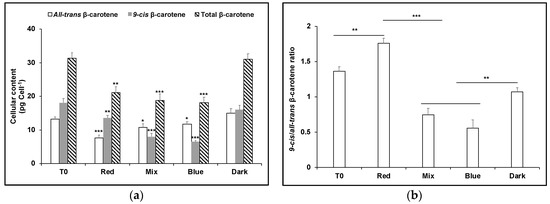
Figure 5.
Production of carotenes in D. salina cultures treated with 5 µM norflurazon. (a) cellular content of 9-cis βC, all-trans βC and total βC and (b) 9-cis/all-trans βC ratio. Cultures were grown for 24 h under white LED light then treated with norflurazon and maintained for a further 48 h under red, blue or a mix of red and blue LED light at 200 µmol photons m−2 s−1 or kept in the dark. T0: time point after growth for 24h under white LED light only, before addition of norflurazon. Results were analysed by one way ANOVA with posterior Dunnett’s test compared to T0 and Tukey multiple pairwise-comparisons. Asterisks represent different levels of significance (*** 0 < p ≤ 0.001, ** 0.001 < p ≤ 0.01, * 0.01 < p ≤ 0.05).
A similarly greater loss of all-trans βC compared to 9-cis βC in red light was obtained using Lee Bright Red, Medium Red or 787 Marius Red filters: these transmitted only a fraction (8.6%, 3.6% and 1.0%) of the light intensity applied with a red LED (1000 µmol m−2 s−1), but importantly excluded light wavelengths below 550 nm (Figure A1b–d). Each increased the total β-carotene pool size and the 9-cis/all-trans βC ratio was higher (Figure 6). With the 787 Marius Red filter, cells received only approximately 10–17 µmol m−2 s−1 light intensity of the red wavelength but this was still sufficient to increase the ratio of 9-cis/all-trans βC ratio, the amount of 9-cis βC per cell and total β-carotene to values approaching those found using white light at 1000 µmol m−2 s−1.
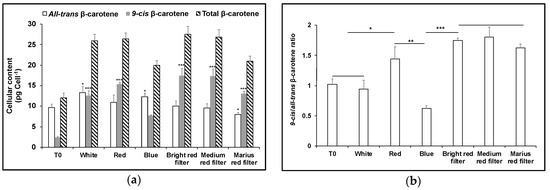
Figure 6.
Cultivation of D. salina using red light filters. D. salina was cultivated under white light to early orange phase (cell density of ~0.5 × 106 cells mL−1; carotenoid: chlorophyll ratio ~3), and then cultures were diluted with fresh medium to a cell density of ~0.2 × 106 cells mL−1 (no nutrient stress) (T0) and then further cultivated for 48 h under white, red or blue LED light at 1000 µmol m−2 s−1 or under white LED light at 1000 µmol m−2 s−1 covered with one of three different red filters (Lee filter 26 Bright red; Lee filter 27 Medium red; or Lee filter 787 Marius red). (a) Cellular content of 9-cis, all-trans and total β-carotene. (b) 9-cis/all-trans β-carotene ratio. Results were analysed by one way ANOVA and Tukey multiple pairwise-comparisons. Asterisks represent different levels of significance (*** 0 < p ≤ 0.001, ** 0.001 < p ≤ 0.01, * 0.01 < p ≤ 0.05).
The co-regulation by light and temperature on the β-carotene production and isomeric composition in D. salina is shown in Figure 7. Cultivation at 15 °C compared to 25 °C increased the 9-cis/all-trans βC ratio, especially under red light, but decreased the pool size of β-carotene measured over the same time frame (48 h).
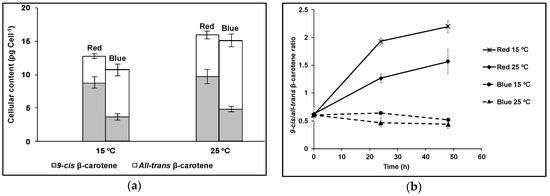
Figure 7.
D. salina cultivated under red or blue light at either 15 ℃ or 25 ℃. (a) Cellular content of 9-cis βC and all-trans βC (b) 9-cis/all-trans βC ratio. Cells were cultured under a light:dark 12h:12h white light growth regime to mid-log phase of the growth cycle (0.1–0.2 × 106 cells mL−1) then transferred to the dark for 24 h before treatment for 48 h at either 15 °C or 25 °C under continuous blue or red LED light at 1000 µmol m−2 s−1. Each culture condition was set up at least in triplicate.
Finally, the effects of blue and red light on the destruction of all-trans βC were evaluated. No reaction of all-trans βC solutions was detected under red light in nitrogen (Figure 8a). Under red light in air, (Figure 8b), 40% destruction of all-trans βC was recorded, whereas in blue light (Figure 8c), all-trans βC was fully destroyed within the same time frame. These data show that blue light is more damaging to all-trans βC than red light.
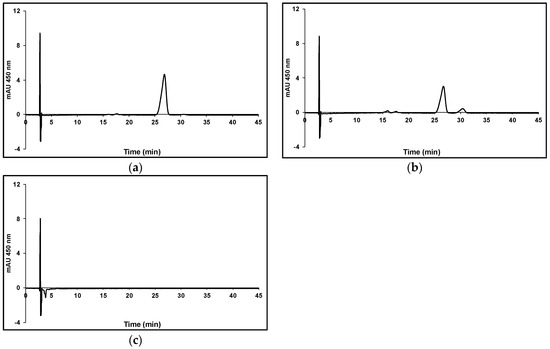
Figure 8.
Effect of red or blue LED light on the photo-destruction of all-trans βC. All-trans βC was dissolved in chloroform to a final concentration of 2.4 μM and vials were thoroughly flushed with either nitrogen or air, sealed and incubated for 24 h at 25 °C under different LED lights at 200 µmol m−2 s−1. (a) Red light under nitrogen; (b) Red light in air; (c) Blue light in air.
4. Discussion
In the present work, we found that under high intensity red LED light (up to 1000 µmol m−2 s−1) but in conditions of nutrient sufficiency, D. salina accumulated carotenoids rapidly within 48 h. Surprisingly, the major accumulated isomer was 9-cis βC, ~twice as much as all-trans βC. In vitro, 9-cis βC is a better scavenger of free radicals than all-trans βC [12], and reportedly degrades more rapidly compared to all-trans βC under both light and dark conditions [28]. Furthermore, chlorophyll absorbs photons in the range of the emission spectrum of the red LED used here (625–680 nm) and therefore in D. salina cultures in high intensity red light, a high rate of photo-oxidation of 9-cis βC might have been anticipated. Carotenoids are known antioxidants synthesized by many microalgae to prevent photoinhibition caused by photo-oxidation of photosynthetic reaction centres. Photooxidative damage occurs when species such as singlet oxygen (1O2) are formed under saturating light conditions as a result of transfer of energy from chlorophyll in the triplet excited state (3Chl*) to the ground state of O2. 1O2 react readily with fatty acids to form lipid peroxides and will set up a chain of oxygen activation events that may eventually lead to a hyperoxidant state and cell death [29]. Carotenoids protect the photosystems in the following ways: (i) by reacting with lipid peroxidation products and terminating free radical chain reactions as a result of the presence of the polyene chain; (ii) by scavenging 1O2 and dissipating the energy as heat; and (iii) by reacting with triplet excited chlorophyll 3Chl* to prevent formation of 1O2 or by dissipation of excess excitation energy through the xanthophyll cycle [3,30,31].
The simplest explanation to resolve the seeming anomaly, namely accumulation of the more readily degraded 9-cis βC under high intensity red light conditions that should be associated with high rates of photo-oxidation, invokes the activity of β-carotene isomerases, the gene transcripts of which are increased in light stress [21]. Davidi et al. [11] showed that all the enzymes in the biosynthetic pathway from phytoene to β-carotene were present in the plastidic lipid globules and included enriched concentrations β-carotene isomerases; two of these, 9-cis-βC-ISO1 and 9-cis-βC-ISO2, were shown to be responsible for the catalytic conversion of all-trans βC to 9-cis βC. Based on the data presented here we propose that the expression of gene transcripts of β-carotene isomerases may be triggered by specific light sensing, possibly through phytochrome.
In red light compared to blue, the apparent loss of 9-cis βC with norflurazon was surprisingly small and the ratio of 9-cis/all-trans βC was 3-fold greater than in blue light (Figure 5). Accumulation of 9-cis βC by phytoene synthase (PSY) gene activation, whose expression has been shown to be greatly increased 6–48 h following stress [11] was precluded by the presence of norflurazon, which blocked phytoene desaturation and consequent carotene synthesis. Under these conditions, the relative increase in pool size of 9-cis βC in red light implies a much higher rate of 9-cis βC formation from extant all-trans βC, caused by increased isomerase activity, than the rate of 9-cis βC destruction (see Figure 5). Carotenes absorb photons in the range 400–550 nm, exactly overlapping the emission spectrum of the blue LED (440–500 nm). However blue light catalysed a much more rapid rate of destruction of carotenes than red light (Figure 8). In blue LED light, 9-cis βC would be destroyed more rapidly than could be replenished by adjustment of the 9-cis/all-trans βC equilibrium position because increased β-carotene isomerase activity from red-light activated gene expression for β-carotene isomerases is not possible in blue light (see Figure 5).
Red light stimulation of the expression of gene transcripts of β-carotene isomerases by a phytochrome to increase the rate of accumulation of 9-cis βC by β-carotene isomerases is also supported by the increase in pool size of 9-cis βC under low intensity red light (Figure 6). Each of the Lee red light filters increased the total β-carotene pool size and the 9-cis/all-trans βC ratio was higher despite the much lower light intensity of the red wavelength compared to the red LED light. The effects of low temperature on 9-cis βC-accumulation in D. salina are also noteworthy, since enzyme catalysis typically shows a Q10 (temperature coefficient) ~2, yet in the present work, formation of 9-cis βC in low temperature compared to high was increased under red light, and had little effect in blue. In higher plants, the activated phytochrome B, a red light photoreceptor, is considered to function as the thermal sensor to sense environmental temperature [32]. Mutants with no phytochromes showed a constitutive warm temperature transcriptome even at low temperatures [33]. Red light sensing to increase the concentration of β-carotene isomerases and catalyse conversion of all-trans βC at low temperatures, as well as high, may play a significant role in photoprotection in D. salina.
We recently proposed that red light enhanced the production of carotenoids in a mechanism dependent on both photon flux density as well as upregulation of phytoene synthase by the red light photoreceptor phytochrome and that chlorophyll absorption of red light photons and subsequent plastoquinone reduction in photosystem II was coupled with oxygen reduction and phytoene desaturation by plastoquinol:oxygen oxidoreductase [1]. According to the findings in the previous work [1], the partitioning electron flux between photosynthesis and carotenoid biosynthesis could be augmented by addition of the regulation of the pool size of 9-cis βC, as seen in the Scheme 1.
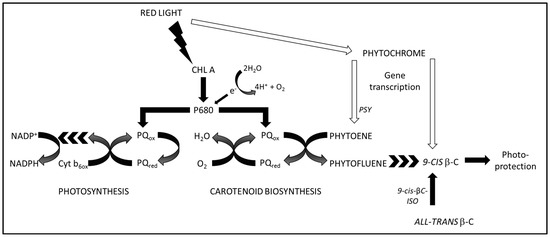
Scheme 1.
Regulation of the pool size of 9-cis βC. Red photon flux intensity controls the partitioning of electrons either for carotenoid biosynthesis or for photosynthesis, via energy absorption by chlorophyll and the PQ pool [1]. Red photon flux also controls phytochrome regulation of the production of gene transcripts for phytoene synthase and β-carotene isomerases. CHL A: chlorophyll a; P680: chlorophyll a, primary electron donor of Photosystem II; PQox: plastoquinone, oxidised form; PQred: plastoquinone, reduced form; Cyt b6ox: cytochrome b6f complex, oxidised form; NADP+: NADP oxidised form; NADPH: NADP reduced form; PSY: phytoene synthase; 9-cis-βC-ISO: 9-cis βC isomerase.
Red light sensing by phytochrome to increase the pool size of phytoene by phytoene synthase has been reported in higher plants [34]. Red light control of carotenoid biosynthesis coupled with the accumulation of the more readily oxidized 9-cis βC as a consequence of isomerisation from all-trans βC reserves would therefore rapidly increase the pool size of anti-oxidant to reduce the rate of formation of ROS under stress (See Scheme 1).
5. Conclusions
Red light availability regulates the isomerisation of all-trans β-carotene to 9-cis β-carotene and upregulates carotenoid biosynthesis in the halotolerant microalga Dunaliella salina. In red light 9-cis βC accumulated, caused by increase in the rate of isomerisation of all-trans βC to 9-cis βC relative to the rate of its destruction. Red light may have industrial value as an energy-efficient light source for production of natural 9-cis βC from D. salina.
Author Contributions
Y.X. and P.J.H. conceived the work, analysed the data, and wrote the article; Y.X. conducted experiments and curated data; P.J.H. agrees to serve as the author responsible for contact and ensures communication.
Funding
This work was supported by EU KBBE.2013.3.2-02 programme (D-Factory: 368 613870) and by the Interreg 2 Seas programme 2014-2020 co-funded by the European Regional Development Fund under subsidy contract No ValgOrize 2S05017.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
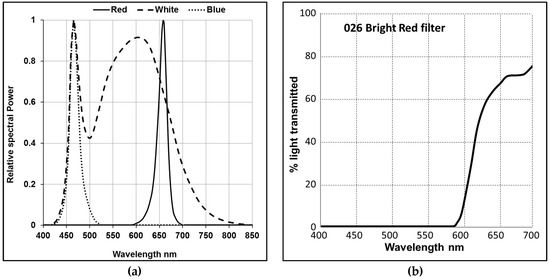
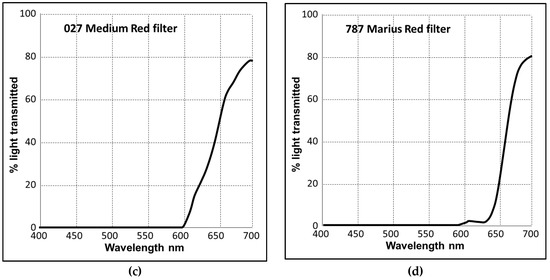
Figure A1.
(a) Typical relative spectral power distribution of white, blue and red LED lights in the Algem bioreactor; (b–d) The light transmission (Y%) for each wavelength (nm) of filters that were used to transmit red light. (b) Lee Filters 026 Bright red (Transmission 8.6%), (c) 027 Medium Red (Transmission 3.6%), (d) 787 Marius Red (Transmission 1.0%).
References
- Xu, Y.; Harvey, P.J. Carotenoid production by Dunaliella salina under red light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Nagendra, P.K.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Sugai, Y.; Uragami, C.; Gardiner, A.T.; Cogdell, R.J. Natural and artificial light-harvesting systems utilizing the functions of carotenoids. J. Photochem. Photobiol. C: Photochem. Rev. 2015, 25, 46–70. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Katz, A.; Avron, M. Accumulation of β-carotene in halotolerant algae: Purification and characterization of β-carotene-rich globules from Dunaliella Bardawil (Chlorophyceae). J. Phycol. 1982, 18, 529–537. [Google Scholar] [CrossRef]
- Ben-Amotz, A. Analysis of carotenoids with emphasis on 9-cis β-carotene in vegetables and fruits commonly consumed in Israel. Food Chem. 1998, 62, 515–520. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol. 1989, 91, 1040–1043. [Google Scholar] [CrossRef]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008, 26, 631–638. [Google Scholar] [CrossRef]
- Jiménez, C.; Pick, U. Differential stereoisomer compositions of β, β-carotene in thylakoids and in pigment globules in Dunaliella. J. Plant Physiol. 1994, 143, 257–263. [Google Scholar] [CrossRef]
- Davidi, L.; Shimoni, E.; Khozin-Goldberg, I.; Zamir, A.; Pick, U. Origin of β-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol. 2014, 164, 2139–2156. [Google Scholar] [CrossRef]
- Davidi, L.; Levin, Y.; Ben-Dor, S.; Pick, U. Proteome analysis of cytoplasmatic and plastidic β-carotene lipid droplets in Dunaliella bardawil. Plant Physiol. 2015, 167, 60–79. [Google Scholar] [CrossRef]
- Levin, G.; Mokady, S. Antioxidant activity of 9-cis compared to all-trans β-carotene in vitro. Free Radic. Biol. Med. 1994, 17, 77–82. [Google Scholar] [CrossRef]
- Jaime, L.; Mendiola, J.A.; Ibáñez, E.; Martin-Alvarez, P.J.; Cifuentes, A.; Reglero, G.; Señoráns, F.J. Beta-carotene isomer composition of sub- and supercritical carbon dioxide extracts. Antioxidant activity measurement. J. Agric. Food Chem. 2007, 55, 10585–10590. [Google Scholar] [CrossRef]
- Greenberger, S.; Harats, D.; Salameh, F.; Lubish, T.; Harari, A.; Trau, H.; Shaish, A. 9-cis-rich β-carotene powder of the alga Dunaliella reduces the severity of chronic plaque psoriasis: A randomized, double-blind, placebo-controlled clinical trial. J. Am. Coll. Nutr. 2012, 31, 320–326. [Google Scholar] [CrossRef]
- Harari, A.; Abecassis, R.; Relevi, N.; Levi, Z.; Ben-Amotz, A.; Kamari, Y.; Harats, D.; Shaish, A. Prevention of atherosclerosis progression by 9-cis-β-carotene rich alga Dunaliella in apoE-deficient mice. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Rotenstreich, Y.; Belkin, M.; Sadetzki, S.; Chetrit, A.; Ferman-Attar, G.; Sher, I.; Harari, A.; Shaish, A.; Harats, D. Treatment with 9-cis β-carotene-rich powder in patients with retinitis pigmentosa: A randomized crossover trial. JAMA Ophthalmol. 2013, 131, 985–992. [Google Scholar] [CrossRef]
- Zolberg Relevy, N.; Bechor, S.; Harari, A.; Ben-Amotz, A.; Kamari, Y.; Harats, D.; Shaish, A. The inhibition of macrophage foam cell formation by 9-cis β-carotene is driven by BCMO1 activity. PLoS ONE 2015, 10, e0115272. [Google Scholar] [CrossRef] [PubMed]
- Weinrich, T.; Xu, Y.; Wosu, C.; Harvey, P.J.; Jeffery, G. Mitochondrial Function, Mobility and Lifespan Are Improved in Drosophila melanogaster by Extracts of 9-cis-β-Carotene from Dunaliella salina. Marine Drugs 2019, 17, 279. [Google Scholar] [CrossRef]
- Sher, I.; Tzameret, A.; Peri-Chen, S.; Edelshtain, V.; Ioffe, M.; Sayer, A.; Buzhansky, L.; Gazit, E.; Rotenstreich, Y. Synthetic 9-cis-beta-carotene inhibits photoreceptor degeneration in cultures of eye cups from rpe65rd12 mouse model of retinoid cycle defect. Sci. Rep. 2018, 8, 6130. [Google Scholar] [CrossRef] [PubMed]
- Shaish, A.; Avron, M.; Ben-Amotz, A. Effect of inhibitors on the formation of stereoisomers in the biosynthesis of β-carotene in Dunaliella bardawil. Plant Cell Physiol. 1990, 31, 689–696. [Google Scholar]
- Davidi, L.; Pick, U. Novel 9-cis/all-trans β-carotene isomerases from plastidic oil bodies in Dunaliella bardawil catalyze the conversion of all-trans to 9-cis β-carotene. Plant Cell Rep. 2017, 36, 807–814. [Google Scholar] [CrossRef]
- Ben-Amotz, A. Effect of low temperature on the stereoisomer composition of β-carotene in the halotolerant alga Dunaliella bardawil (chlorophyta). J. Phycol. 1996, 32, 272–275. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Lers, A.; Avron, M. Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. The wavelength dependence of massive carotene synthesis in Dunaliella bardawil (Chlorophyceae). J. Phycol. 1989, 25, 175–178. [Google Scholar] [CrossRef]
- Orset, S.C.; Young, A.J. Exposure to low irradiances favors the synthesis of 9-cis beta, beta-carotene in Dunaliella salina (Teod.). Plant Physiol. 2000, 122, 609–618. [Google Scholar] [CrossRef]
- Gómez, P.I.; González, M.A. The effect of temperature and irradiance on the growth and carotenogenic capacity of seven strains of Dunaliella salina (Chlorophyta) cultivated under laboratory conditions. Biol. Res. 2005, 38, 151–162. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal growth media and sources of algal cultures. In Micro-algal Biotechnology, 1st ed.; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 456–465. [Google Scholar]
- Jimenez, C.; Pick, U. Differential reactivity of β-carotene isomers from Dunaliella bardawil toward oxygen radicals. Plant Physiol. 1993, 101, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hansberg, W.; Aguirre, J. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J. Theor. Biol. 1990, 142, 201–221. [Google Scholar] [CrossRef]
- Yokthongwattana, K.; Jin, E.; Melis, A. Chloroplast acclimation, photodamage and repair reactions of Photosystem-II in the model green alga, Dunaliella salina. In The Alga Dunaliella Biodiversity, Physiology, Genomics and Biotechnology, 1st ed.; Ben-Amotz, A., Polle, E.W., Subba Rao, D.V., Eds.; CRC Press: Enfield, NH, USA, 2009; pp. 273–299. [Google Scholar]
- Erickson, E.; Wakao, S.; Niyogi, K.K. Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J. 2015, 82, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Legris, M.; Burgie, E.S.; Rojas, C.C.R.; Neme, M.; Wigge, P.A.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Viczián, A.; Klose, C.; Ádám, É.; Nagy, F. New insights of red light-induced development. Plant Cell Environ. 2017, 40, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Welsch, R.; Beyer, P.; Hugueney, P.; Kleinig, H.; von Lintig, J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 2000, 211, 846–854. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).